The Dynamics of Seizures After Microsurgical Treatment of Brain AVMs in Patients with Symptomatic Epilepsy: A Single-Center Experience over 10 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
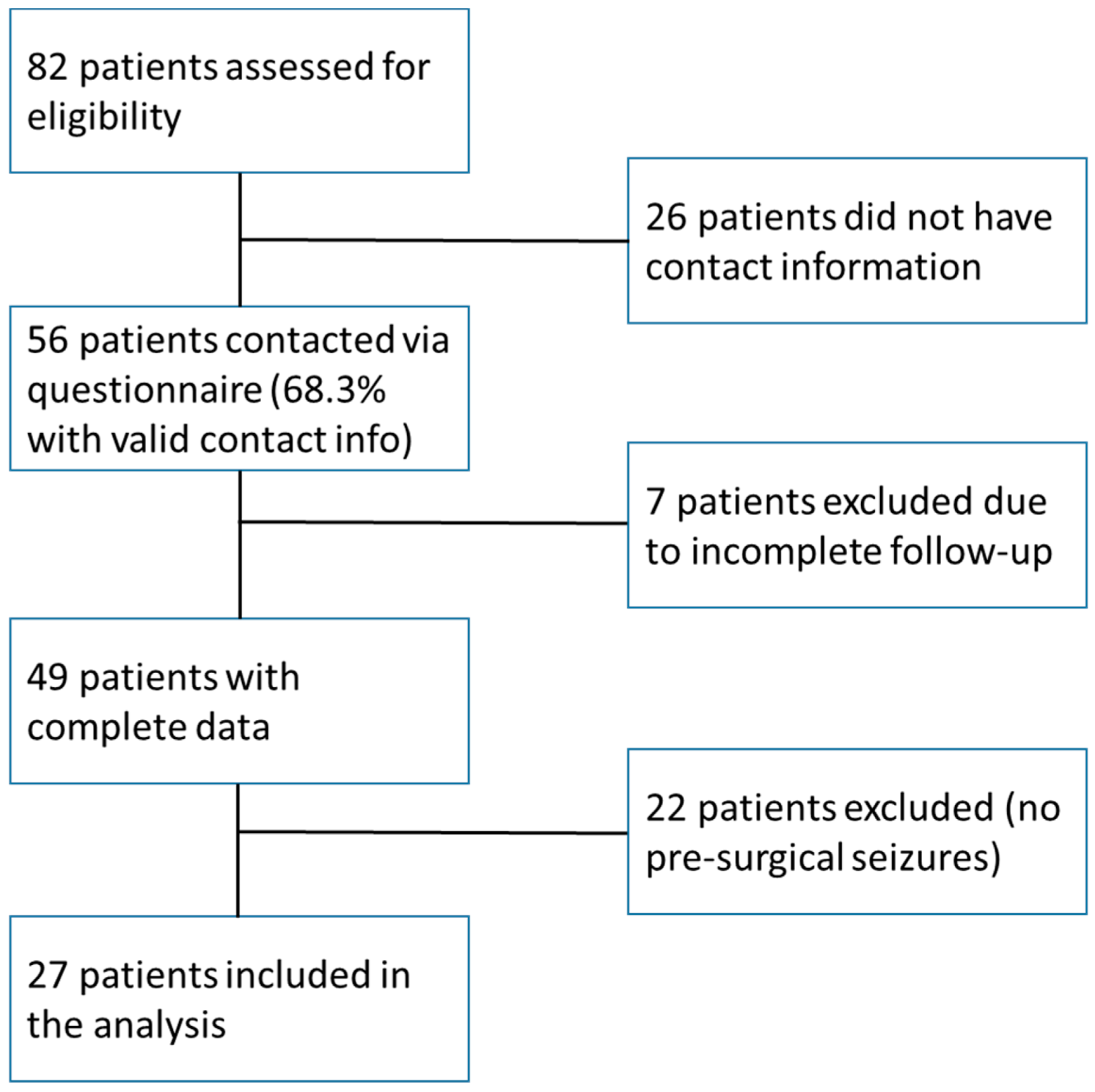
