Investigation of the Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) and Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction
Abstract
1. Introduction
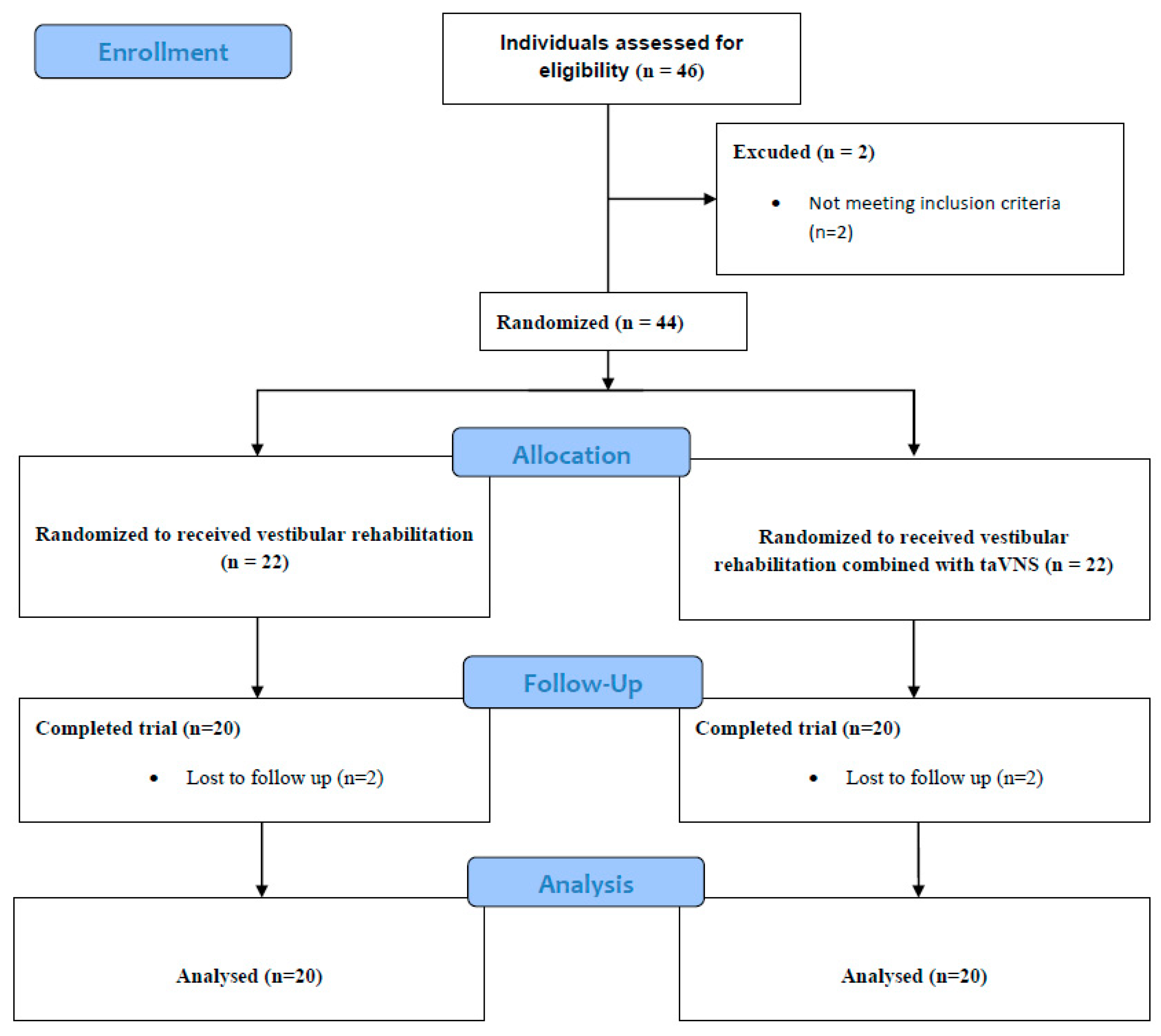
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measurements
2.3.1. Demographic Information Form
2.3.2. LOS
2.3.3. m-CTSIB
2.3.4. Static Balance Tests
2.3.5. DVA
2.3.6. VAS
2.3.7. TSK
2.3.8. HADS
2.3.9. DHI
2.4. Treatment Programs
2.4.1. Vestibular Rehabilitation Group (Group 1, n = 20)
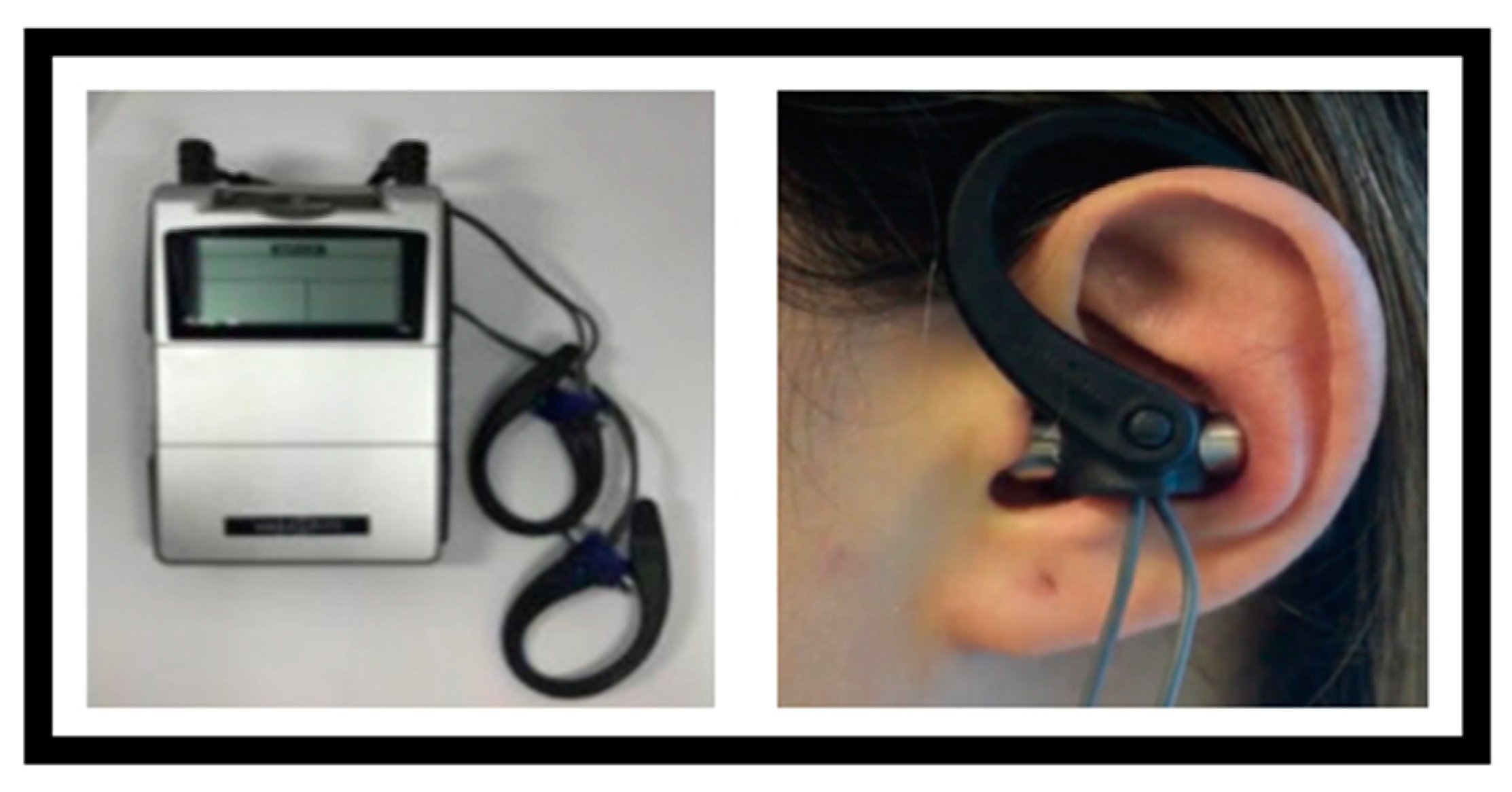
2.4.2. Vestibular Rehabilitation Group Combined with taVNS (Group 2, n = 20)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- Renga, V. Clinical Evaluation of Patients with Vestibular Dysfunction. Neurol. Res. Int. 2019, 2019, 3931548. [Google Scholar] [CrossRef]
- Herdman, S.J.; Schubert, M.C.; Das, V.E.; Tusa, R.J. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch. Otolaryngol. Head. Neck Surg. 2003, 129, 819–824. [Google Scholar] [CrossRef]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.; Goebel, J.A.; Shepard, N.T.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: From The American Physical Therapy Association Neurology Section. J. Neurol. Phys. Ther. 2016, 40, 124–155. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.L.; Alghwiri, A.A.; Alghadir, A. An overview of vestibular rehabilitation. Handb. Clin. Neurol. 2016, 137, 187–205. [Google Scholar] [CrossRef]
- Han, B.I.; Song, H.S.; Kim, J.S. Vestibular rehabilitation therapy: Review of indications, mechanisms, and key exercises. J. Clin. Neurol. 2011, 7, 184–196. [Google Scholar] [CrossRef]
- Black, F.O.; Pesznecker, S.C. Vestibular adaptation and rehabilitation. Curr. Opin. Otolaryngol. Head. Neck Surg. 2003, 11, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Lilios, A.; Chimona, T.; Nikitas, C.; Papadakis, C.; Chatziioannou, I.; Skoulakis, C. The Effect of Supervision in Vestibular Rehabilitation in Patients with Acute or Chronic Unilateral Vestibular Dysfunction: A Systematic Review. Otol. Neurotol. 2021, 42, e1422–e1431. [Google Scholar] [CrossRef]
- Gonzalez, H.F.J.; Yengo-Kahn, A.; Englot, D.J. Vagus Nerve Stimulation for the Treatment of Epilepsy. Neurosurg. Clin. N. Am. 2019, 30, 219–230. [Google Scholar] [CrossRef]
- Badran, B.W.; Austelle, C.W. The Future Is Noninvasive: A Brief Review of the Evolution and Clinical Utility of Vagus Nerve Stimulation. Focus (Am. Psychiatr. Publ.) 2022, 20, 3–7. [Google Scholar] [CrossRef]
- Usichenko, T.; Hacker, H.; Lotze, M. Transcutaneous auricular vagal nerve stimulation (taVNS) might be a mechanism behind the analgesic effects of auricular acupuncture. Brain Stimul. 2017, 10, 1042–1044. [Google Scholar] [CrossRef]
