Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Stratification
2.2. Statistical Analysis
3. Results
3.1. Clinical Presentation and Risk Factors
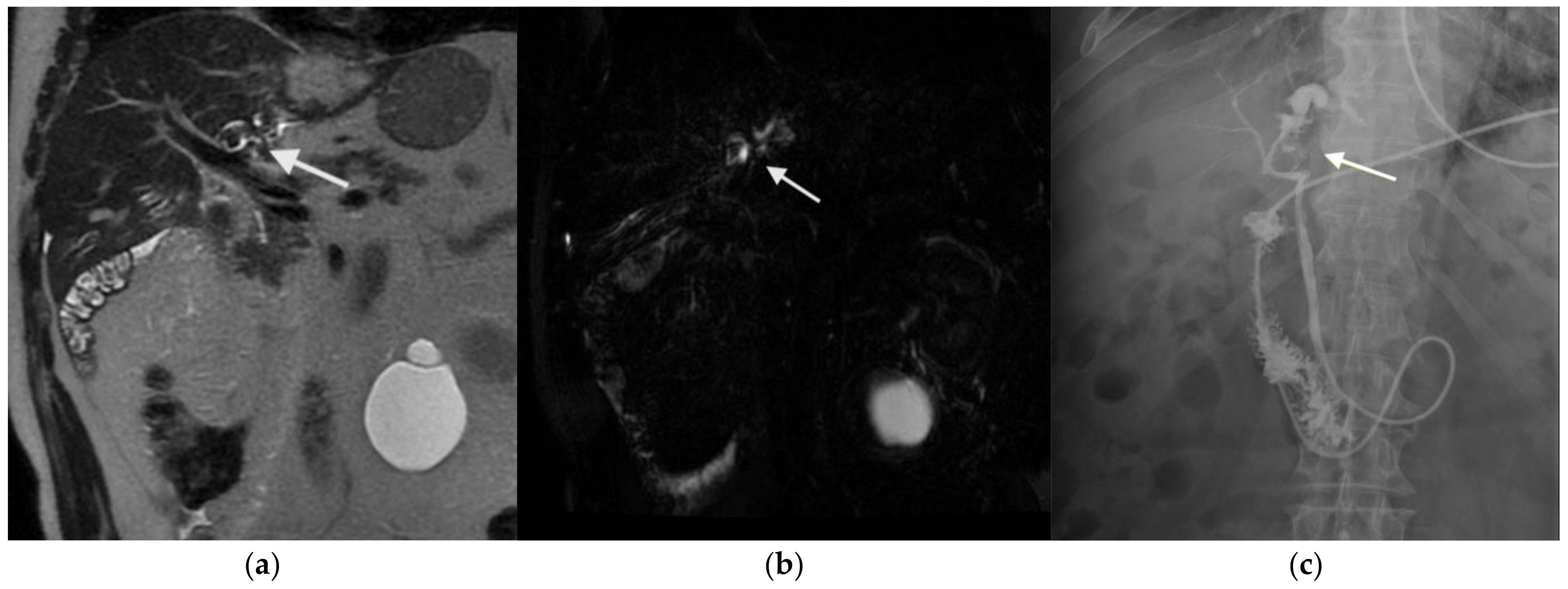
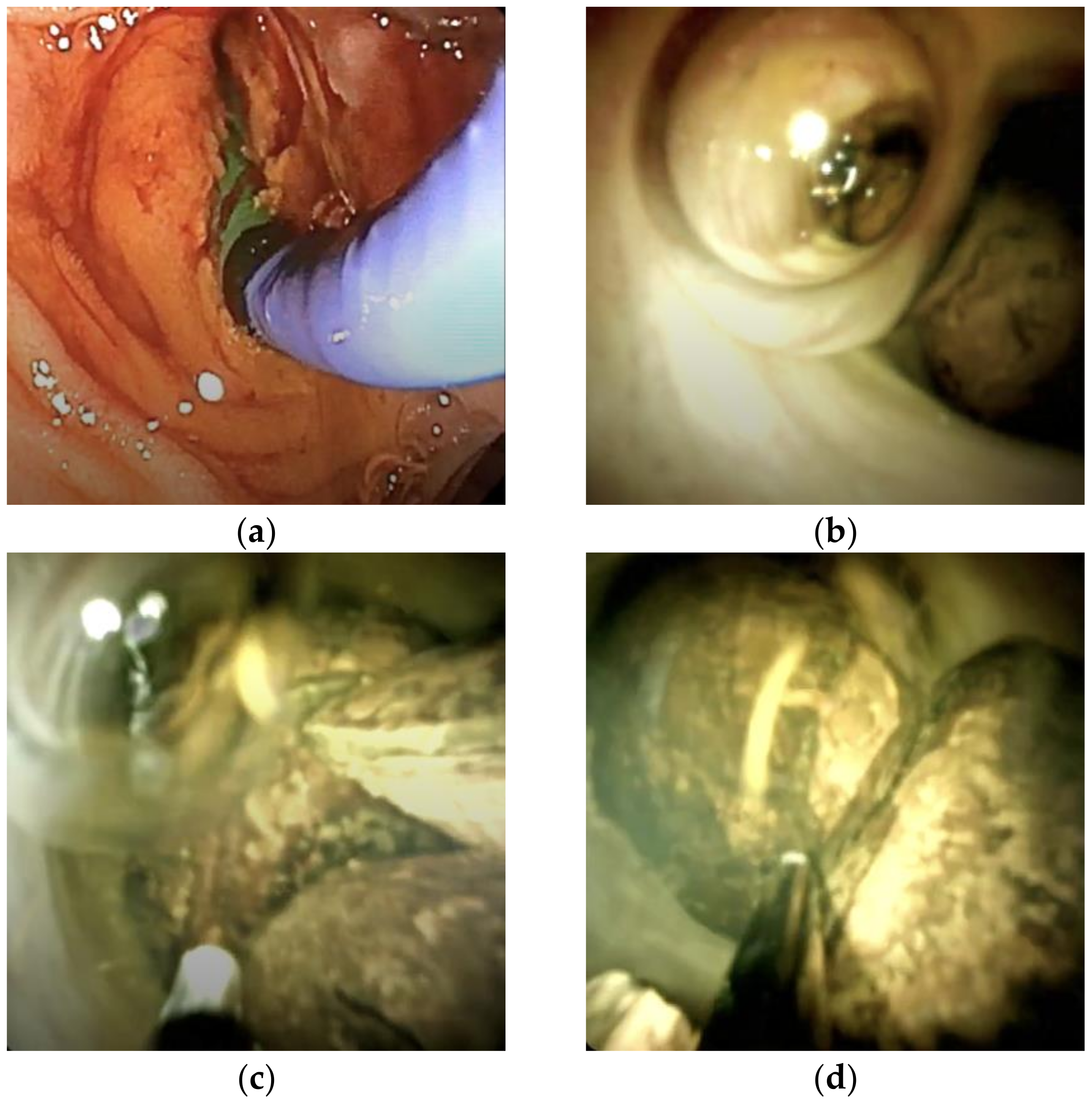
3.2. Figures, Tables, and Schemes
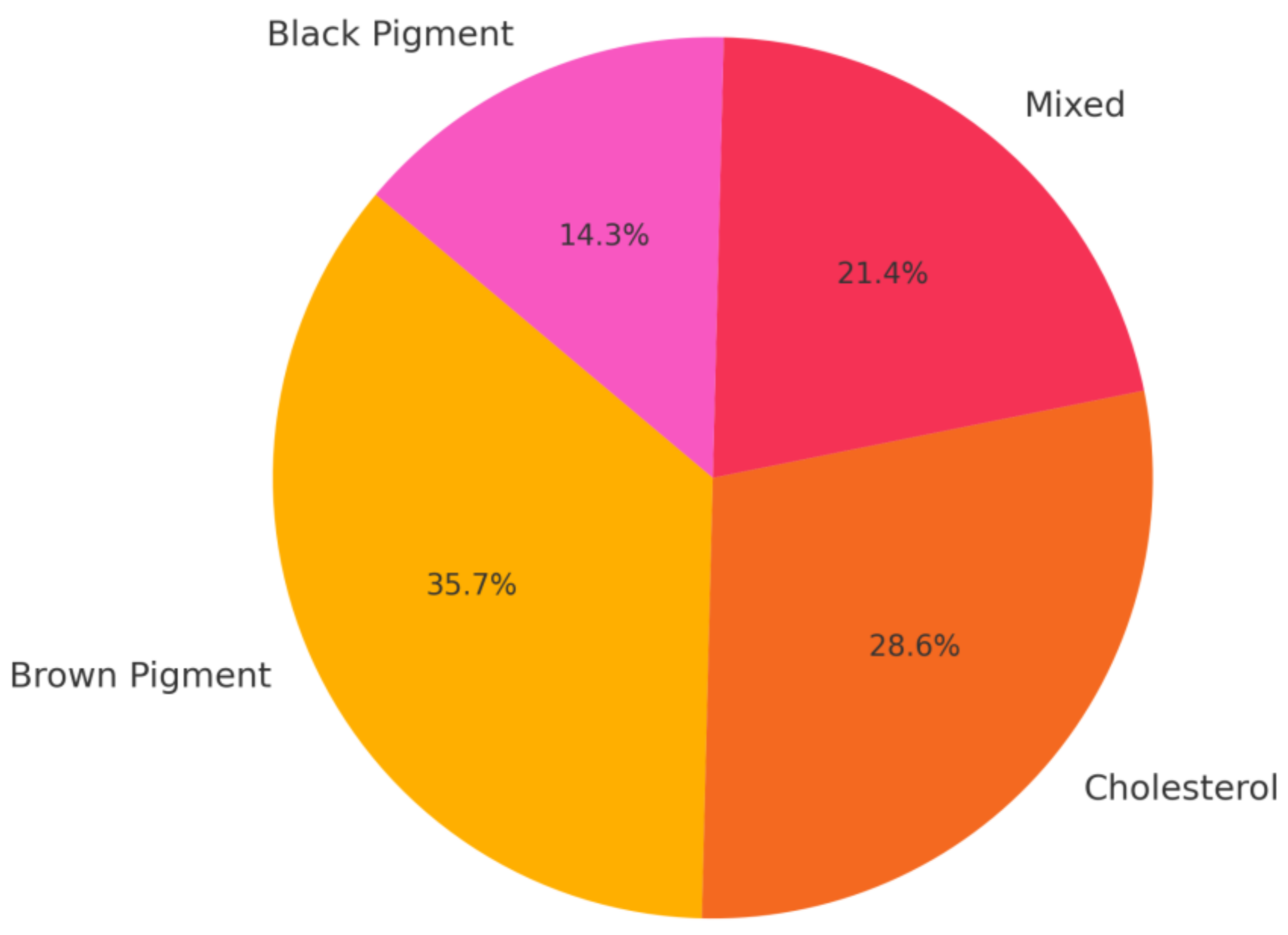
3.3. Stone Composition
3.4. Microbiological Findings
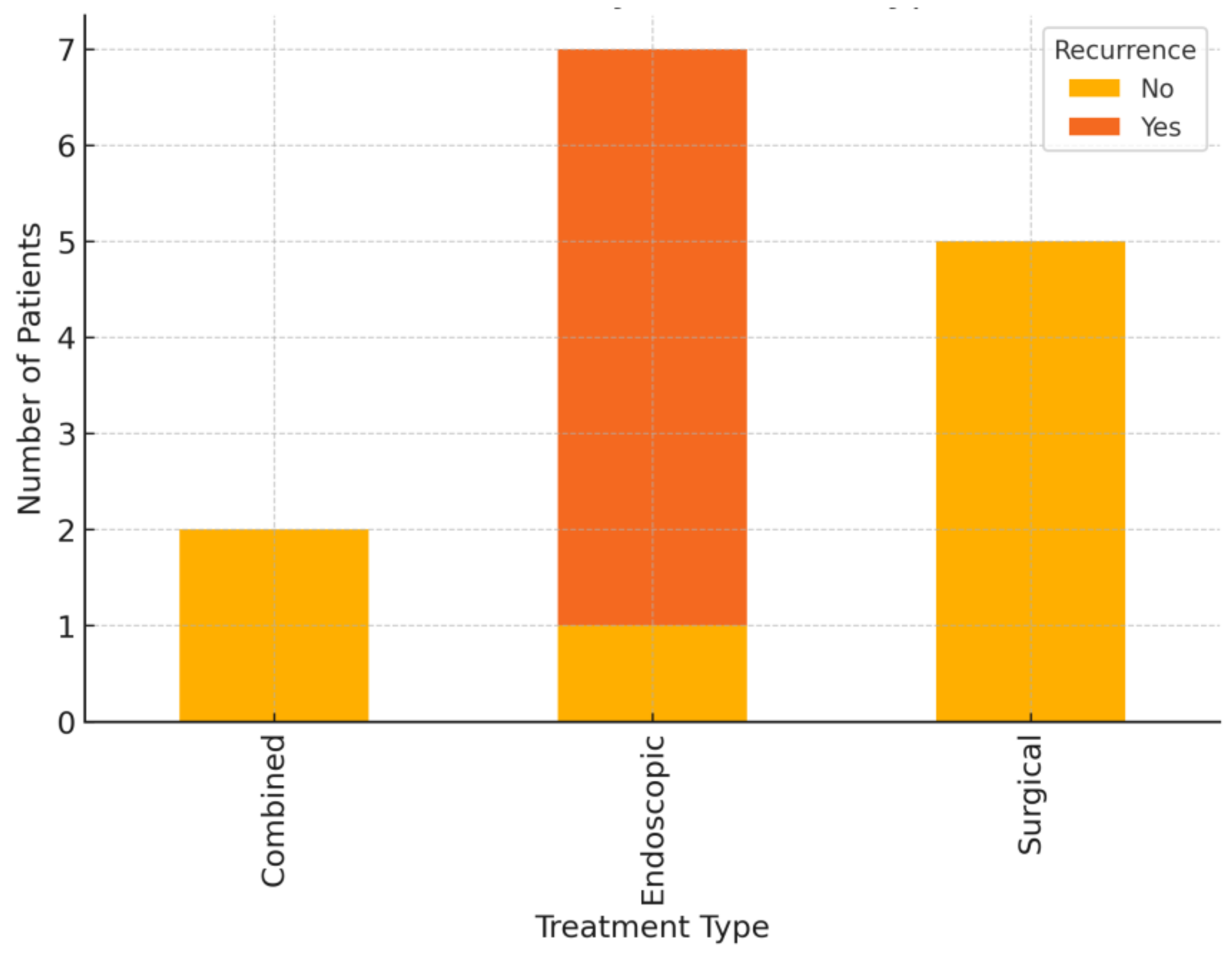
3.5. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HL | Hepatolithiasis |
| MRCP | Magnetic resonance cholangiopancreatography |
| CT | Computed tomography |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| PTC | Percutaneous transhepatic cholangiography |
| EUS | Endoscopic ultrasound |
| UDCA | Ursodeoxycholic acid |
References
- Motta, R.V.; Saffioti, F.; Mavroeidis, V.K. Hepatolithiasis: Epidemiology, presentation, classification and management of a complex disease. World J. Gastroenterol. 2024, 30, 1836–1850. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira1, R.S.; da Silva, P.; Queiroz, C.A.S.; Terra-Júnior, J.A.; Crema, E. Prevalence of bacteriobilia in patients undergoing elective colecystectomy. ABCD Arq. Bras. Cir. Dig. 2018, 31, e1392. [Google Scholar] [CrossRef] [PubMed]
- Tazuma, S.; Nakanuma, Y. Clinical features of hepatolithiasis: Analyses of multicenter-based surveys in Japan. Lipids Health Dis. 2015, 14, 129. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, Z.-Y.; Ma, J.-J. Effect of cholesterol metabolism on hepatolithiasis. World J. Gastroenterol. 2025, 31, 99960. [Google Scholar] [CrossRef]
- Shoda, J. Hepatolithiasis-epidemiology and pathogenesis update. Front. Biosci. 2003, 8, 1091. [Google Scholar] [CrossRef]
