1. Introduction
Bone loss is the most common type of aging-related bone defect, which has a long-term influence on quality of life, high health costs, and social burdens [
1,
2]. Recent pertinent data demonstrate that aging-related bone loss, such as osteopenia and osteoporosis, increased in prevalence from 21.47% to 56.23% and from 0.89% to 17.23%, respectively [
3]. Aging-related bone loss results in insufficient jawbone mass in the elderly, which affects the implementation and quality of implant surgery. A useful solution for improving bone homeostasis in aging bodies is still lacking. Bone marrow-derived mesenchymal stem cells (BMSCs) constitute a key cell population in bone marrow and are associated with the maintenance of bone homeostasis [
4]. One study reported that the osteogenic ability of aging BMSCs is impaired due to the accumulation of aging-related stressors, such as telomere attrition and DNA replication stress [
5]. During the aging process, exposure to these stressors leads to an aging-dependent decline in the multipotent properties of BMSCs, including cell viability, senescence-associated marker levels, and osteogenic differentiation ability [
6]. The modulation of multipotent properties in aging BMSCs might provide a solution to slow or reverse aging. Therefore, it is still necessary to clarify aging-associated DNA replication stress and the underlying key regulatory factors in aging BMSCs.
The transcribed single-stranded RNA inevitably invades the template DNA strands and rehybridizes with double-stranded DNA to form a DNA/RNA structure, which is referred to as the R-loop [
7]. A high level of RNA transcription makes MSCs particularly vulnerable to perturbations in gene expression, which is associated with increased R-loop formation [
8]. Although reports on R-loops and aging BMSCs are very limited, genomic R-loop coverage significantly increases during the aging process, and the age-related distribution is associated with specific features, such as transcript levels and GC content [
8]. R-loop formation is closely related to DNA replication stress and blocks the progression of the replication fork in key transcription factors [
7]. Runt-related transcription factor 2 (Runx2) is known as the key transcription factor for osteogenic differentiation. However, the details of the R-loops in these key osteogenesis-related genes are still lacking. How R-loops influence the osteogenic differentiation of BMSCs is still unclear.
R-loops are closely associated with DNA-binding proteins. A previous study revealed the different families of DNA-binding proteins that regulate R-loops, including HMGA family proteins [
9]. High-mobility group A-2 (Hmga2) is a non-histone chromosomal HMGA family protein that alters chromatin structure through DNA binding [
10]. Hmga2 contains three separate DNA-binding domains that consist of 8 or 9 amino acids and have high affinity for short AT-rich sequences [
11]. Hmga2 reduces the SA-β-gal level and improves the migration ability of aging-derived BMSCs (24-month-old rats) [
12]. Hmga2 mutation strongly reduces the bone phenotype and body size of mice [
11,
13]. Hmga2 knockout suppresses the differentiation of preosteoblast MC3T3-E1 cells into osteoblasts [
14]. Hmga2 overexpression rescues the suppressed osteoblast differentiation of mBMSCs and increases new bone regeneration in injured mouse femurs [
15,
16,
17]. These results indicate that Hmga2 is related to the nuclear abnormalities of aging MSCs and might play a pivotal role in bone regeneration. However, how Hmga2 regulates the transcription of key osteogenic genes in aging BMSCs via R-loops remains unclear.
In this study, the role and mechanism of Hmga2 in the osteogenic differentiation of aging mBMSCs were investigated. Hmga2 significantly improved the osteogenic differentiation ability of aging mBMSCs by eliminating the highly accumulated R-loop formation of key osteogenesis-related genes, including runt-related transcription factor 2 (Runx2), osterix (Osx), alkaline phosphatase (Alpl), collagen type I alpha 1 (Col1a1), osteocalcin (Ocn), and bone morphogenetic protein 2 (Bmp2).
2. Materials and Methods
2.1. Animals
C57BL/6J mice (6–8 weeks old) were purchased from Cyagen Biosciences (Suzhou, China). The mice were carefully cared for and raised until they reached 20 months of age. The animal care and experimental procedures were performed following the guidelines of the Beijing Experimental Animal Management Ordinance (Approval of Animal Ethical and Welfare: KQYY-2022). Male mice (20 months old) exhibiting normal grooming and walking behavior were obtained and selected for this study.
2.2. Cell Isolation and Culture
Aging mBMSCs were obtained from the bone marrow of the femurs of aging mice. Briefly, single-cell suspensions were obtained by repeated blowing, and monocytes were collected with Percoll (specific gravity of 1.082; Invitrogen, Carlsbad, CA, USA) and then cultured in a humidified environment containing 5% CO2 at 37 °C. The culture medium was changed every 3 days and consisted of alpha-modified Eagle’s medium (α-MEM) (Invitrogen) supplemented with 15% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). Passage 3–5 cells were used for analysis.