- Steidel, K.; Krause, K.; Menzler, K.; Strzelczyk, A.; Immisch, I.; Fuest, S.; Gorny, I.; Mross, P.; Hakel, L.; Schmidt, L.; et al. Transcutaneous auricular vagus nerve stimulation influences gastric motility: A randomized, double-blind trial in healthy individuals. Brain Stimul. 2021, 14, 1126–1132. [Google Scholar] [CrossRef]
- Austelle, C.W.; O’Leary, G.H.; Thompson, S.; Gruber, E.; Kahn, A.; Manett, A.J.; Short, B.; Badran, B.W. A Comprehensive Review of Vagus Nerve Stimulation for Depression. Neuromodulation 2022, 25, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Kaniusas, E.; Kampusch, S.; Tittgemeyer, M.; Panetsos, F.; Gines, R.F.; Papa, M.; Kiss, A.; Podesser, B.; Cassara, A.M.; Tanghe, E.; et al. Current Directions in the Auricular Vagus Nerve Stimulation II—An Engineering Perspective. Front. Neurosci. 2019, 13, 772. [Google Scholar] [CrossRef]
- Kreisberg, E.; Esmaeilpour, Z.; Adair, D.; Khadka, N.; Datta, A.; Badran, B.W.; Bremner, J.D.; Bikson, M. High-resolution computational modeling of the current flow in the outer ear during transcutaneous auricular Vagus Nerve Stimulation (taVNS). Brain Stimul. 2021, 14, 1419–1430. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.Y.; Wang, D.; Wu, M.Z.; He, J.K.; Zhang, J.L.; Zhao, B.; Hou, L.W.; Wang, J.Y.; Wang, L.; et al. Transcutaneous Auricular Vagus Nerve Stimulation: From Concept to Application. Neurosci. Bull. 2021, 37, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Tona, K.D.; Ouwerkerk, L.; van Paridon, J.; Poletiek, F.; van Steenbergen, H.; Bosch, J.A.; Nieuwenhuis, S. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 2019, 12, 635–642. [Google Scholar] [CrossRef]
- Chakravarthy, K.V.; Xing, F.; Bruno, K.; Kent, A.R.; Raza, A.; Hurlemann, R.; Kinfe, T.M. A Review of Spinal and Peripheral Neuromodulation and Neuroinflammation: Lessons Learned Thus Far and Future Prospects of Biotype Development. Neuromodulation 2019, 22, 235–243. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef]
- Beh, S.C. Emerging evidence for noninvasive vagus nerve stimulation for the treatment of vestibular migraine. Expert. Rev. Neurother. 2020, 20, 991–993. [Google Scholar] [CrossRef]
- Wu, D.; Liu, B.; Wu, Y.; Wang, Y.; Sun, J.; Yang, J.; Duan, J.; Liu, G.; Cao, K.; Zhang, Y.; et al. Meniere Disease treated with transcutaneous auricular vagus nerve stimulation combined with betahistine Mesylate: A randomized controlled trial. Brain Stimul. 2023, 16, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Beh, S.C. Noninvasive Vagus Nerve Stimulation for Nausea Prevention During BPPV Treatment. Neuromodulation 2020, 23, 886–887. [Google Scholar] [CrossRef]
