Management of Myomectomy Scar Pregnancy: A Scoping Review
Abstract
1. Background
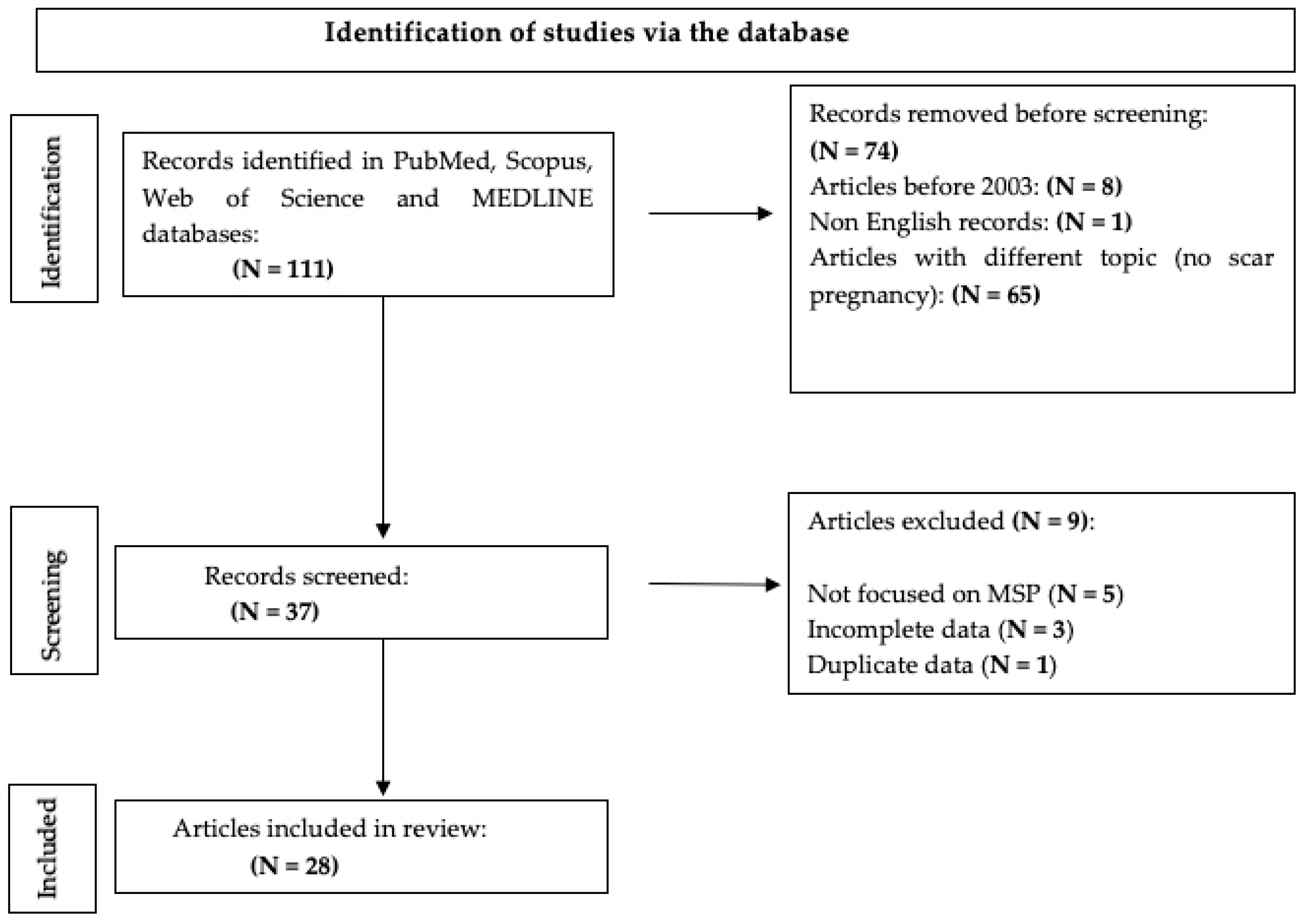
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion Criteria
- Studies reporting on myomectomy scar pregnancies (MSP);
- Case reports, retrospective studies, and reviews discussing the diagnosis, management, and outcomes of MSP;
- Articles published in English within the defined period.
2.3. Exclusion Criteria
- Studies not specifically addressing MSP;
- Articles not in English or lacking available translations;
- Papers with incomplete or low-quality data.