To package viral constructs, human embryonic kidney 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen).
2.3. Plasmid Construction and Viral Infection
As previously described [
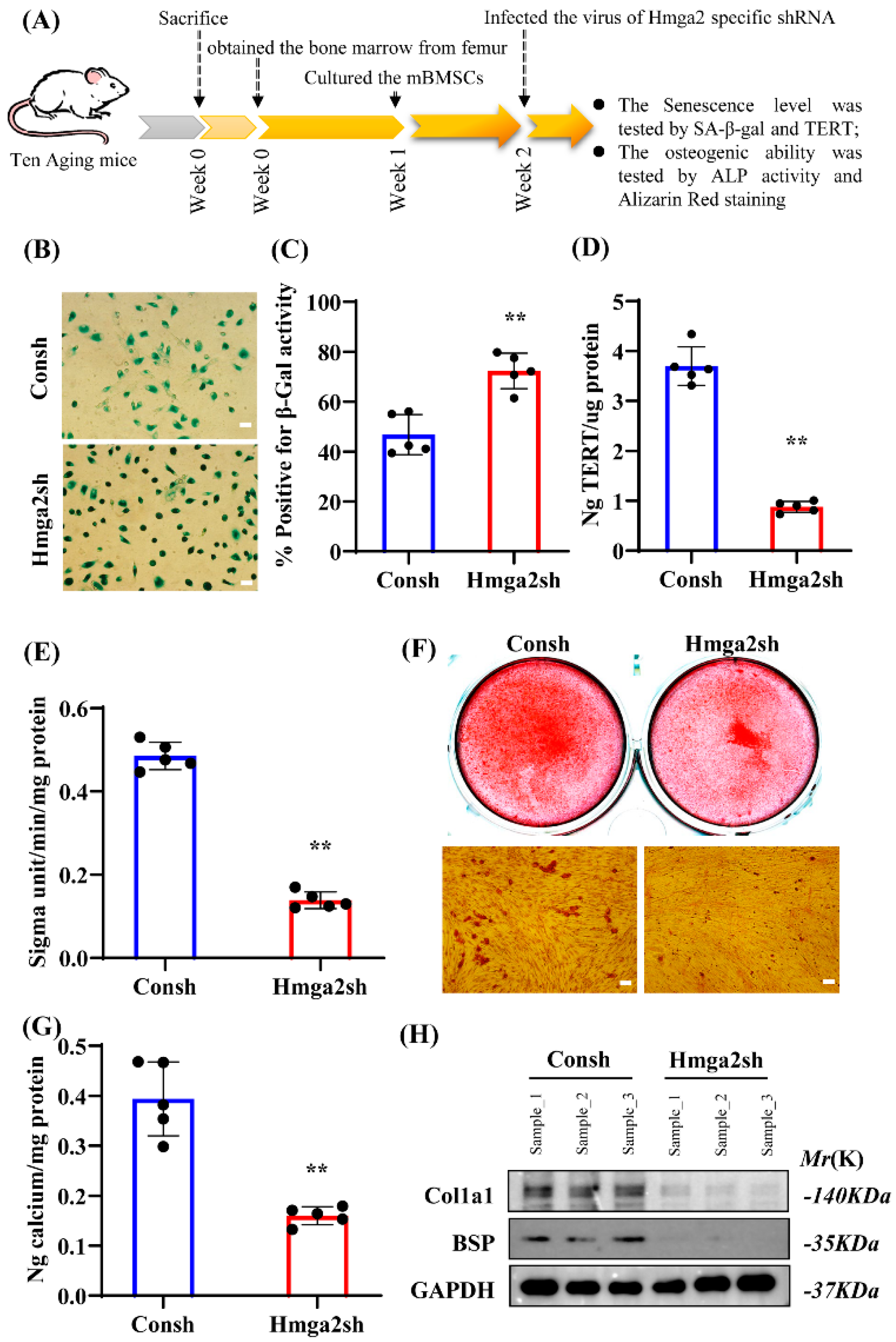
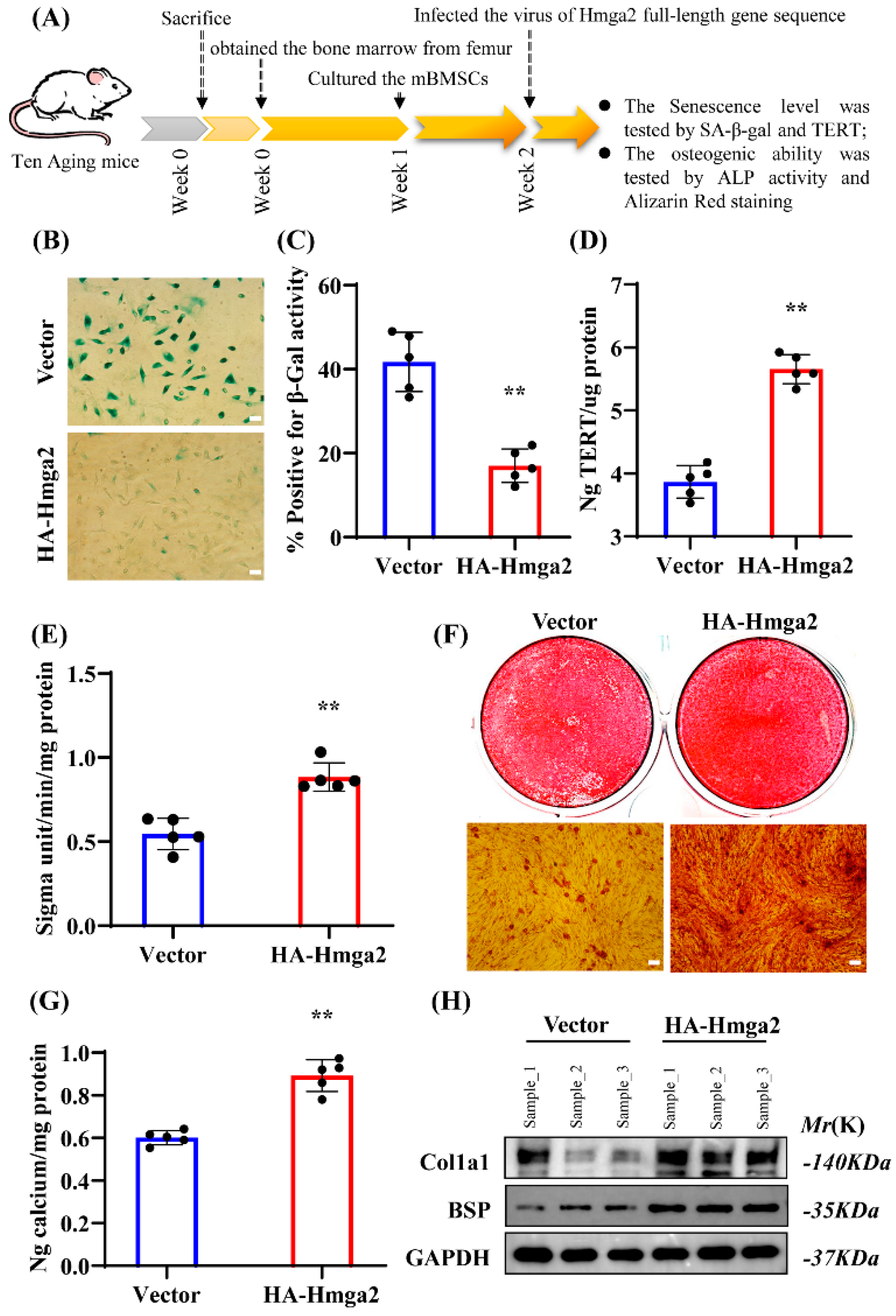
18], plasmids containing short hairpin RNA (shRNA) with a specific target sequence of Hmga2 (pLKO.1 lentiviral vector; Addgene, Cambridge, MA, USA) and a full-length gene sequence with an HA tag of Hmga2 (HA-pQCXIN retroviral vector; BD Biosciences, Franklin Lakes, NJ, USA) were constructed. Then, the virus was packaged in 293T cells, and the aging mBMSCs were infected with the retro- or lentivirus. The control shRNA (Consh, Houston, TX, USA) was purchased from Addgene (Cambridge, MA, USA). The target shRNA sequence of Hmga2 (Hmga2sh) was as follows: 5′-AGACCCAGAGGAAGACCCAAA-3′.
2.4. Real-Time Reverse Transcriptase–Polymerase Chain Reaction (Real-Time RT–PCR)
Briefly, total RNA was extracted from aging mBMSCs, and cDNA synthesis was performed as previously described [
18]. Real-time RT–PCR was performed via the standard protocol of the CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany), and an Icycler iQ Multicolor Real-Time RT–PCR detection system. The sequences of the primers used in this study are listed in
Supplementary Table S1.
2.5. Western Blot
The total protein of aged BMSCs was extracted as previously described [
18]. The nuclear protein was extracted according to the manufacturer’s recommendations (NE-PER™ Nuclear and Cytoplasmic Extraction Kit; Catalog No. 78833; Thermo Scientific, Waltham, MA, USA). The extracted protein was analyzed using SDS–polyacrylamide gel electrophoresis [
18]. Briefly, the primary antibodies used in this study were anti-HA (catalog no. ab9110; Abcam, Cambridge, UK), anti-Hmga2 (catalog no. ab207301; Abcam), anti-Runx2 (catalog no. ab192256; Abcam), anti-collagen-1 (catalog no. ab316222; Abcam), and anti-BSP (catalog no. EPR23243-30; Abcam). Anti-GAPDH (catalog no. ab8245; Abcam) and anti-histone H3 (catalog no. ab1791; Abcam) were used as total and nuclear protein markers, respectively.