- Sheen-Chen, S.-M.; Cheng, Y.-F.; Chen, F.-C.; Chou, F.-F.; Lee, T.-Y. Ductal dilatation and stenting for residual hepatolithiasis: A promising treatment strategy. Gut 1998, 42, 708–710. [Google Scholar] [CrossRef]
- Dorrance, H.R.; Lingam, M.K.; Hair, A.; Oien, K.; O’Dwyer, P.J. Acquired abnormalities of the biliary tract from chronic gallstone disease11No competing interests declared. J. Am. Coll. Surg. 1999, 189, 269–273. [Google Scholar] [CrossRef]
- Sharma, C.K. Significant Microbial Pathogenesis Perspective of Biliary Diseases. Infect. Disord. Drug Targets 2025, 25, E18715265302000. [Google Scholar] [CrossRef]
- Melichar, B.; Zezulová, M. The significance of altered gastrointestinal permeability in cancer patients. Curr. Opin. Support. Palliat. Care 2011, 5, 47–54. [Google Scholar] [CrossRef]
- Li, G.; Gu, R.; Wen, X.; Wei, D.; Ming, X.; Chen, H. The Effect of Early Enteral Nutrition on Hyperthermic Intraoperative Intraperitoneal Chemotherapy–Induced Mucosal Permeability Following Gastrectomy. J. Parenter. Enter. Nutr. 2012, 36, 213–218. [Google Scholar] [CrossRef]
- Krentz, T.; Allen, S. Bacterial translocation in critical illness. J. Small Anim. Pract. 2017, 58, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Yadav, H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab. 2017, 71, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kiecka, A.; Szczepanik, M. Proton pump inhibitor-induced gut dysbiosis and immunomodulation: Current knowledge and potential restoration by probiotics. Pharmacol. Rep. 2023, 75, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Fickert, P.; Wagner, M. MDR3 (ABCB4) Defects: A Paradigm for the Genetics of Adult Cholestatic Syndromes. Semin. Liver Dis. 2007, 27, 077–098. [Google Scholar] [CrossRef]