- Eren, O.E.; Filippopulos, F.; Sonmez, K.; Mohwald, K.; Straube, A.; Schoberl, F. Non-invasive vagus nerve stimulation significantly improves quality of life in patients with persistent postural-perceptual dizziness. J. Neurol. 2018, 265 (Suppl. S1), 63–69. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Liu, Y.; Zhong, J.; Jiang, L.; Liu, Y.; Chen, Q.; Xie, Y.; Zhang, Q. Electroacupuncture for Tinnitus: A Systematic Review. PLoS ONE 2016, 11, e0150600. [Google Scholar] [CrossRef] [PubMed]
- Beh, S.C. Nystagmus and Vertigo in Acute Vestibular Migraine Attacks: Response to Non-Invasive Vagus Nerve Stimulation. Otol. Neurotol. 2021, 42, e233–e236. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, H.; Yan, Z.; Zhang, Y.; Liu, X.; Hou, X.; Chen, W.; Tu, Y.; Hodges, S.; et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: An fMRI study. Reg. Anesth. Pain. Med. 2021, 46, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Blake, J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am. J. Manag. Care 2017, 23 (Suppl. S17), S312–S316. [Google Scholar]
- Kilic, G.; Celikyurt, C.; Algun, Z.C. The Effect of Vestibular Rehabilitation on Balance and Quality of Life in Patients with Bilateral Vestibular Hypofunction. Indian. J. Otolaryngol. Head. Neck Surg. 2023, 75, 2091–2099. [Google Scholar] [CrossRef]
- Clark, S.; Rose, D.J.; Fujimoto, K. Generalizability of the limits of stability test in the evaluation of dynamic balance among older adults. Arch. Phys. Med. Rehabil. 1997, 78, 1078–1084. [Google Scholar] [CrossRef]
- Ertunc Gulcelik, G.; Tarakci, D.; Gedik Soyuyuce, O.; Gence Gumus, Z.; Korkut, N.; Algun, Z.C. Research on the Effects of a Web-Based System With Oculomotor and Optokinetic Stimuli on Vestibular Rehabilitation. Am. J. Phys. Med. Rehabil. 2021, 100, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Dannenbaum, E.; Paquet, N.; Hakim-Zadeh, R.; Feldman, A.G. Optimal parameters for the clinical test of dynamic visual acuity in patients with a unilateral vestibular deficit. J. Otolaryngol. 2005, 34, 13–19. [Google Scholar] [CrossRef]
- Genc, S.G.; Budak, M.; Yilmaz, M.S.; Algun, Z.C. Effects of structured exercise program on severity of dizziness, kinesiophobia, balance, fatigue, quality of sleep, activities of daily living, and quality of life in bilateral vestibular hypofunction. Medicine 2023, 102, e34435. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head. Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Anson, E.R.; Carender, W.J.; Hoppes, C.W.; Cass, S.P.; Christy, J.B.; Cohen, H.S.; Fife, T.D.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Updated Clinical Practice Guideline From the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J. Neurol. Phys. Ther. 2022, 46, 118–177. [Google Scholar] [CrossRef]
