Comorbidity Burden in Chronic Thromboembolic Pulmonary Hypertension: Implications and Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistics
3. Results
3.1. Treatment Strategies
3.2. Comorbidities
3.3. The Relationship of Comorbidities with Baseline Hemodynamics and 6MWD
3.4. Outcome
3.4.1. Survival
3.4.2. Survivors vs. Non-Survivors
3.5. 6MWD and WHO-FC in First Year of Follow-Up
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting Survival in Pulmonary Arterial Hypertension. Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Poms, A.D.; Turner, M.; Farber, H.W.; Meltzer, L.A.; McGoon, M.D. Comorbid Conditions and Outcomes in Patients With Pulmonary Arterial Hypertension. Chest 2013, 144, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Dwivedi, K.; Pausch, C.; Lewis, R.A.; Olsson, K.M.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Vizza, C.D.; et al. Phenotyping of idiopathic pulmonary arterial hypertension: A registry analysis. Lancet Respir. Med. 2022, 10, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Elliott, C.G.; Chin, K.; Benza, R.L.; Channick, R.N.; Davis, R.D.; He, F.; LaCroix, A.; Madani, M.M.; McLaughlin, V.V.; et al. Results From the United States Chronic Thromboembolic Pulmonary Hypertension Registry. Chest 2021, 160, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, N.; Espitia, H.G.; Velazquez-Moreno, H.; Macuil-Chazaro, B.; Serrano-Lopez, A.; Vega-Barrientos, R.S.; Hill, N.S.; Preston, I.R. Chronic Thromboembolic Pulmonary Hypertension: Experience from a Single Center in Mexico. Lung 2016, 194, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Tanabe, N.; Jujo, T.; Kato, F.; Anazawa, R.; Yamamoto, K.; Naito, A.; Kasai, H.; Nishimura, R.; Suda, R.; et al. Long-Term Outcome of Chronic Thromboembolic Pulmonary Hypertension at a Single Japanese Pulmonary Endarterectomy Center. Circ. J. 2018, 82, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Kearney, K.; Gold, J.; Corrigan, C.; Dhital, K.; Boshell, D.; Haydock, D.; McGiffin, D.; Wilson, M.; Collins, N.; Cordina, R.; et al. Chronic thromboembolic pulmonary hypertension in Australia and New Zealand: An analysis of the PHSANZ registry. Respirology 2021, 26, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Dzikowska-Diduch, O.; Mroczek, E.; Mularek-Kubzdela, T.; Chrzanowski, Ł.; Skoczylas, I.; Tomaszewski, M.; Peregud-Pogorzelska, M.; Karasek, D.; Lewicka, E.; et al. Characteristics and outcomes of patients with chronic thromboembolic pulmonary hypertension in the era of modern therapeutic approaches: Data from the Polish multicenter registry (BNP-PL). Ther. Adv. Chronic Dis. 2021, 12, 20406223211002961. [Google Scholar] [CrossRef] [PubMed]
- Kjellström, B.; Bouzina, H.; Björklund, E.; Beaudet, A.; Edwards, S.C.; Hesselstrand, R.; Jansson, K.; Nisell, M.; Rådegran, G.; Sandqvist, A.; et al. Five year risk assessment and treatment patterns in patients with chronic thromboembolic pulmonary hypertension. ESC Heart Fail. 2022, 9, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, S.M.; Shin, J.W.; Choi, B.W.; Kim, H.; Lee, J.S.; Park, S.S.; Moon, H.S.; Park, Y.B. Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: Results from the Korean registry. Korean J. Intern. Med. 2016, 31, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Kerr, K.; Fedullo, P. Chronic Thromboembolic Pulmonary Hypertension. Epidemiol. Risk Factors. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 3), S201–S206. [Google Scholar] [CrossRef] [PubMed]
- Gerges, C.; Pistritto, A.M.; Gerges, M.; Friewald, R.; Hartig, V.; Hofbauer, T.M.; Reil, B.; Engel, L.; Dannenberg, V.; Kastl, S.P.; et al. Left Ventricular Filling Pressure in Chronic Thromboembolic Pulmonary Hypertension. J. Am. Coll. Cardiol. 2023, 81, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2009, 34, 1219–1263. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity Measures for Use with Administrative Data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]

| Total N = 187 | PEA (+) N = 64 | PEA (−) N = 123 | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 60.2 ± 14.6 | 51.6 ± 14.1 | 64.7 ± 12.8 | <0.001 |
| Female | 118 (63.1) | 38 (59.4) | 80 (65.0) | 0.273 |
| Associated medical conditions | ||||
| Previous acute PE history | 129 (69.0) | 57 (89.1) | 72 (59.5) | <0.001 |
| Deep vein thrombosis history | 56 (30) | 27 (42.2) | 29 (23.6) | 0.008 |
| Malignancy history | 12 (6.5) | 2 (3.2) | 10 (8.3) | 0.156 |