2.6. Dot Blotting
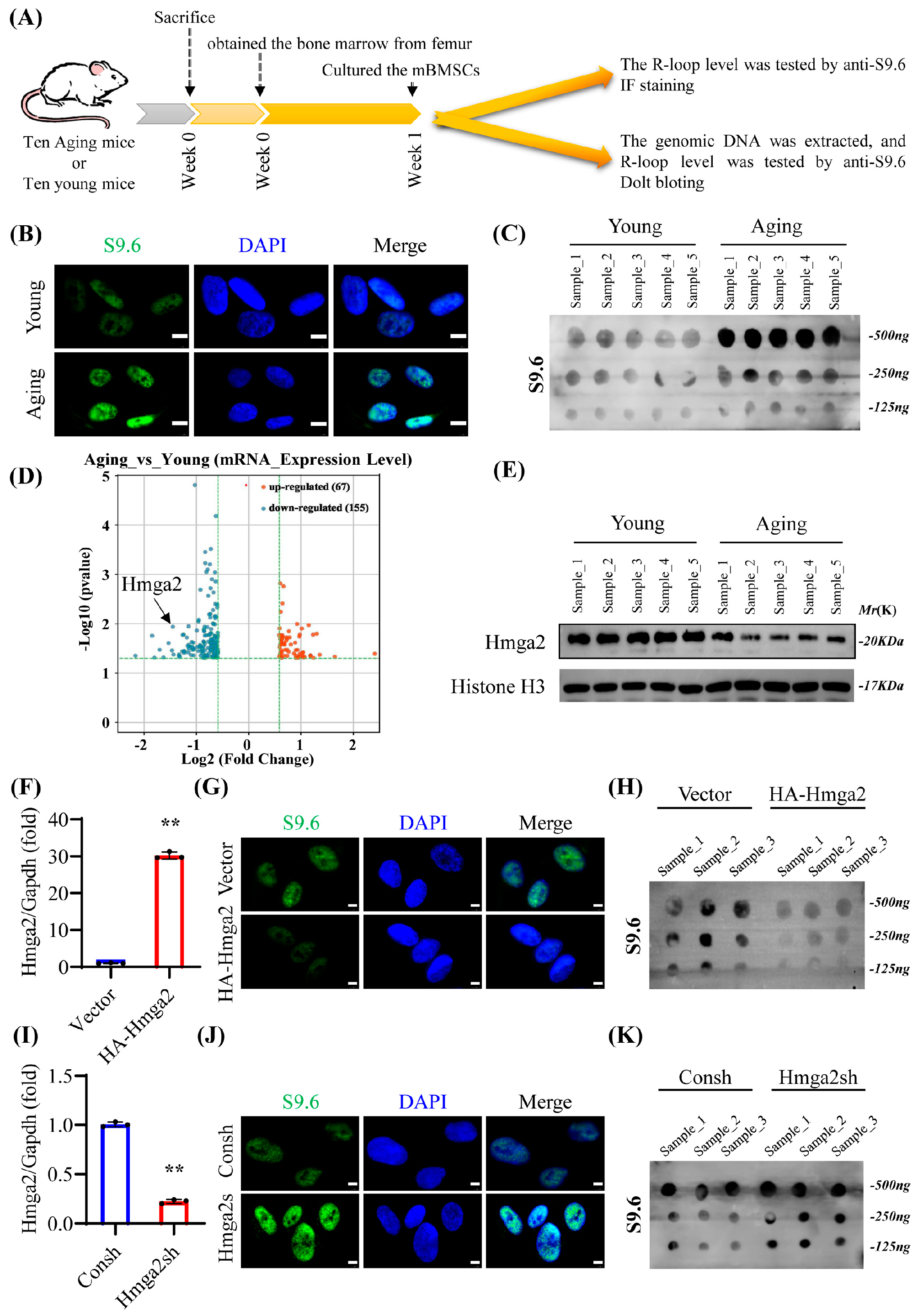
The genomic DNA of aging mBMSCs was extracted according to the manufacturer’s protocol for the GenElute™ Mammalian Genomic DNA Miniprep Kit (catalog No. G1N70, Millipore, Darmstadt, Germany). Approximately 2 μg of each DNA sample was incubated with 20× SSC and 37% formaldehyde at 65 °C for 15 min and then immediately transferred to ice. The DNA sample mixture was placed on the activated PVDF membrane, subjected to UV crosslinking, and irradiated for 15 min. After being washed with 1× TBST solution, the PVDF membrane was incubated in 5% non-fat milk for 2 h and then incubated with the primary antibody anti-S9.6 (catalog no. MABE1095; Millipore) at 4 °C overnight. The film was exposed to HRP-conjugated chemiluminescence, and the results were recorded.