- Konakoglu, G.; Ozden, A.V.; Solmaz, H.; Bildik, C. The effect of auricular vagus nerve stimulation on electroencephalography and electromyography measurements in healthy persons. Front. Physiol. 2023, 14, 1215757. [Google Scholar] [CrossRef] [PubMed]
- Demircioglu, G.; Ozden, A.V.; Genc, H. Comparison of the efficacy of auricular vagus nerve stimulation and conventional low back rehabilitation in patients with chronic low back pain. Complement. Ther. Clin. Pract. 2024, 56, 101862. [Google Scholar] [CrossRef]
- Kutlu, N.; Ozden, A.V.; Alptekin, H.K.; Alptekin, J.O. The Impact of Auricular Vagus Nerve Stimulation on Pain and Life Quality in Patients with Fibromyalgia Syndrome. Biomed. Res. Int. 2020, 2020, 8656218. [Google Scholar] [CrossRef]
- Kimbrell, T.A.; Ketter, T.A.; George, M.S.; Little, J.T.; Benson, B.E.; Willis, M.W.; Herscovitch, P.; Post, R.M. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol. Psychiatry 2002, 51, 237–252. [Google Scholar] [CrossRef]
- Brody, A.L.; Saxena, S.; Mandelkern, M.A.; Fairbanks, L.A.; Ho, M.L.; Baxter, L.R. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 2001, 50, 171–178. [Google Scholar] [CrossRef]
- George, M.S.; Sackeim, H.A.; Rush, A.J.; Marangell, L.B.; Nahas, Z.; Husain, M.M.; Lisanby, S.; Burt, T.; Goldman, J.; Ballenger, J.C. Vagus nerve stimulation: A new tool for brain research and therapy. Biol. Psychiatry 2000, 47, 287–295. [Google Scholar] [CrossRef]
- Frangos, E.; Ellrich, J.; Komisaruk, B.R. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Frangos, E.; Komisaruk, B.R. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: fMRI Evidence in Healthy Humans. Brain Stimul. 2017, 10, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Kario, K. The insular cortex and cardiovascular system: A new insight into the brain-heart axis. J. Am. Soc. Hypertens. 2010, 4, 174–182. [Google Scholar] [CrossRef] [PubMed]
- de Morree, H.M.; Rutten, G.J.; Szabo, B.M.; Sitskoorn, M.M.; Kop, W.J. Effects of Insula Resection on Autonomic Nervous System Activity. J. Neurosurg. Anesthesiol. 2016, 28, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, E.S.; Papacostas, S.S.; Charalambous, M.; Eracleous, E.; Thodi, C.; Pantzaris, M. Vertigo and imbalance caused by a small lesion in the anterior insula. Electromyogr. Clin. Neurophysiol. 2006, 46, 185–192. [Google Scholar]
- Kirsch, V.; Keeser, D.; Hergenroeder, T.; Erat, O.; Ertl-Wagner, B.; Brandt, T.; Dieterich, M. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct. Funct. 2016, 221, 1291–1308. [Google Scholar] [CrossRef]