| Active malignancy | 5 (2.7) | 1 (1.6) | 4 (3.3) | 0.434 |
| Pacemaker | 3 (1.6) | 0 (0) | 3 (2.5) | 0.275 |
| Intracardiac mass | 3 (1.6) | 1 (1.6) | 2 (1.7) | 0.722 |
| Right infective endocarditis | 1 | 0 (0.5) | 1 (0.8) | 0.648 |
| Splenectomy | 2 (1.1) | 0 (0) | 2 (1.7) | 0.426 |
| Thrombophilic disorder | 21 (12.1) | 11 (18) | 10 (8.9) | 0.080 |
| Antiphospholipid syndrome | 8 (4.6) | 8 (13.1) | 0 (0) | <0.001 |
| Inflammatory bowel disease | 4 (2.2) | 1 (1.6) | 3 (2.5) | 0.567 |
| Clinical Findings | ||||
| Body mass index, kg/m2 | 29.4 ± 6.1 | 28.7 ± 4.9 | 29.9 ± 6.7 | 0.225 |
| WHO-FC I | 1 (0.5) | 0 (0) | 1 (0.8) | 0.493 |
| II | 34 (18.2) | 14 (21.9) | 20 (16.3) | |
| III | 134 (71.7) | 46 (71.9) | 88 (71.5) | |
| IV | 18 (9.6) | 4 (6.3) | 14 (11.4) | |
| 6MWD, m [patient number] | 278 ± 145 [164] | 309 ± 143 [58] | 261 ± 144 [106] | 0.044 |
| Hemodynamics | ||||
| Mean RA pressure, mmHg | 10.9 ± 4.8 | 11.2 ± 5.3 | 10.7 ± 4.5 | 0.569 |
| PA systolic pressure, mmHg | 67.2 ± 20.5 | 76.8 ± 20.7 | 62.2 ± 18.6 | <0.001 |
| PA mean pressure, mmHg | 41.4 ± 11.0 | 46.6 ± 11.7 | 38.6 ± 9.6 | <0.001 |
| PA wedge pressure, mmHg | 11.6 ± 4.8 | 11.2 ± 11.0 | 11.8 ± 11.0 | 0.397 |
| PVR, Wood units | 7.7 ± 4.5 | 9.1 ± 4.3 | 7.0 ± 4.4 | 0.001 |
| Cardiac index, L/min/m2 | 2.4 ± 0.8 | 2.3 ± 0.7 | 2.5 ± 0.8 | 0.380 |
| Medical therapy | ||||
| PH-specific therapy at any time | 138 (73.8) | 37 (57.8) | 101 (82.1) | <0.001 |
| Riociguat | 122 (65.2) | 31 (48.4) | 92 (75.4) | <0.001 |
| Phosphodiesterase 5 inhibitors | 10 (5.3) | 3 (4.7) | 7 (5.7) | 0.529 |
| ERAs | 29 (15.6) | 8 (12.5) | 21 (17.2) | 0.412 |
| Iloprost inhaler | 8 (4.3) | 7 (10.9) | 1 (0.8) | 0.003 |
| IV/Sc prostacycline | 5 (2.7) | 2 (3.1) | 3 (2.5) | 0.562 |
| Anticoagulation | 180 (96.3) | 61 (95.3) | 119 (96.7) | 0.578 |
| N/N/N | No Comorbidity a | 1–2 Comorbidity b | 3 or More Comorbidity c | p | |
|---|---|---|---|---|---|
| Age, years | 16/79/92 | 46.2 ± 17.4 | 56.5 ± 14.5 | 65.8 ± 11.3 | <0.001 * |
| Age groups | 16/79/92 | <0.001 | |||
| 18–45 years | 10 (62.5) | 20 (25.3) | 6 (6.5) | ||
| 46–65 years | 3 (18.8) | 34 (43.0) | 37 (40.2) | ||
| ≥66 years | 3 (18.8) | 25 (31.6) | 49 (53.3) | ||