2.2. Sample Size
2.3. Treatment Approaches
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
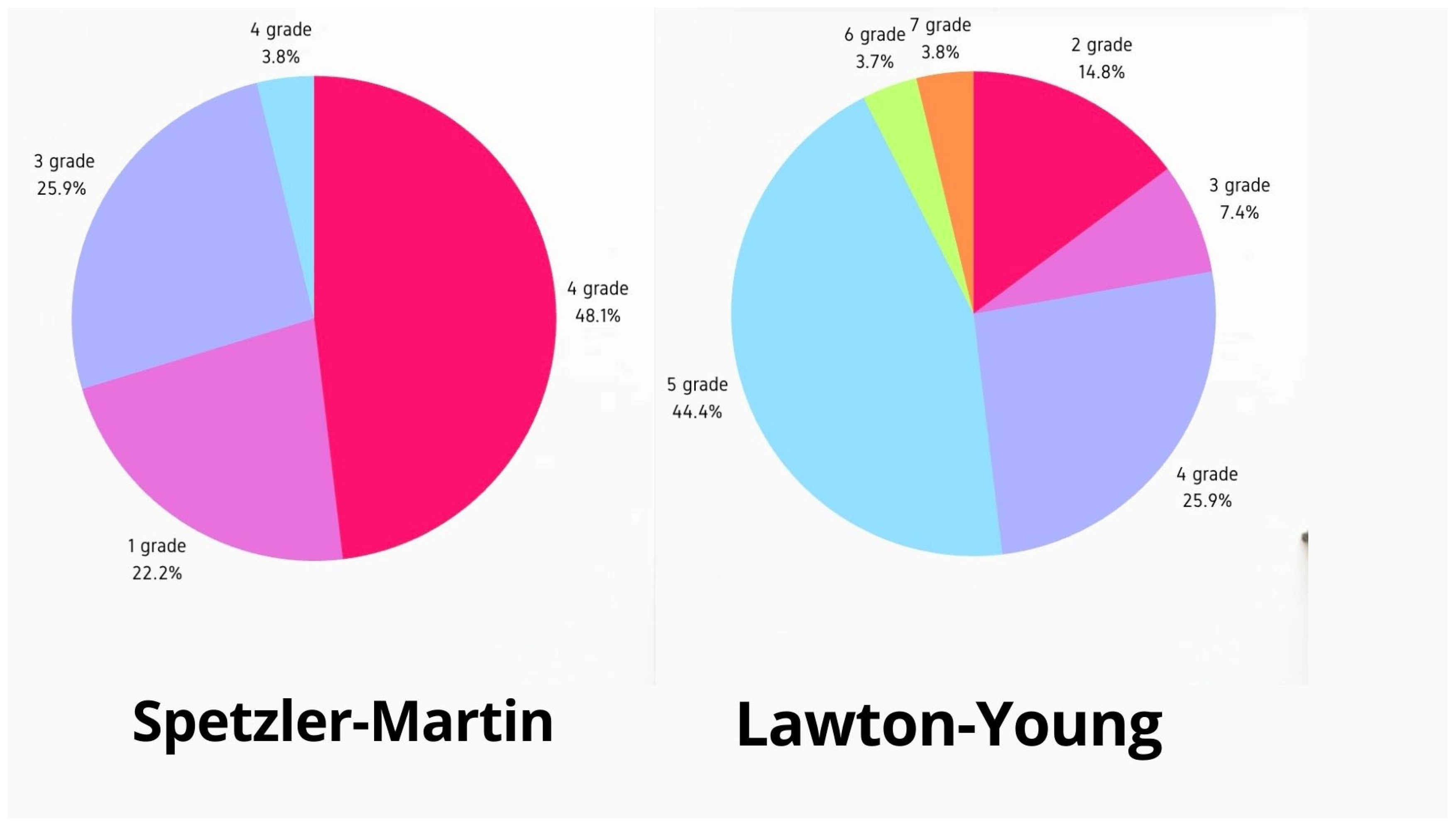
3.1. Patient Characteristics
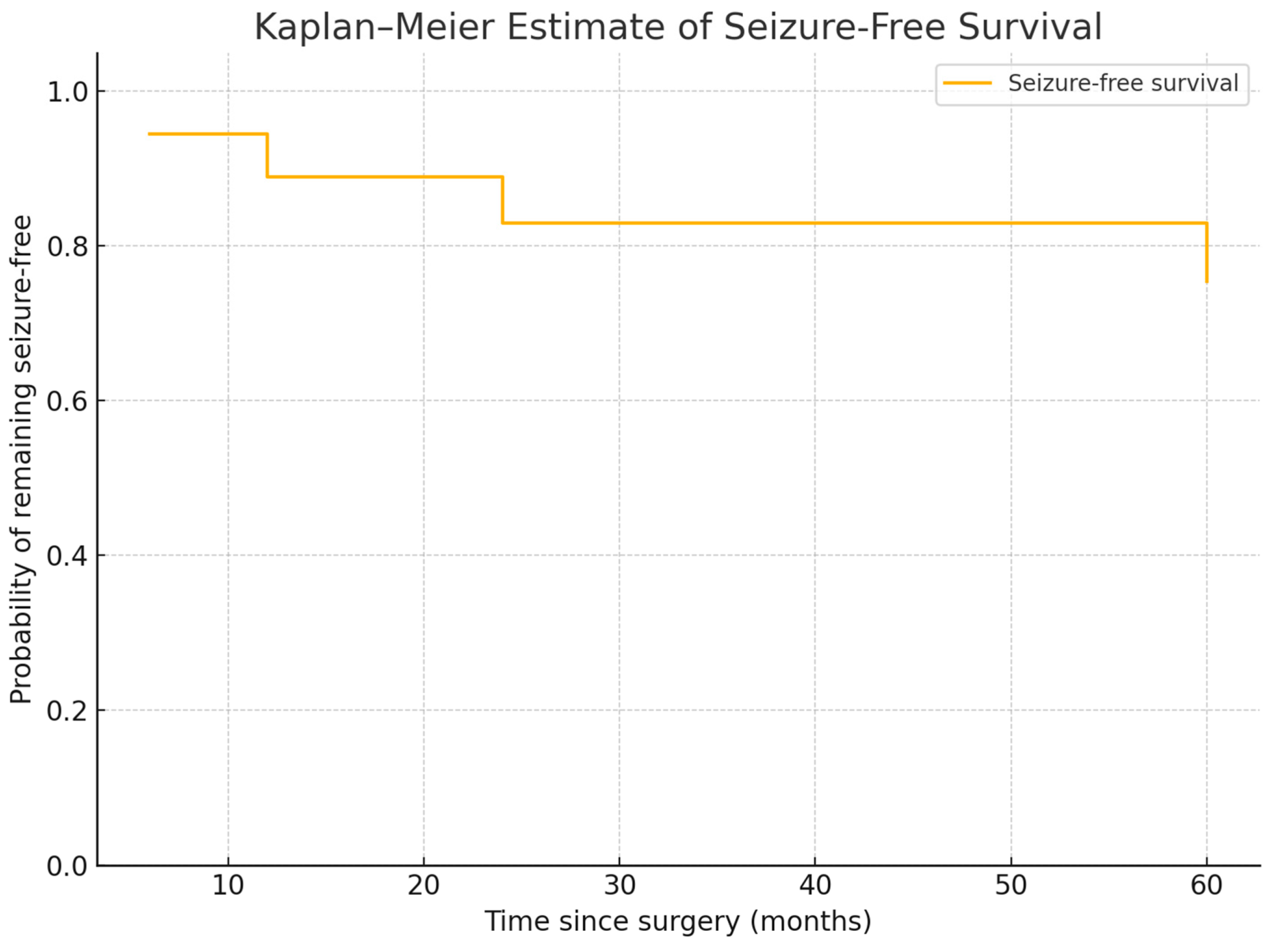
3.2. Seizure Outcomes
- 78% achieved seizure freedom (Engel IA).
- 11% experienced reduced seizure frequency (Engel IIA, IIIA).
- 11% had persistent seizures (Engel IVB).
- Generalized seizures (59%) responded more favorably than focal seizures (41%).