- Roa, J.C.; García, P.; Kapoor, V.K.; Maithel, S.K.; Javle, M.; Koshiol, J. Gallbladder cancer. Nat. Rev. Dis. Prim. 2022, 8, 69. [Google Scholar] [CrossRef]
- Wang, P.; He, Y.; Ma, X.; Sun, B.; Huang, B.; Zhu, C.; Liu, Y. Expression and Significance of COX-2 and Ki-67 in Hepatolithiasis with Bile Duct Carcinoma. Med. Sci. Monit. 2015, 21, 2943–2949. [Google Scholar] [CrossRef]
- Vetrone, G.; Ercolani, G.; Grazi, G.L.; Ramacciato, G.; Ravaioli, M.; Cescon, M.; Varotti, G.; Del Gaudio, M.; Quintini, C.; Pinna, A.D. Surgical Therapy for Hepatolithiasis: A Western Experience. J. Am. Coll. Surg. 2006, 202, 306–312. [Google Scholar] [CrossRef]
- Chen, M.-F.; Jan, Y.-Y.; Jeng, L.-B.; Hwang, T.-L.; Wang, C.-S.; Chen, S.-C.; Chao, T.-C.; Chen, H.-M.; Lee, W.-C.; Yeh, T.-S.; et al. Intrahepatic cholangiocarcinoma in Taiwan. J. Hepato-Biliary-Pancreatic Surg. 1999, 6, 136–141. [Google Scholar] [CrossRef]
- Ohta, T.; Elnemr, A.; Yasui, T.; Kitagawa, H.; Kayahara, M.; Fushida, S.; Nishimura, G.-I.; Nagakawa, T.; Miwa, K.; Yamamoto, M.; et al. Expression of nerve growth factor in hepatolithiasis. Liver 1999, 19, 489–494. [Google Scholar] [CrossRef]
- Ohta, T.; Nagakawa, T.; Konishi, I.; Ueno, K.; Kanno, M.; Akiyama, T.; Kayahara, M.; Izumi, R.; Konishi, K.; Miyazaki, I.; et al. Clinical experience of intrahepatic cholangiocarcinoma associated with hepatolithiasis. JPN J. Surg. 1988, 18, 47–53. [Google Scholar] [CrossRef]