| Female, n (%) | 16/79/92 | 9 (56.3) | 50 (63.3) | 59 (64.1) | 0.631 |
| WHO-FC III or IV | 16/79/92 | 11 (68.8) | 66 (83.5) | 74 (80.5) | 0.638 |
| 6MWD, m | 16/66/85 | 359.4 ± 150.2 | 301.3 ± 148.4 | 234.8 ± 137.6 | <0.001 # |
| ProBNP | 11/47/68 | 1591 ± 1554 | 785 ± 1123 | 2087 ± 4234 | 0.109 |
| BNP | 0/11/16 | - | 378 ± 562 | 571 ± 1240 | 0.634 |
| Treatment | |||||
| Pulmonary endarterectomy | 16/79/92 | 10 (62.5) | 30 (38.0) | 24 (26.1) | 0.007 |
| Balloon pulmonary angioplasty | 16/79/92 | 1 (6.3) | 3 (3.8) | 5 (5.4) | 0.849 |
| PH-specific treatment | 16/78/92 | 12 (75.0) | 57 (72.2) | 69 (75.0) | 0.768 |
| Riociguat | 11 (68.8) | 51 (65.4) | 61 (66.3) | 0.965 | |
| PDE5-I | 0 (0) | 4 (5.1) | 6 (6.5) | 0.368 | |
| ERAs | 5 (31.2) | 7 (9.0) | 17 (18.5) | 0.05 | |
| Iloprost inh | 0 (0) | 3 (3.8) | 5 (5.4) | 0.425 | |
| IV/Sc prostacycline | 0 (0) | 1 (1.3) | 4 (4.3) | 0.298 | |
| Combination treatment | 16/78/92 | 4 (25.0) | 10 (12.7) | 19 (20.7) | 0.561 |
| Hemodynamics | |||||
| Mean RA pressure, mmHg | 16/78/91 | 8.2 ± 4.2 | 9.8 ± 4.3 | 12.3 ± 4.8 | <0.001 † |
| PA systolic pressure, mmHg | 16/79/92 | 77.6 ± 17.7 | 65.2± 20.0 | 67.0 ± 21.1 | 0.086 |
| PA mean pressure, mmHg | 16/79/92 | 46.1 ± 10.9 | 40.2 ± 10.3 | 41.5 ± 11.5 | 0.149 |
| PA wedge pressure, mmHg | 16/75/89 | 9.2 ± 3.1 | 10.3 ± 3.4 | 13.2 ± 5.6 | <0.001 § |
| PVR, WU | 16/78/90 | 8.5 ± 2.5 | 8.1 ± 4.6 | 7.3 ± 4.6 | 0.372 |
| Cardiac index, L/min/m2 | 15/65/87 | 2.5 ± 0.8 | 2.4 ± 0.8 | 2.3 ± 0.8 | 0.716 |
| OUTCOMES of all and according to PEA status | |||||
| Outcomes of all patients | |||||
| First-year 6MWD, m | 14/50/70 | 443 ± 99 | 361 ± 137 | 295 ± 140 | <0.001 ¢ |
| First-year 6MVD, m | 14/45/68 | 80.0 ± 117.6 | 72.7 ± 135.3 | 51.5 ± 118.5 | 0.578 |
| First-year FC improved n (%) | 15/62/78 | 8 (53.3) | 39 (62.9) | 38 (48.7) | 0.244 |
| Mortality n (%) | 16/78/90 | 3 (18.8) | 19 (24.4) | 19 (21.1) | 0.893 |
| Outcomes of PEA (+) patients | |||||
| First-year 6MWD, m | 9/21/20 | 470 ± 76 | 420 ± 111 | 334 ± 172 | 0.030 £ |
| First-year 6MVD, m | 10/25/18 | 95 ± 120 | 87.9 ± 104.5 | 94.7 ± 165.5 | 0.984 |
| First-year FC improved n (%) | 32/21 | 5 (50.0) | 16 (72.7) | 12 (57.1) | 0.387 |
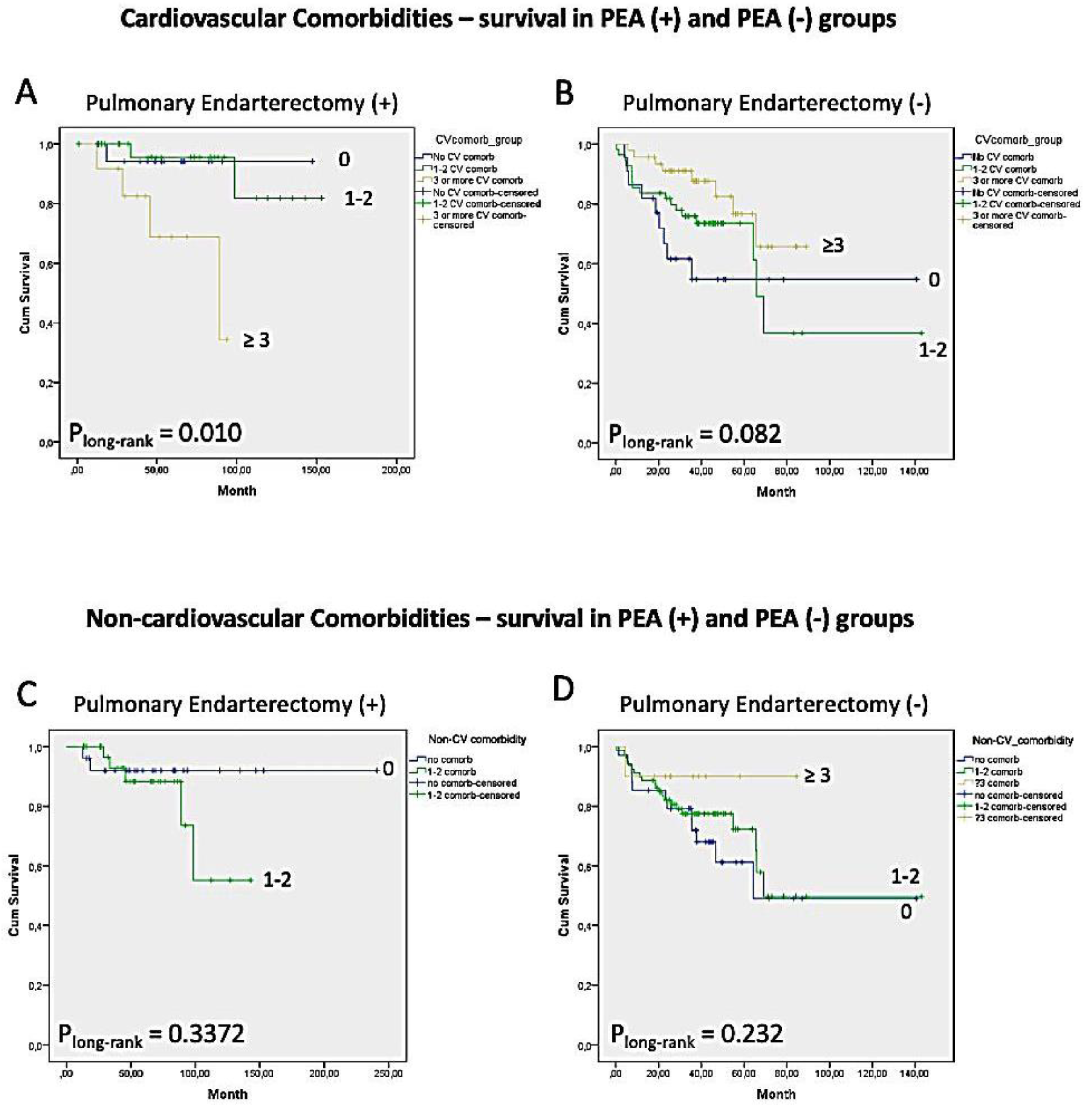