4. Representative Case
5. Discussion
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkins, R.H. Natural history of intracranial vascular malformations: A review. Neurosurgery 1985, 16, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Al-Shahi Salman, R. Interventions for treating brain arteriovenous malformations in adults. Cochrane Database Syst. Rev. 2010, CD003436. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.L.; Cordato, D.J.; Morgan, M.K.; Herkes, G.K. Postoperative seizure outcome in a series of 114 patients with supratentorial arteriovenous malformations. J. Clin. Neurosci. 2000, 7, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, D.L.; Awad, I.A. Vascular malformations and epilepsy: Clinical considerations and basic mechanisms. Epilepsia 1994, 35 (Suppl. S6), S30–S43. [Google Scholar] [CrossRef]
- Englot, D.J.; Young, W.L.; Han, S.J.; McCulloch, C.E.; Chang, E.F.; Lawton, M.T. Seizure predictors and control after microsurgical resection of supratentorial arteriovenous malformations in 440 patients. Neurosurgery 2012, 71, 572–580. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Aledo-Serrano, A.; Coppola, A.; Didelot, A.; Bates, E.; Sainz-Fuertes, R.; Lawthom, C. The impact of epilepsy on quality of life: Findings from a European survey. Epilepsy Behav. 2023, 142, 109179. [Google Scholar] [CrossRef]
- Hoh, B.L.; Chapman, P.H.; Loeffler, J.S.; Carter, B.S.; Ogilvy, C.S. Results of multimodality treatment for 141 patients with brain arteriovenous malformations and seizures: Factors associated with seizure incidence and seizure outcomes. Neurosurgery 2002, 51, 303–311. [Google Scholar] [CrossRef]
- Josephson, C.B.; Leach, J.P.; Duncan, R.; Roberts, R.; Counsell, C.; Salman, R.A.-S. Seizure risk from cavernous or arteriovenous malformations: Prospective population-based study. Neurology 2011, 76, 1548–1554. [Google Scholar] [CrossRef]
- de Tisi, J.; Bell, G.S.; Peacock, J.L.; McEvoy, A.W.; Harkness, W.F.; Sander, J.W.; Duncan, J.S. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: A cohort study. Lancet 2011, 378, 1388–1395. [Google Scholar] [CrossRef]
- Devinsky, O.; Pacia, S. Epilepsy surgery. Neurol. Clin. 1993, 11, 951–971. [Google Scholar] [CrossRef]
- Lawton, M.T.; Kim, H.; McCulloch, C.E.; Mikhak, B.; Young, W.L. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery 2010, 66, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, V.N.; Duncan, J.S.; Witt, J.; Elger, C.E.; Staba, R.; Engel, J. Getting the best outcomes from epilepsy surgery. Ann. Neurol. 2018, 83, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Mukhtarova, K.; Nurimanov, C.; Zholdybayeva, E.; Makhambetov, Y.; Akshulakov, S. Associations of Brain Arteriovenous Malformation-Related Factors with Epileptic Seizure Presentations. Diagnostics 2024, 14, 1077. [Google Scholar] [CrossRef]