2.7. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
The Senescence β-Galactosidase Staining Kit protocol (catalog No. 9860, Cell Signaling Technology, Danvers, MA, USA) was used and SA-β-gal in aging mBMSCs was detected. Briefly, aging mBMSCs were grown on coverslips and fixed with 4% paraformaldehyde. After washing, β-Gal staining solution was added, and the mixture was incubated at 37 °C for 2 h. Microscopy (Olympus, Tokyo, Japan) was used to examine the degree of staining, and Image-Pro Plus 6.0 (ImageJ-FIJI-Trmk, Tokyo, Japan) was used to measure the percentage of positively stained cells.
2.8. Telomerase Reverse Transcriptase (TERT) Level Analysis
The TERT level was assessed via a TERT enzyme-linked immunosorbent assay (ELISA, Helsinki, Finland) kit according to the manufacturer’s recommendations (Teloeras Reverse ELISA Kit, CUSABIO, Houston, TX, USA). In brief, a 200 μg/100 μL protein sample was added to the analysis well and incubated at 37 °C for 2 h. Then, 100 μL of biotin-conjugated antibody, 100 μL of HRP-conjugated streptavidin, and 90 μL of TMB substrate were added. Finally, 50 μL of Stop Solution was added to each well, and the optical density of each well was read at 450 nm within 5 min.
2.9. Alkaline Phosphatase Activity and Alizarin Red Detection
For osteogenic induction, 2 × 105 cells were plated in each well of a 6-well plate, and a StemPro osteogenesis differentiation kit (Invitrogen) was used. For the ALP activity assay, the cells were induced for 5 days and then incubated with 1× BCIP/NBT buffer (Sigma, Kanagawa, Japan). For alizarin red staining, the cells were induced for 2 weeks and then stained with 2% alizarin red (Sigma). For Ca2+ concentration analysis, 10% cetylpyridinium chloride (CPC) was used to destain the alizarin red for 30 min at room temperature. The absorbance at 562 nm was measured, and the concentration was determined.
2.10. Bioinformatic Analysis
2.11. Chromatin Immunoprecipitation (ChIP) and DNA–RNA Immunoprecipitation (DRIP) Assays
A ChIP assay kit (catalog no. MABE1095; Millipore) was used according to the manufacturer’s protocol. Briefly, each reaction employed 2 × 106 aging mBMSCs, which were cross-linked with formaldehyde. After being incubated at 4 °C and centrifuged, the cells were resuspended in lysis buffer (1% Triton X-100; 50 mM KOH, pH 7.5; 1 mM EDTA, pH 8; and 140 mM NaCl; protease inhibitor) and lysed via ultrasonication. The extracted cells were subsequently centrifuged and resuspended in lysis buffer supplemented with 0.3% SDS. IP extraction was subsequently performed.
For DRIP, anti-S9.6 (catalog no. MABE1095; Millipore) was used to detect the RNA/DNA hybrids and to measure R-loop levels. IP extraction (1 mg/mL) was precleared, and the mixture was incubated with protein A beads (Invitrogen) overnight at 4 °C. For ChIP, anti-Hmga2 (catalog no. ab207301; Abcam) was used. Normal rabbit IgG (catalog no. ab172730; Abcam) and normal mouse IgG (catalog no. sc-2025; Santa Cruz Biotechnology, Dallas, TX, USA) were used as negative controls. In addition, 5% IP extraction was used as the input. The beads were washed for 10 min at 4 °C, followed by lysis buffer, high NaCl buffer (lysis buffer with 500 mM NaCl), LiCl buffer (250 mM LiCl; 1 mM EDTA; 10 mM Tris-HCl, pH 8.0; 1% sodium deoxycholate and NP-40), and TE buffer (pH 8.0). Bead-bound DNA was eluted twice using 150 μL of elution buffer (1% SDS; 50 mM Tris-HCl, pH 7.5; and 10 mM EDTA, pH 8.0) for 10 min and then treated with proteinase K (0.75 mg/mL; Invitrogen) overnight at 65 °C. Finally, DNA was extracted via the phenol–chloroform method. The precipitated DNA was quantified via real-time RT–PCR analysis. The final data are expressed as the percentage of input DNA.