- Ohta, T.; Nagakawa, T.; Ueda, N.; Nakamura, T.; Akiyama, T.; Ueno, K.; Miyazaki, I. Mucosal dysplasia of the liver and the intraductal variant of peripheral cholangiocarcinoma in hepatholithiasis. Cancer 1991, 68, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xia, D.; Yan, L. Liver transplantation for hepatolithiasis: Is terminal hepatolithiasis suitable for liver transplantation? Clin. Transpl. 2016, 30, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yang, A.-M. Caroli disease: An update on pathogenesis. Chin. Med. J. 2021, 134, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Sakpal, S.V.; Babel, N.; Chamberlain, R.S. Surgical management of hepatolithiasis. HPB 2009, 11, 194–202. [Google Scholar] [CrossRef]
- Calomino, N.; Scheiterle, M.L.P.F.; Fusario, D.; La Francesca, N.; Martellucci, I.; Marrelli, D. Porcelain gallbladder and its relationship to cancer. Eur. Surg. 2021, 53, 311–316. [Google Scholar] [CrossRef]
- D’Souza, L.S.; Bucobo, J.C. Determining the Indeterminate in Biliary Strictures. Clin. Gastroenterol. Hepatol. 2020, 18, 300–301. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Saeian, K. Caroli’s disease: Identification and treatment strategy. Curr. Gastroenterol. Rep. 2007, 9, 151–155. [Google Scholar] [CrossRef]
- Leung, J.W.; Yu, A.S. Hepatolithiasis and biliary parasites. Baillieres Clin. Gastroenterol. 1997, 11, 681–706. [Google Scholar] [CrossRef]
- Bull, L.N.; Thompson, R.J. Progressive Familial Intrahepatic Cholestasis. Clin. Liver Dis. 2018, 22, 657–669. [Google Scholar] [CrossRef]
- Binda, C.; Gibiino, G.; Coluccio, C.; Sbrancia, M.; Dajti, E.; Sinagra, E.; Capurso, G.; Sambri, V.; Cucchetti, A.; Ercolani, G.; et al. Biliary Diseases from the Microbiome Perspective: How Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms 2022, 10, 312. [Google Scholar] [CrossRef]