- Strozyk, D.; Nogueira, R.G.; Lavine, S.D. Endovascular treatment of intracranial arteriovenous malformation. Neurosurg. Clin. N. Am. 2009, 20, 399–418. [Google Scholar] [CrossRef]
- Nurimanov, C.; Babi, A.; Menlibayeva, K.; Makhambetov, Y.; Kaliyev, A.; Zholdybayeva, E.; Akshulakov, S. Effect of Targeted Embolization on Seizure Outcomes in Patients with Brain Arteriovenous Malformations. Diagnostics 2022, 13, 47. [Google Scholar] [CrossRef]
- Heros, R.C.; Korosue, K.; Diebold, P.M. Surgical excision of cerebral arteriovenous malformations: Late results. Neurosurgery 1990, 26, 570–578. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef]
- Pikus, H.J.; Beach, M.L.; Harbaugh, R.E. Microsurgical treatment of arteriovenous malformations: Analysis and comparison with stereotactic radiosurgery. J. Neurosurg. 1998, 88, 641–646. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. Rate of re-bleeding of arteriovenous malformations in the first year after rupture. J. Clin. Neurosci. 2012, 19, 1087–1088. [Google Scholar] [CrossRef]
- von der Brelie, C.; Malter, M.P.; Niehusmann, P.; Elger, C.E.; von Lehe, M.; Schramm, J. Surgical management and long-term seizure outcome after epilepsy surgery for different types of epilepsy associated with cerebral cavernous malformations. Epilepsia 2013, 54, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Choque-Velasquez, J.; Tagle-Vega, U.; García-Mendoza, F.D.J.; Machado-Musri, E.; Guerrero-Ocampo, M.; Valenzuela-Rangel, A.F. Current advances in epilepsy among patients with arteriovenous malformations. Explor. Neurosci. 2024, 3, 175–197. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Q. Relationship between chronic hypoxia and seizure susceptibility. CNS Neurosci. Ther. 2022, 28, 1689–1705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Beijnum, J.; van der Worp, H.B.; Buis, D.R.; Salman, R.A.-S.; Kappelle, L.J.; Rinkel, G.J.E.; van der Sprenkel, J.W.B.; Vandertop, W.P.; Algra, A.; Klijn, C.J.M. Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA 2011, 306, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Korja, M.; Bervini, D.; Assaad, N.; Morgan, M.K. Role of surgery in the management of brain arteriovenous malformations: Prospective cohort study. Stroke 2014, 45, 3549–3555. [Google Scholar] [CrossRef]
- Malik, G.M.; Seyfried, D.M.; Morgan, J.K. Temporal lobe arteriovenous malformations: Surgical management and outcome. Surg. Neurol. 1996, 46, 106–115. [Google Scholar] [CrossRef]
- Abla, A.A.; Rutledge, W.C.; Seymour, Z.A.; Guo, D.; Kim, H.; Gupta, N.; Sneed, P.K.; Barani, I.J.; Larson, D.; McDermott, M.W.; et al. A treatment paradigm for high-grade brain arteriovenous malformations: Volume-staged radiosurgical downgrading followed by microsurgical resection. J. Neurosurg. 2015, 122, 419–432. [Google Scholar] [CrossRef]


| Baseline Characteristics of the Patient Cohort | ||
|---|---|---|
| Gender | ||
| Male | 14 | 52% |
| Female | 13 | 48% |