- Martins, E.S.D.C.; Bastos, V.H.; de Oliveira Sanchez, M.; Nunes, M.K.; Orsini, M.; Ribeiro, P.; Velasques, B.; Teixeira, S.S. Effects of vestibular rehabilitation in the elderly: A systematic review. Aging Clin. Exp. Res. 2016, 28, 599–606. [Google Scholar] [CrossRef]
- Wipperman, J. Dizziness and vertigo. Prim. Care 2014, 41, 115–131. [Google Scholar] [CrossRef]
- Yardley, L.; Beech, S.; Zander, L.; Evans, T.; Weinman, J. A randomized controlled trial of exercise therapy for dizziness and vertigo in primary care. Br. J. Gen. Pract. 1998, 48, 1136–1140. [Google Scholar]
- Tramontano, M.; Belluscio, V.; Bergamini, E.; Allevi, G.; De Angelis, S.; Verdecchia, G.; Formisano, R.; Vannozzi, G.; Buzzi, M.G. Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors 2022, 22, 8553. [Google Scholar] [CrossRef]
- Abasi, A.; Raji, P.; Friedman, J.H.; Hadian, M.R.; Hoseinabadi, R.; Abbasi, S.; Baghestani, A. Effects of Vestibular Rehabilitation on Fatigue and Activities of Daily Living in People with Parkinson’s Disease: A Pilot Randomized Controlled Trial Study. Parkinsons Dis. 2020, 2020, 8624986. [Google Scholar] [CrossRef]
- Ghaffari, A.; Asadi, B.; Zareian, A.; Akbarfahimi, M.; Raissi, G.R.; Fathali Lavasani, F. The Effects of Vestibular Rehabilitation on Poststroke Fatigue: A Randomized Controlled Trial Study. Stroke Res. Treat. 2022, 2022, 3155437. [Google Scholar] [CrossRef]
- Sever, E.; Kilic, G.; Algun, Z.C. The Effects of Vestibular Rehabilitation on Kinesiophobia and Balance with Individuals Who has Vestibular Hypofunction. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74 (Suppl. S3), 4319–4324. [Google Scholar] [CrossRef]
- Fang, J.; Rong, P.; Hong, Y.; Fan, Y.; Liu, J.; Wang, H.; Zhang, G.; Chen, X.; Shi, S.; Wang, L.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2016, 79, 266–273. [Google Scholar] [CrossRef]
- Guinand, N.; Boselie, F.; Guyot, J.P.; Kingma, H. Quality of life of patients with bilateral vestibulopathy. Ann. Otol. Rhinol. Laryngol. 2012, 121, 471–477. [Google Scholar] [CrossRef]
| Group 1 (n = 20) | Group 2 (n = 20) | ||
|---|---|---|---|
| Gender (n/%) | Female | 16/80 | 7/35 |
| Male | 4/20 | 13/65 | |
| Age (mean ± SD) | 46.40 ± 13.28 | 45.00 ± 13.21 | |
| Cigarettes (n/%) | Yes | 4/20 | 10/50 |
| No | 16/80 | 10/50 | |
| Alcohol (n/%) | Yes | 0 | 2/10 |
| No | 20/100 | 18/90 |
| Definition | Exercises | |
|---|---|---|
| Vestibular adaptation exercises | VOR*1 VOR*2 The VOR response is developed by activating the adaptation mechanism. Head movements are made during visual fixation. Includes saccadic and pursuit eye movements. |
|
| Oculomotor exercises | Develops the visual system. Includes saccadic and pursuit eye movements. |
|