- Sheen-Chen, S.-M.; Chen, W.-J.; Eng, H.-L.; Sheen, C.-W.; Chou, F.-F.; Cheng, Y.-F.; Lee, T.-Y. Bacteriology and antimicrobial choice in hepatolithiasis. Am. J. Infect. Control 2000, 28, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.R.; Anderson, M.A.; Itani, M.; Sharbidre, K.G.; Lalwani, N.; Paspulati, R.M. Secondary sclerosing cholangitis: Mimics of primary sclerosing cholangitis. Abdom. Radiol. 2022, 48, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J. Gastroenterol. 2015, 21, 13418. [Google Scholar] [CrossRef]
- Itani, M.; Lalwani, N.; Anderson, M.A.; Arif-Tiwari, H.; Paspulati, R.M.; Shetty, A.S. Magnetic resonance cholangiopancreatography: Pitfalls in interpretation. Abdom. Radiol. 2021, 48, 91–105. [Google Scholar] [CrossRef]
- Subhash, A.; Buxbaum, J.L.; Tabibian, J.H. Peroral cholangioscopy: Update on the state-of-the-art. World J. Gastrointest. Endosc. 2022, 14, 63–76. [Google Scholar] [CrossRef]
- Padmore, G.; Sutherland, F.R.; Ball, C.G. The art and craft of biliary T-tube Use. J. Trauma. Acute Care Surg. 2021, 91, e46–e49. [Google Scholar] [CrossRef]
- Pizzicannella, M.; Boskoski, I.; Perretta, S. Peroral Cholangioscopy: How Technology and Imaging Have Changed ERCP. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 973–979. [Google Scholar] [CrossRef]
- Giovannini, M. EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2019, 8, 35. [Google Scholar] [CrossRef]
- Li, R.; Shan, B.; Tian, K.; Zhang, X.; Xie, X. Biliary tract exploration via left hepatic duct stump versus the common bile duct incision in left-sided hepatolithiasis: A meta-analysis. ANZ J. Surg. 2021, 91, E439–E445. [Google Scholar] [CrossRef]