| Seizure character | ||
| Focal | 11 | 41% |
| Generalized | 16 | 59% |
| Presence of aura | ||
| Yes | 11 | 41% |
| No | 16 | 59% |
| Presurgical embolization | ||
| Total | 15 | 55.5% |
| Once | 12 | 44.4% |
| Three-times | 3 | 11.1% |
| ENGEL Classification | ||
|---|---|---|
| IA | 17 | 62.96% |
| IB | 3 | 11.1% |
| IIA | 2 | 7.41% |
| IIIA | 3 | 11.1% |
| IVB | 2 | 7.41% |
| ILAE classification | ||
| 1 | 16 | 59% |
| 3 | 8 | 29.9% |
| 5 | 3 | 11.1% |
| ENGEL Outcomes by Groups | Total | ||||
|---|---|---|---|---|---|
| Positive | Moderate | Negative | |||
| Years with seizures | <5 years | 11 | 1 | 0 | 12 |
| 5–10 years | 4 | 3 | 0 | 7 | |
| >10 years | 3 | 1 | 4 | 8 | |
| Total | 18 | 5 | 4 | 27 | |
| p-Value 0.005 | |||||
| ILAE Class | Total | ||||
|---|---|---|---|---|---|
| 1 | 3 | 5 | |||
| Years with seizures | <5 years | 10 | 2 | 0 | 12 |
| 5–10 years | 4 | 3 | 0 | 7 | |
| >10 years | 2 | 3 | 3 | 8 | |
| Total | 16 | 8 | 3 | 27 | |
| p-Value 0.03 | |||||
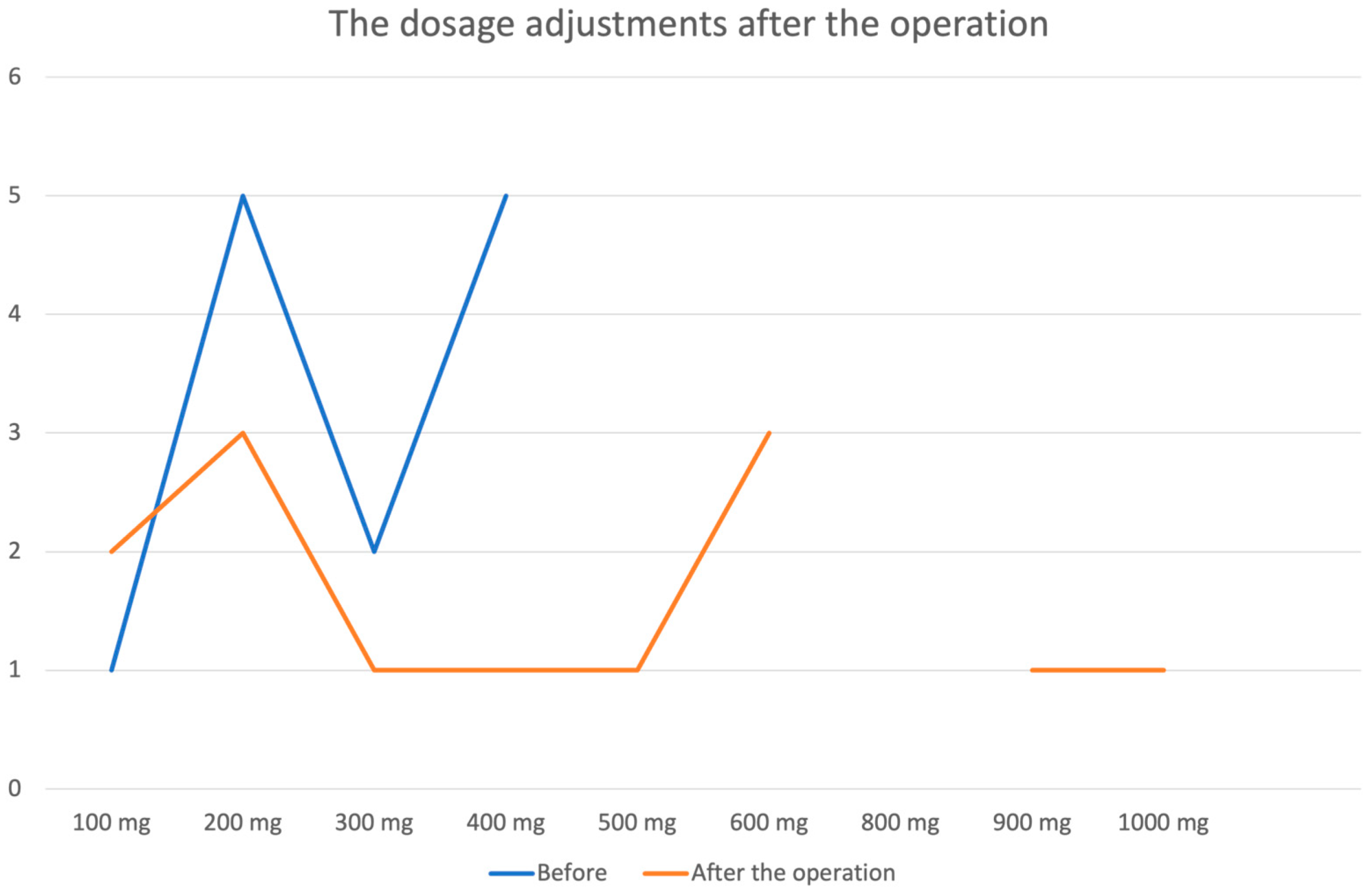
| Antiseizure Medication Usage | ||
|---|---|---|
| Before | After the Operation | |
| Yes | 17 | 14 |
| No | 10 | 13 |
| The name of antiseizure medication | ||
| Before | After the operation | |
| Finlepsin | 6 | 1 |
| Valproic acid | 3 | 3 |
| Carbamazepine | 8 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhambetov, Y.; Maidan, A.; Mammadinova, I.; Menlibayeva, K.; Kunakbayev, B.; Dyussembaev, S.; Nurakay, N.; Makhambetov, N.; Almabayeva, A.; Nurimanov, C. The Dynamics of Seizures After Microsurgical Treatment of Brain AVMs in Patients with Symptomatic Epilepsy: A Single-Center Experience over 10 Years. Medicina 2025, 61, 856. https://doi.org/10.3390/medicina61050856
Makhambetov Y, Maidan A, Mammadinova I, Menlibayeva K, Kunakbayev B, Dyussembaev S, Nurakay N, Makhambetov N, Almabayeva A, Nurimanov C. The Dynamics of Seizures After Microsurgical Treatment of Brain AVMs in Patients with Symptomatic Epilepsy: A Single-Center Experience over 10 Years. Medicina. 2025; 61(5):856. https://doi.org/10.3390/medicina61050856
Chicago/Turabian StyleMakhambetov, Yerbol, Aiman Maidan, Iroda Mammadinova, Karashash Menlibayeva, Baurzhan Kunakbayev, Serik Dyussembaev, Nurtay Nurakay, Nursultan Makhambetov, Aigul Almabayeva, and Chingiz Nurimanov. 2025. "The Dynamics of Seizures After Microsurgical Treatment of Brain AVMs in Patients with Symptomatic Epilepsy: A Single-Center Experience over 10 Years" Medicina 61, no. 5: 856. https://doi.org/10.3390/medicina61050856
APA StyleMakhambetov, Y., Maidan, A., Mammadinova, I., Menlibayeva, K., Kunakbayev, B., Dyussembaev, S., Nurakay, N., Makhambetov, N., Almabayeva, A., & Nurimanov, C. (2025). The Dynamics of Seizures After Microsurgical Treatment of Brain AVMs in Patients with Symptomatic Epilepsy: A Single-Center Experience over 10 Years. Medicina, 61(5), 856. https://doi.org/10.3390/medicina61050856