| Mortality n (%) | 10/30/24 | 1 (10.0) | 0 (0) | 6 (27.3) | 0.010 |
| Outcomes of PEA (−) patients | |||||
| First-year 6MWD, m | 5/29/50 | 394 ± 125 | 318 ± 140 | 280 ± 123 | 0.119 |
| First-year 6MVD, m | 5/25/49 | 53 ± 121.6 | 60.5 ± 156.7 | 34.8 ± 91.0 | 0.663 |
| First-year FC improved n (%) | 5/40/57 | 3 (60.0) | 23 (57.5) | 26 (45.6) | 0.472 |
| Mortality n (%) | 6/49/68 | 2 (33.3) | 19 (38.8) | 13 (19.1) | 0.061 |
| N/N | Survivors N = 143 | Non-Survivors N = 41 | p | |
|---|---|---|---|---|
| Age | 143/41 | 58.5 ± 14.9 | 65.9 ± 12.5 | 0.002 |
| Female | 143/41 | 91 (63.6) | 26 (63.4) | 0.979 |
| Comorbidities | ||||
| Sytemic hypertension | 142/41 | 66 (46.5) | 17 (41.5) | 0.570 |
| Diabetes mellitus | 142/41 | 33 (23.2) | 10 (24.4) | 0.878 |
| Smoking | 142/41 | 29 (20.4) | 10 (24.4) | 0.585 |
| Obesity | 132/32 | 53 (40.2) | 12 (37.5) | 0.783 |
| Coronary artery disease | 143/41 | 24 (16.8) | 6 (14.6) | 0.743 |
| Atrial fibrillation | 141/39 | 22 (15.6) | 5 (12.8) | 0.667 |
| Heart Failure | 143/41 | 24 (16.8) | 8 (19.5) | 0.684 |
| Stroke | 142/41 | 8 (5.6) | 1 (2.4) | 0.686 |
| Lung disease | 143/40 | 56 (39.2) | 11 (27.5) | 0.176 |
| Anemia | 139/39 | 50 (36) | 16 (41) | 0.564 |
| Chronic kidney disease | 143/41 | 24 (16.8) | 11 (26.8) | 0.148 |
| Connective tissue disease | 143/41 | 12 (8.4) | 0 (0) | 0.071 |
| Active cancer | 143/41 | 4 (2.8) | 1 (2.4) | 1.000 |
| Thyroid replacement therapy | 143/41 | 11 (7.7) | 2 (4.9) | 0.736 |
| Comorbidity burden | ||||
| Total number of comorbidities | 143/41 | 2.8 ± 1.9 | 2.6 ± 1.7 | 0.626 |
| ≥3 comorbidites | 143/41 | 71 (49.7) | 19 (46.3) | 0.709 |
| ≥3 CV comorbidities | 141/41 | 45 (31.9) | 12 (29.3) | 0.748 |
| ≥3 non-CV comorbidities | 143/41 | 9 (6.3) | 1 (2.4) | 0.462 |
| Functional capacity | ||||
| WHO-FC I–II III–IV | 143/41 | 31 (21.7) 112 (78.3) | 4 (9.8) 37 (90.2) | 0.086 |
| Initial 6MWD, m [51/6] | 133/32 | 290.6 ± 150.9 | 209.4 ± 116.0 | 0.001 |
| First-year 6MWD, m [43/7] | 108/26 | 359.2 ± 130.6 | 235.5 ± 150.5 | <0.001 |
| 6MVD, m | 105/22 | 69.2 ± 111.6 | 28.7 ± 171.6 | 0.269 |
| Hemodynamics | ||||
| Mean RA pressure, mmHg | 141/41 | 11.0 ± 4.9 | 10.2 ± 4.3 | 0.341 |