| Static and dynamic balance exercises | This includes balance exercises in which the support area, support surface, arm position, head and eye movements are varied. |
|
| Posture exercises | This includes exercises especially for the neck, shoulder, and back muscles. |
|
| Walking exercises | It includes walking and balance exercises with different tasks. |
|
| Group 1 | Group 2 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | p | Before Treatment | After Treatment | p | ||||
| LOS | MV (°/s) | Forward | 4.55 ± 0.8 | 4.59 ± 0.55 | 0.883 | 4.66 ± 0.59 | 4.91 ± 0.75 | 0.250 | 0.260 |
| Right Forward | 4.42 ± 0.8 | 4.71 ± 0.89 | 0.290 | 4.97 ± 0.84 | 5.04 ± 0.68 | 0.646 | 0.788 | ||
| Right | 3.67 ± 0.87 | 3.94 ± 0.58 | 0.166 | 3.92 ± 0.59 | 4.07 ± 1.04 | 0.444 | 0.255 | ||
| Right Backward | 2 ± 0.51 | 2.16 ± 0.81 | 0.585 | 2.14 ± 0.73 | 2.31 ± 0.83 | 0.513 | 0.684 | ||
| Backward | 1.1 ± 0.32 | 1.16 ± 0.26 | 0.739 | 1.14 ± 0.35 | 1.21 ± 0.35 | 0.581 | 0.488 | ||
| Left Backward | 1.99 ± 0.46 | 2.11 ± 0.51 | 0.475 | 2.4 ± 0.51 | 2.29 ± 0.71 | 0.485 | 0.946 | ||
| Left | 3.59 ± 0.66 | 3.7 ± 0.8 | 0.626 | 4.01 ± 0.59 | 4.18 ± 0.63 | 0.350 | 0.178 | ||
| Left Forward | 4.76 ± 0.83 | 4.99 ± 0.87 | 0.266 | 4.95 ± 0.72 | 5.43 ± 0.91 | 0.020 * | 0.068 | ||
| EP (%) | Forward | 70.45 ± 19.7 | 73 ± 11.75 | 0.659 | 77.15 ± 12.11 | 84.05 ± 10.17 | 0.081 | 0.059 | |
| Right Forward | 77.8 ± 16.87 | 81.95 ± 17.42 | 0.433 | 88.2 ± 19.56 | 90.05 ± 14.17 | 0.590 | 0.734 | ||
| Right | 72.4 ± 18.99 | 77.25 ± 12.53 | 0.309 | 76.75 ± 14.92 | 80.8 ± 9.34 | 0.227 | 0.311 | ||
| Right Backward | 69.25 ± 25.2 | 79.55 ± 18.79 | 0.088 | 73.45 ± 18.79 | 79 ± 17.29 | 0.259 | 0.337 | ||
| Backward | 70.95 ± 20.12 | 79.9 ± 18.51 | 0.088 | 68.5 ± 21.41 | 76.4 ± 20.66 | 0.191 | 0.242 | ||
| Left Backward | 71.85 ± 21.22 | 79.3 ± 17.71 | 0.238 | 80.4 ± 14.38 | 79.05 ± 13.74 | 0.743 | 0.763 | ||
| Left | 72.5 ± 13.96 | 76.1 ± 14.4 | 0.446 | 79.2 ± 9.33 | 80.35 ± 11.71 | 0.709 | 0.733 | ||
| Left Forward | 86.2 ± 20.22 | 89.9 ± 19.58 | 0.409 | 89.1 ± 16.56 | 93.75 ± 16.79 | 0.160 | 0.383 | ||
| MXE (%) | Forward | 79.45 ± 15.46 | 80.7 ± 11.31 | 0.755 | 82.95 ± 13.25 | 89.75 ± 7.4 | 0.070 | 0.054 | |
| Right Forward | 87.45 ± 9.96 | 88.65 ± 11.59 | 0.684 | 93.65 ± 17.29 | 94.8 ± 12.35 | 0.779 | 0.735 | ||
| Right | 80.05 ± 15.04 | 81.45 ± 8.96 | 0.709 | 82.55 ± 10.46 | 82.65 ± 7.75 | 0.965 | 0.973 | ||
| Right Backward | 74.4 ± 22.7 | 83.8 ± 17.88 | 0.032 * | 82.9 ± 12.23 | 90.75 ± 9.84 | 0.004 ** | 0.031 * | ||
| Backward | 86.45 ± 32.74 | 85.6 ± 13.82 | 0.727 | 79.4 ± 18.5 | 86.9 ± 14.8 | 0.097 | 0.165 | ||
| Left Backward | 77.1 ± 16.87 | 84.5 ± 14.76 | 0.154 | 86.6 ± 8.85 | 87.1 ± 10.08 | 0.801 | 0.868 | ||