- Ishihara, Y.; Matsumoto, K.; Kato, H.; Tsutsumi, K.; Tomoda, T.; Matsumi, A.; Miyamoto, K.; Yamazaki, T.; Saragai, Y.; Fujii, Y.; et al. Treatment outcomes, including risk factors of stone recurrence, for hepatolithiasis using balloon-assisted endoscopy in patients with hepaticojejunostomy (with video). Surg. Endosc. 2021, 35, 1895–1902. [Google Scholar] [CrossRef]
- Feng, X. Classification and management of hepatolithiasis: A high-volume, single-center’s experience. Intractable Rare Dis. Res. 2012, 1, 151–156. [Google Scholar] [CrossRef]
- Ros, E.; Navarro, S.; Bru, C.; Gilabert, R.; Bianchi, L.; Bruguera, M. Ursodeoxycholic acid treatment of primary hepatolithiasis in Caroli’s syndrome. Lancet 1993, 342, 404–406. [Google Scholar] [CrossRef]

| Patient ID | Age | Sex | Stone Location | Stone Type | Bile Culture | Treatment | Recurrence | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| P1 | 62 | F | Left | Cholesterol | Negative | Surgical | No | 5.0 |
| P2 | 58 | M | Left | Brown Pigment | Positive | Endoscopic | Yes | 6.2 |
| P3 | 45 | F | Right | Cholesterol | Negative | Surgical | No | 8.1 |
| P4 | 74 | F | Left | Mixed | Positive | Combined | No | 4.2 |
| 4.7 | 69 | F | Bilateral | Brown Pigment | Positive | Endoscopic | Yes | 4.7 |
| P6 | 55 | M | Left | Black Pigment | Negative | Endoscopic | Yes | 5.3 |
| P7 | 60 | F | Right | Brown Pigment | Positive | Endoscopic | Yes | 2.1 |
| P8 | 32 | F | Left | Cholesterol | Negative | Surgical | No | 7.6 |
| P9 | 70 | M | Bilateral | Mixed | Positive | Combined | No | 1.8 |