| PA systolic pressure, mmHg | 143/41 | 65.5 ± 20.7 | 72.1 ± 19.8 | 0.069 |
| PA mean pressure, mmHg | 143/41 | 40.5 ± 11.1 | 43.7 ± 10.3 | 0.099 |
| PA wedge pressure, mmHg | 138/39 | 11.4 ± 4.5 | 12.4 ± 5.6 | 0.236 |
| PVR, Wood units | 142/39 | 7.5 ± 4.4 | 8.4 ± 4.8 | 0.282 |
| Cardiac index, L/min/m2 | 131/33 | 2.40 ± 0.76 | 2.35 ± 0.8 | 0.747 |
| Treatment | ||||
| Pulmonary endarterectomy | 143/41 | 54 (37.8) | 7 (17.1) | 0.013 |
| Pulmonary vasodilator drugs | 143/41 | 104 (72.7) | 33 (80.5) | 0.315 |
| International Prospect Registry 2011, 2016 [12,14] | Korean Registry Park SY, 2016 [13] | Al-Naamani, et al. A Mexican Center 2016 [7] | Miwa H, et al. A Japanese Center, 2018 [8] | PHSANZ Registry Kearney K, et al. Australia and New Zealand, 2020 [9] | US-CTEPH Registry Kerr KM, et al. 2021 [6] | Polish Registry, BNP-PL, Kopec G, et al. 2021 [10] | Swedish Registry, Kjellstrom B, et al. 2022 [11] | Gerges C, et al. An Austrian Center, 2023 [16] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N = 679 | PEA (+) N = 404 | PEA (−) N = 275 | Total N = 134 | Total N = 50 | Total N = 280 | PEA (+) N = 146 | PEA (−) N = 240 | Total N = 750 | PEA (+) N = 566 | PEA (−) N = 184 | Total N = 516 | Total N = 311 | PEA (+) N = 98 | PEA (−) N = 213 | Total N = 593 | |
| Age, mean ± SD or median [interquartile range] | 63 [51–72] | 60 | 67 | 58.3 ± 15.9 | 63 [53–75] | 57 ± 12.5 | 55 ± 16 | 62 ± 16 | 59 [46–69] | 57 [44–67] | 67 [54–73] | 63.8 ± 15.4 | 70 [62–76] | 65 [52–72] | 72 [65–77] | 58.7 ± 15.5 |
| Female, % | 40.9 | 45 | 57 | 56.7 | 58 | 71 | 47.2 | 56.7 | 49.2 | 52.7 | 55 | 50.4 | 50 | 37 | 56 | 49.9 |
| Systemic HT, % | 21.6 | 34 | 26 | 35 | 36.1 | 35.7 | 37.5 | 58.7 | 41 | 32 | 45 | 48.7 | ||||
| Diabetes, % | 5.2 # | 6.7 | 14 | 0.7 (severe DM) | 6.8 | 12 | 15.1 | 13.6 | 19.6 | 17 | 8 | 2 | 10 | 12 | ||
| Smoking, % | 34 | 34 | 43.8 | 33.8 | 40.3 | 38.7 | 45.1 | 4.7 (present) 27.7 (past) | ||||||||
| Obesity, % | 17.6 | 17 | 20 | 40 | 49.2 | 51.1 | 43.5 | 31.6 | 22 | 19 | 24 | 18.9 | ||||
| Coronary artery dis., % | 11.8 | 10 | 15 | 2.1 | 19.2 | 10.4 | 10.9 | 9.5 | 15.2 | 18.6 | 10 | 3 | 13 | 15.3 | ||
| Atrial fibrillation, % | 14 | 7.1 | 7.1 | 7 | 14.7 | 10 | 5 | 13 | 10.1 | |||||||