| Left | 80.6 ± 12.12 | 126.95 ± 195.11 | 0.239 | 81.65 ± 8.68 | 84.1 ± 8.33 | 0.275 | 0.368 | ||
| Left Forward | 91 ± 17.86 | 95.75 ± 15.23 | 0.303 | 93.65 ± 13.19 | 97.9 ± 11.57 | 0.098 | 0.286 | ||
| RT (s) | Forward | 1.25 ± 0.3 | 1.17 ± 0.36 | 0.147 | 1.18 ± 0.49 | 0.92 ± 0.41 | 0.044 * | 0.317 | |
| Right Forward | 1.01 ± 0.39 | 0.98 ± 0.23 | 0.501 | 0.99 ± 0.25 | 0.92 ± 0.27 | 0.286 | 0.267 | ||
| Right | 0.95 ± 0.22 | 0.88 ± 0.27 | 0.881 | 0.95 ± 0.18 | 0.93 ± 0.16 | 0.709 | 0.946 | ||
| Right Backward | 1.2 ± 0.32 | 0.95 ± 0.33 | 0.014 * | 0.86 ± 0.42 | 0.88 ± 0.17 | 0.616 | 0.329 | ||
| Backward | 1.04 ± 0.24 | 0.96 ± 0.26 | 0.376 | 0.95 ± 0.23 | 0.9 ± 0.11 | 0.267 | 0.154 | ||
| Left Backward | 1 ± 0.27 | 0.86 ± 0.26 | 0.079 | 0.99 ± 0.2 | 0.9 ± 0.2 | 0.014 * | 0.072 | ||
| Left | 0.95 ± 0.35 | 0.94 ± 0.15 | 0.629 | 0.88 ± 0.27 | 0.87 ± 0.19 | 0.055 | 0.227 | ||
| Left Forward | 1.43 ± 2.13 | 0.96 ± 0.11 | 0.324 | 1.01 ± 0.33 | 0.99 ± 0.16 | 0.871 | 0.875 | ||
| DC (%) | Forward | 83.35 ± 12 | 79.8 ± 10.07 | 0.165 | 81.1 ± 10.49 | 79.95 ± 13.84 | 0.837 | 0.914 | |
| Right Forward | 68.55 ± 14.04 | 67.95 ± 16.87 | 0.914 | 73.85 ± 11.61 | 73 ± 13.47 | 0.629 | 0.989 | ||
| Right | 69.6 ± 11.35 | 70.75 ± 14.6 | 0.499 | 76.9 ± 12.74 | 76.6 ± 9.39 | 0.935 | 0.933 | ||
| Right Backward | 64.2 ± 14.41 | 63.6 ± 15.83 | 0.908 | 62.15 ± 19.05 | 67.3 ± 14.02 | 0.422 | 0.310 | ||
| Backward | 62.95 ± 23.87 | 72.55 ± 19.59 | 0.260 | 76 ± 16.43 | 70.2 ± 16.95 | 0.324 | 0.228 | ||
| Left Backward | 68.25 ± 15.78 | 67.25 ± 14.92 | 0.823 | 69.05 ± 14.28 | 67.85 ± 14.85 | 0.867 | 0.560 | ||
| Left | 74.7 ± 17.81 | 76.5 ± 12.32 | 0.763 | 70.25 ± 14.52 | 71.6 ± 13.88 | 0.735 | 0.765 | ||
| Left Forward | 70.6 ± 17.15 | 73.3 ± 12.24 | 0.643 | 74.25 ± 10.71 | 72.7 ± 11.56 | 0.685 | 0.662 | ||
| m-CTSIB (mm/s) | Hard | EO | 9.47 ± 2.17 | 8.93 ± 2 | 0.161 | 11.09 ± 3.2 | 9.48 ± 2.01 | 0.045 * | 0.065 |
| EC | 13.55 ± 5.59 | 12.22 ± 4.11 | 0.205 | 14.63 ± 5.55 | 11.39 ± 4.06 | 0.004 ** | 0.016 * | ||
| Soft | EO | 12.95 ± 3.37 | 11.94 ± 3.07 | 0.062 | 12.29 ± 2.34 | 11.85 ± 2 | 0.372 | 0.532 | |
| EC | 21.38 ± 8.5 | 18.74 ± 7.4 | 0.179 | 22.27 ± 8.72 | 16.28 ± 5.01 | 0.002 ** | 0.019 * | ||
| Group 1 | Group 2 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | p | Before Treatment | After Treatment | p | ||||
| Tandem (s) | EO | 28.73 ± 3.07 | 30 ± 0 | 0.068 | 26.7 ± 8.11 | 30 ± 0 | 0.109 | ||
| EC | 14.27 ± 8.25 | 26.13 ± 5.92 | 0.000 ** | 16.85 ± 13.28 | 27.96 ± 4.7 | 0.003 ** | 0.377 | ||
| OLS (s) | Hard EO | Right foot | 23.64 ± 9.54 | 28.41 ± 5.07 | 0.008 ** | 25.98 ± 9.24 | 29.18 ± 2.73 | 0.068 | 0.917 |