2.12. Adeno-Associated Virus (AAV)-Mediated Gene Transduction in Aging Mice
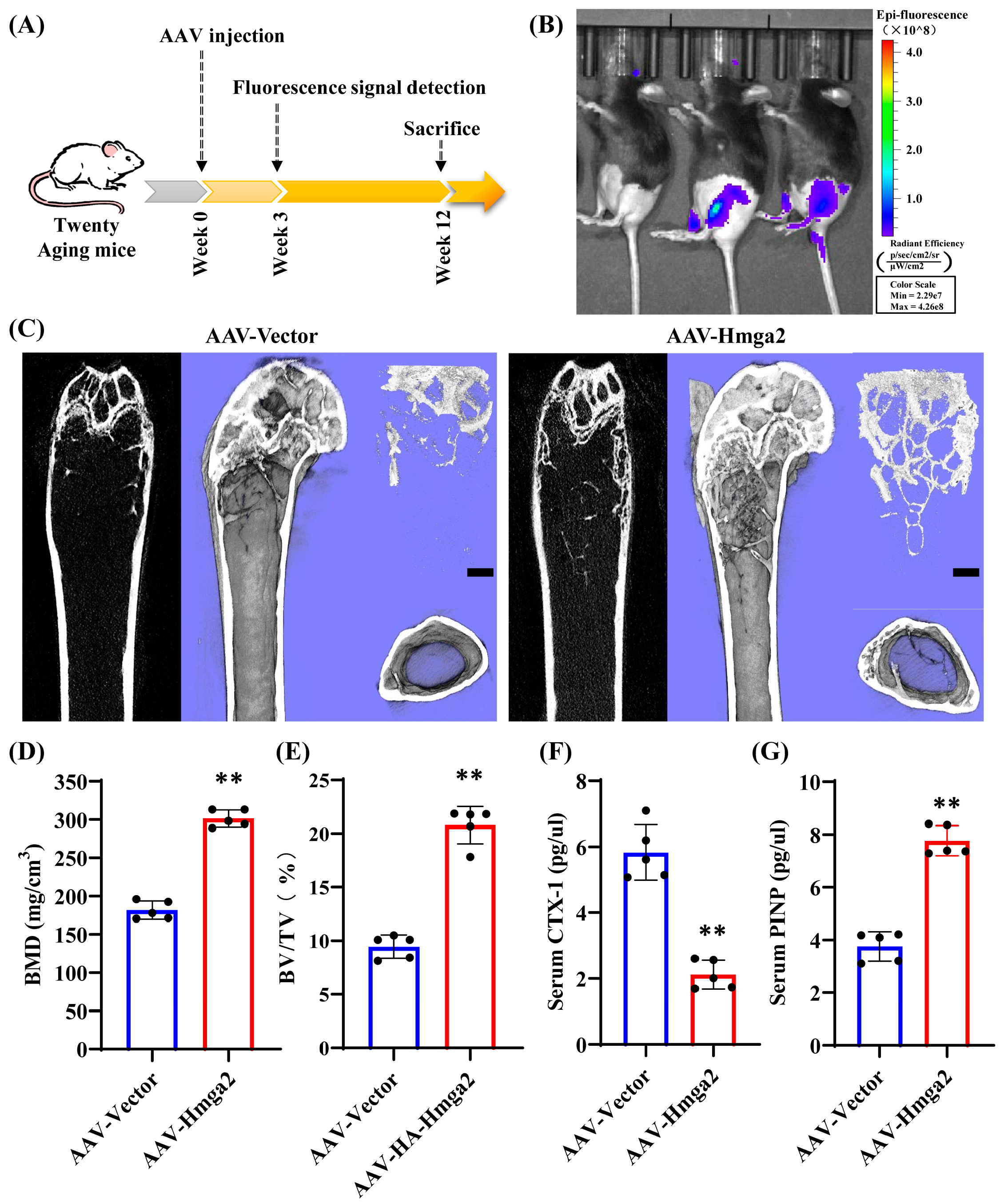
Briefly, the DNA sequence of mouse Hmga2 was obtained and fused to the N-terminus of the rAAV9 vector, which was used for rAAV production (6 × 1012 GC/mL). Twenty aging mice (male, 20 months old, Cyagen Biosciences, C57BL/6J) were randomly divided into two groups: the rAAV9-Vector injection group and the rAAV9-HA-Hmga2 injection group. Anesthesia with 90 mg/kg ketamine and xylazine 10 mg/kg 1% sodium pentobarbital (90 mg/kg) was conducted via an intraperitoneal injection, and approximately 100 μL of rAAV9 virus (1 × 1011 GC per site) was injected into the proximal joint end of the femur of the aging mice. After injection for 3 weeks, the fluorescence expression in individual tissues of mice immobilized by inhalation anesthesia of isoflurane was monitored via IVIS-100 optical imaging. After injection for 12 weeks, the fluorescence expression in individual tissues of mice immobilized under inhalation anesthesia with isoflurane was monitored via IVIS-100 optical imaging.