| Heart failure, % | 6.5 * | 5 | 9 | 4.5 Congestive HF | 12 Cardiomyopathy | 3.6 | 2.5 | 7.1 | ||||||||
| Stroke, % | 6 | 5.5 | 5.1 | 6.5 | 5 | 7 | 4 | |||||||||
| Lung disease, % | 10.8 9.5 1.3 - | 8 | 13 | 32 | 2.1 | 29.1 | 27.2 | 34.8 | ||||||||
| COPD | 22 | 15.2 | 12.4 | 23.9 | 9.5 | |||||||||||
| ILD | 6 | 1.6 | 1.4 | 2.2 | 1.6 | |||||||||||
| Asthma | 2.9 | 4 | 12.3 | 13.6 | 8.7 | 5.8 | ||||||||||
| Anemia, % | ||||||||||||||||
| Chronic kidney dis., % | 0.5 dialy dep. | 0.4 dialy dep. | 1.8 | 24.6 | 4 (eGFR < 30) | 1 | 6 | 41.8 | ||||||||
| Connective tissue dis,% | 2.2 | 2.6 | 2.9 | 1.6 | ||||||||||||
| Any cancer, % | 12.7 | 12 | 16 | 2.2 | 2 | 5.7 | 9.2 | 7.4 | 14.7 | 1.9 (present) 7.6 (past) | 13.8 | |||||
| Thyroid disorder and HRT % | 8.4 | 6 | 11.1 | 11.1 | 11.0 | 12 hypothyroidism 5.6 hyperthyroidism | 18.5 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilickiran Avci, B.; Basarici, I.; Akbulut, M.; Atas, H.; Yaylali, Y.T.; Sinan, U.Y.; Atahan, E.; Meric, M.; Kaya, B.; Ohtaroglu Tokdil, K.; et al. Comorbidity Burden in Chronic Thromboembolic Pulmonary Hypertension: Implications and Outcome. Medicina 2025, 61, 827. https://doi.org/10.3390/medicina61050827
Kilickiran Avci B, Basarici I, Akbulut M, Atas H, Yaylali YT, Sinan UY, Atahan E, Meric M, Kaya B, Ohtaroglu Tokdil K, et al. Comorbidity Burden in Chronic Thromboembolic Pulmonary Hypertension: Implications and Outcome. Medicina. 2025; 61(5):827. https://doi.org/10.3390/medicina61050827
Chicago/Turabian StyleKilickiran Avci, Burcak, Ibrahim Basarici, Mehmet Akbulut, Halil Atas, Yalin Tolga Yaylali, Umit Yasar Sinan, Ersan Atahan, Murat Meric, Baris Kaya, Kardelen Ohtaroglu Tokdil, and et al. 2025. "Comorbidity Burden in Chronic Thromboembolic Pulmonary Hypertension: Implications and Outcome" Medicina 61, no. 5: 827. https://doi.org/10.3390/medicina61050827
APA StyleKilickiran Avci, B., Basarici, I., Akbulut, M., Atas, H., Yaylali, Y. T., Sinan, U. Y., Atahan, E., Meric, M., Kaya, B., Ohtaroglu Tokdil, K., Calay, O., Tokdil, H., Mutlu, B., Kucukoglu, M. S., & Ongen, Z. (2025). Comorbidity Burden in Chronic Thromboembolic Pulmonary Hypertension: Implications and Outcome. Medicina, 61(5), 827. https://doi.org/10.3390/medicina61050827