| Left foot | 25.84 ± 9.03 | 28.56 ± 4.45 | 0.043 * | 23.8 ± 10.49 | 28.65 ± 3.28 | 0.028 * | 0.486 | ||
| Hard EC | Right foot | 9.14 ± 8.69 | 20.44 ± 9.8 | 0.000 ** | 10.06 ± 8.32 | 25.42 ± 7.75 | 0.000 ** | 0.101 | |
| Left foot | 8.28 ± 7.56 | 19.24 ± 10.32 | 0.000 ** | 8.81 ± 7.74 | 23.05 ± 8.7 | 0.000 ** | 0.216 | ||
| Soft EO | Right foot | 19.86 ± 11.37 | 27.12 ± 5.61 | 0.003 ** | 25.12 ± 7.75 | 28.21 ± 4.24 | 0.018 * | 0.579 | |
| Left foot | 19.2 ± 12.49 | 26.95 ± 6.41 | 0.004 ** | 21.74 ± 9.84 | 28.4 ± 4.24 | 0.003 ** | 0.283 | ||
| Soft EC | Right foot | 5.78 ± 6.57 | 15.39 ± 9.92 | 0.000 ** | 5.85 ± 4.32 | 20.1 ± 8.57 | 0.000 ** | 0.079 | |
| Left foot | 6.12 ± 7.71 | 15.23 ± 10.28 | 0.000 ** | 4.76 ± 3.4 | 18.06 ± 8.06 | 0.000 ** | 0.242 | ||
| DVA | 0.35 ± 0.34 | 0.15 ± 0.26 | 0.003 ** | 0.53 ± 0.35 | 0.32 ± 0.4 | 0.013 * | 0.146 | ||
| Dizziness | VAS | 7.33 ± 1.33 | 1.57 ± 1.08 | 0.000 ** | 6.24 ± 1.9 | 0.18 ± 0.46 | 0.000 ** | 0.000 ** | |
| Fatigue | VAS | 7.38 ± 1.67 | 2.8 ± 1.3 | 0.000 ** | 7.36 ± 2.18 | 1.09 ± 1.3 | 0.000 ** | 0.000 ** | |
| TSK | 50.2 ± 3.61 | 27.2 ± 1.74 | 0.000 ** | 49.3 ± 3.74 | 24.35 ± 1.73 | 0.000 ** | 0.000 ** | ||
| HADS | 21.2 ± 5.18 | 13.1 ± 5.1 | 0.000 ** | 21.15 ± 5.86 | 7.9 ± 4.02 | 0.000 ** | 0.003 ** | ||
| HADS-A | 11.9 ± 2.55 | 7.3 ± 2.6 | 0.000 ** | 11.8 ± 2.76 | 4.6 ± 2.37 | 0.000 ** | 0.001 ** | ||
| HADS-D | 9.3 ± 3.06 | 5.8 ± 2.78 | 0.000 ** | 9.35 ± 3.5 | 3.3 ± 2.15 | 0.000 ** | 0.004 ** | ||
| DHI | 58.4 ± 25.48 | 5.5 ± 7.37 | 0.000 ** | 82.1 ± 19.63 | 6 ± 6.62 | 0.000 ** | 0.761 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türk Kalkan, T.; Tarakçi, D.; Kiliç, G.; Çelikyurt, C. Investigation of the Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) and Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction. Medicina 2025, 61, 872. https://doi.org/10.3390/medicina61050872
Türk Kalkan T, Tarakçi D, Kiliç G, Çelikyurt C. Investigation of the Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) and Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction. Medicina. 2025; 61(5):872. https://doi.org/10.3390/medicina61050872
Chicago/Turabian StyleTürk Kalkan, Tuğba, Devrim Tarakçi, Gamze Kiliç, and Cengiz Çelikyurt. 2025. "Investigation of the Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) and Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction" Medicina 61, no. 5: 872. https://doi.org/10.3390/medicina61050872
APA StyleTürk Kalkan, T., Tarakçi, D., Kiliç, G., & Çelikyurt, C. (2025). Investigation of the Effectiveness of Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) and Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction. Medicina, 61(5), 872. https://doi.org/10.3390/medicina61050872