2.13. Microcomputed Tomography (Micro-CT) Analysis
For trabecular and cortical bone mass and microarchitecture evaluation, mouse femur bones were harvested, fixed overnight in 4% paraformaldehyde, and then scanned for micro-CT analysis (eXplore Locus SP; GE Healthcare, Chicago, IL, USA). The scanning parameters were 70 kV and 80 μA, and the thickness of the tomography was 6 μm. A three-dimensional structure of each femur bone was established. Briefly, the data of the trabecular bone area of interest (AOI) were obtained from a 3 mm region of the distal metaphysis, approximately 0.3 mm away from the epiphysis. The data of the cortical AOI were obtained from the midshaft, approximately 4 mm away from the epiphysis. The data were analyzed with the DataView software (version 2.1.2), and the quantification parameters of bone mineral density (BMD) and bone volume per tissue volume (BV/TV) were determined using the CTan software (version 1.15).
2.14. Serum Enzyme-Linked Immunosorbent (ELISA)
Approximately 500 μL of whole peripheral blood was collected from the retro-orbital venous plexus of each mouse before necropsy. The serum was isolated through centrifugation at 1000× g for 10 min followed by 5000× g for 10 min to remove cell debris at 4 °C. Bone turnover-sensitive biomarkers, including the bone formation marker procollagen I N-terminal peptide (PINP) and the bone resorption marker cross-linked C-telopeptide of type 1 collagen (CTX-1), were detected in serum using murine ELISA kits according to the manufacturers’ instructions (R&D Systems, Minneapolis, MN, USA).
2.15. Histology and Histomorphometry
The tissue slices were deparaffinized and hydrated with distilled water. For hematoxylin–eosin (HE) staining, deparaffinized tissue slices were incubated with Weigert’s iron hematoxylin solution at room temperature (RT) for 5 min, acidified within seconds, and incubated in 0.1% eosin staining solution for 2 min. For picrosirius red staining, the Picrosirius Red Stain Kit (MaoKang Bio, Shanghai, China) was used. Briefly, deparaffinized tissue slices were adequately incubated with picrosirius red solution at RT for 60 min. The nucleus was dyed with Mayer’s hematoxylin solution for 10 min. For Masson’s trichrome staining, a Masson’s Staining Kit (Sigma–Aldrich, St. Louis, MO, USA) was used. Briefly, deparaffinized tissue slices were incubated with Weigert’s iron hematoxylin solution at RT for 5 min and acidified within seconds. Then, the slices were incubated with Ponceau S at RT for 5 min and rinsed quickly with 0.5% glacial acetic acid. The sections were washed with 1% aqueous aluminum phosphate and stained with aniline blue. Microscopy (Zeiss AxioX-4, Zeiss, Jena, Germany) was used to examine staining after sealing with neutral resin, and Image-Pro Plus 6.0 (ImagJ-FIJI-Trmk, Tokyo, Japan) was used to measure the positive staining area.
2.16. Immunofluorescence Analysis
Deparaffinization of the tissue slices was performed via proteolytic antigen retrieval (PIER) treatment via HistoReveal regent (ab103720, Abcam) at room temperature (RT) for 10 min. Then, the tissue slices were permeabilized with Triton X-100 (5%, v/v) at RT for 5 min and incubated in H2O2 (0.5%, v/v) at RT for 30 min. Each treatment was followed by washing with PBS buffer at RT 3 times for 10 min each. Next, the slices were blocked with BSA (5%, w/v) at RT for 30 min, after which immunostaining was performed. The primary antibodies used included anti-Runx2 (catalog no. ab76956; Abcam) and anti-S9.6 (catalog no. MABE1095; Millipore), and the secondary antibodies used were goat anti-rabbit IgG H&L (Alexa Fluor® 488, ab150073; Abcam) and goat anti-mouse IgG H&L (Alexa Fluor® 594, ab150116; Abcam). Normal rabbit IgG (catalog no. ab172730; Abcam) and normal mouse IgG (catalog no. sc-2025; Santa Cruz Biotechnology, USA) were used as negative controls. Nuclei were stained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, Invitrogen). A microscope (Olympus, Tokyo, Japan) was used to examine the fluorescence staining.
2.17. Statistical Analysis
All the statistical calculations used in this study were analyzed using the SPSS 10 statistical software. Statistical significance was analyzed using one-way ANOVA or Student’s t-test; p ≤ 0.05 was regarded as significant.
4. Discussion
Aging-related bone loss leads to insufficient jawbone mass in the elderly, which affects the implementation and quality of implant surgery. Improving bone homeostasis in aging bodies still requires further solutions. As BMSCs undergo the aging process, R-loop-induced DNA replication stress unavoidably occurs during the transcription of osteogenesis-related genes. Therefore, exploring aging-related R-loop modification changes is expected to provide more evidence for improving BMSCs’ ability to generate new bones. Here, we investigated the role of Hmga2 in the osteogenic differentiation ability of aging BMSCs.