| P10 | 64 | F | Right | Brown Pigment | Positive | Endoscopic | Yes | 9.0 |
| P11 | 48 | M | Left | Cholesterol | Negative | Surgical | No | 4.5 |
| P12 | 79 | F | Bilateral | Mixed | Positive | Endoscopic | No | 2.9 |
| P13 | 53 | M | Left | Black Pigment | Negative | Endoscopic | Yes | 3.8 |
| P14 | 82 | F | Left | Brown Pigment | Positive | Surgical | No | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calomino, N.; Carbone, L.; Kelmendi, E.; Piccioni, S.A.; Poto, G.E.; Bagnacci, G.; Resca, L.; Guarracino, A.; Tripodi, S.; Barbato, B.; et al. Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center. Medicina 2025, 61, 860. https://doi.org/10.3390/medicina61050860
Calomino N, Carbone L, Kelmendi E, Piccioni SA, Poto GE, Bagnacci G, Resca L, Guarracino A, Tripodi S, Barbato B, et al. Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center. Medicina. 2025; 61(5):860. https://doi.org/10.3390/medicina61050860
Chicago/Turabian StyleCalomino, Natale, Ludovico Carbone, Engjell Kelmendi, Stefania Angela Piccioni, Gianmario Edoardo Poto, Giulio Bagnacci, Luca Resca, Annalisa Guarracino, Sergio Tripodi, Bina Barbato, and et al. 2025. "Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center" Medicina 61, no. 5: 860. https://doi.org/10.3390/medicina61050860
APA StyleCalomino, N., Carbone, L., Kelmendi, E., Piccioni, S. A., Poto, G. E., Bagnacci, G., Resca, L., Guarracino, A., Tripodi, S., Barbato, B., Brillanti, S., Roviello, F., Adani, G. L., & Marrelli, D. (2025). Western Experience of Hepatolithiasis: Clinical Insights from a Case Series in a Tertiary Center. Medicina, 61(5), 860. https://doi.org/10.3390/medicina61050860