The prominent hallmark of aging cells is the accumulation of different types of stress [
1]. During cell aging, R-loop formation is closely related to DNA replication stress, which is associated with DNA-binding proteins [
7]. To investigate the role of Hmga2 in aging mBMSCs, we first detected several key senescence markers, including SA-β-gal and TERT. Our study revealed that Hmga2 significantly decreased the percentage of SA-β-gal-positive cells in aging mBMSCs. A previous study reported that Hmga2 expression was decreased during the repeated subculture-induced senescence of human mBMSCs [
9] and human umbilical cord blood-derived MSCs (hUCB-MSCs) [
11,
19,
20]. Hmga2 reduced the in vitro aging process of hUCB-MSCs [
21]. The overexpression of Hmga2 reduced the percentage of SA-β-gal-positive cells in aging rats [
12]. Additionally, the ability of miR-98-5p to inhibit the viability of preosteoblast MC3T3-E1 cells was reversed by Hmga2 overexpression [
17]. These studies are consistent with our results, indicating that Hmga2 has a positive role in preventing BMSC senescence and may be a critical factor or molecular marker for altering MSC senescence.
Further, our results show that Hmga2 significantly enhanced the ALP activity and mineralization ability in aging mBMSCs and promoted the expression of the key osteogenic differentiation marker Runx2. A previous study revealed that Hmga2 is involved in the differentiation of hBMSCs [
15]. Knockout of Hmga2 suppresses the differentiation of preosteoblast MC3T3-E1 cells into osteoblasts [
14]. The overexpression of Hmga2 rescues the MiR-495-suppressed osteogenic differentiation of mBMSCs [
16]. Additionally, the ability of miR-98-5p to inhibit the osteogenic differentiation of MC3T3-E1 cells was reversed by increasing Hmga2 expression [
17]. These findings are consistent with our observed phenomena and indicate that Hmga2 can promote the osteogenic differentiation ability of aging BMSCs.
Moreover, the application of Hmga2 in aging bodies was tentatively explored using an AAV delivery system. We constructed a natural aging mouse (20 months old) model, and the ELISA results show that bone resorption marker CTX-1 was significantly decreased, while bone formation marker PINP was significantly increased after Hmga2 treatment. The histological analysis revealed that Hmga2 treatment increased the collagen remodeling in the bone marrow matrix. This study reported that Hmga2 mutation highly altered body size in both mice and humans [
13]. Hmga2-/- mice exhibited a reduction in bone size [
14]. Hmga2-knockout mice displayed a pigmy phenotype, whereas the overexpression of Hmga2 led to gigantism and somatic overgrowth [
11]. Overexpression of Hmga2 increased new bone regeneration in a Dill-hole injury mouse femur model [
16]. Although further studies are needed to investigate the effects of Hmga2 treatment on the long-term physical function of aging, these findings suggest that Hmga2 was needed to maintain the bone homeostasis of aging.
R-loop formation is closely associated with the accumulation of DNA replication stress during the aging process [
1]. Excessive R-loop formation leads to replication fork collisions and transcription blockade [
18]. For more details, the RLFS in the Runx2, a key transcription factor in osteogenic differentiation, was predicted [
22,
23,
24]. R-loop formation (GC skew-triggered) is often observed between the TSS and the first exon–intron junction. This type of R-loop also produces the G-G-G-G secondary structure on the non-template DNA strand, which further stabilizes the R-loop and suppresses the gene transcription process [
25,
26]. Hmga2 has three distinct DNA-binding domains, each consisting of 8 or 9 amino acids, which have a high affinity for short A/T-rich sequences [
11]. The RLFS region was located mainly at the transcriptional front end of the Runx2 gene. Hmga2 significantly reduced R-loop formation and upregulated the mRNA expression of Runx2. The ChIP and DRIP assays in the RLFS region of Runx2 revealed a significant increase in Hmga2 binding and a decrease in R-loop formation. These suggest that Hmga2 upregulates Runx2 expression by repressing R-loop formation in aging mBMSCs.
To confirm our hypothesis, we tested the level of Runx2 expression and R-loop formation in the bone marrow of aging mice. The immunofluorescence results reveal a decrease in R-loop level and an increase in Runx2 expression after Hmga2 treatment. The colocalization of R-loop and Runx2 did not occur simultaneously. During the osteogenic process, Runx2 is involved in the expression and maintenance of bone matrix proteins, such as Col1a1 and Ocn [
23,
24]. Additionally, we found that Hmga2 treatment significantly increased the total collagen content in aging mice. These findings support our observation that Runx2 expression is upregulated in bone marrow and provide a basis for further investigation of the Hmga2-regulated spatiotemporal expression patterns of osteogenesis-related genes during bone remodeling. In conclusion, our study indicates that Hmga2 might be a critical regulator of the senescence and osteogenic differentiation of aging BMSCs by affecting the formation of R-loops in the genome. Additionally, Hmga2 promotes Runx2 expression in aging BMSCs by decreasing the formation of R-loops in the RLFS region.