3. Results and Discussion
4. Discussion
5. Conclusions
- establishing standardized diagnostic criteria and reporting formats, including FIGO classification for myoma location and size;
- developing evidence-based treatment algorithms that differentiate between stable and emergency cases;
- creating multicenter registries to gather prospective data;
- evaluating the long-term reproductive outcomes of different treatment modalities, including conservative and fertility-sparing approaches.
6. Limits of the Paper
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freytag, D.; Günther, V.; Maass, N.; Alkatout, I. Uterine Fibroids and Infertility. Diagnostics 2021, 11, 1455. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Ura, B.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Peterlunger, I.; Stabile, G.; et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. J. Clin. Med. 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.B.; Chow, J.S.; Chang-Lee, W.; Hill, J.A.; Doubilet, P.M. Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester. J. Clin. Ultrasound 2001, 29, 261–264. [Google Scholar] [CrossRef]
- Coronado, G.D.; Marshall, L.M.; Schwartz, S.M. Complications in pregnancy, labor, and delivery with uterine leiomyomas: A population-based study. Obstet. Gynecol. 2000, 95, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Whynott, R.M.; Vaught, K.C.C.; Segars, J.H. The Effect of Uterine Fibroids on Infertility: A Systematic Review. Semin. Reprod. Med. 2017, 35, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Medical Treatment of Uterine Leiomyoma. Reprod. Sci. 2012, 19, 339–353. [Google Scholar] [CrossRef]
- Flyckt, R.; Coyne, K.; Falcone, T. Minimally Invasive Myomectomy. Clin. Obstet. Gynecol. 2017, 60, 252–272. [Google Scholar] [CrossRef]
- Tsakos, E.; Xydias, E.M.; Ziogas, A.C.; Sorrentino, F.; Nappi, L.; Vlachos, N.; Daniilidis, A. Multi-Port Robotic-Assisted Laparoscopic Myomectomy: A Systematic Review and Meta-Analysis of Comparative Clinical and Fertility Outcomes. J. Clin. Med. 2023, 12, 4134. [Google Scholar] [CrossRef]
- Vitale, S.G.; Saponara, S.; Sicilia, G.; Klarić, M.; Sorrentino, F.; D’Alterio, M.N.; Nappi, L.; Angioni, S. Hysteroscopic diode laser myolysis: From a case series to literature review of incisionless myolysis techniques for managing heavy menstrual bleeding in premenopausal women. Arch. Gynecol. Obstet. 2023, 309, 949–959. [Google Scholar] [CrossRef]
- Patel, M.A. Scar Ectopic Pregnancy. J. Obstet. Gynaecol. India 2015, 65, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Timor-Tritsch, I.E.; Monteagudo, A.; Santos, R.; Tsymbal, T.; Pineda, G.; Arslan, A.A. The diagnosis, treatment and follow-up of cesarean scar pregnancy. Am. J. Obstet. Gynecol. 2012, 207, 44–46. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Georg, A.V.; Marçal, L.C.A.; Nieto-Calvache, A.J.; Adu-Bredu, T.; D’Antonio, F.; Palacios-Jaraquemada, J.M. Placenta Accreta Spectrum Disorders: Current Recommendations from the Perspective of Antenatal Imaging. Rev. Bras. Ginecol. Obstet. 2023, 45, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ballas, J.; Pretorius, D.; Hull, A.D.; Resnik, R.; Ramos, G.A. Identifying sonographic markers for placenta accreta in the first trimester. J. Ultrasound Med. 2012, 31, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, Y.; Lin, B.L.; Kim, S.H.; Higuchi, T.; Iwata, S.; Nakada, S. The Association between Placenta Implantation at Prior Myomectomy Locations and Perinatal Outcomes in Pregnant Women Who Previously Underwent One-Step Hysteroscopic Myomectomy. Gynecol. Minim. Invasive Ther. 2020, 9, 54–58. [Google Scholar] [CrossRef]
- Kocher, M.R.; Sheafor, D.H.; Bruner, E.; Newman, C.; Mateus Nino, J.F. Diagnosis of abnormally invasive posterior placentation: The role of MR imaging. Radiol. Case Rep. 2017, 12, 295–299. [Google Scholar] [CrossRef]
- Mathiesen, E.; Hohenwalter, M.; Basir, Z.; Peterson, E. Placenta increta after hysteroscopic myomectomy. Obstet. Gynecol. 2013, 122 Pt 2, 478–481. [Google Scholar] [CrossRef]
- Lo, T.K.; Lam, C.H.; Cheung, K.W.; Ng, G.H.; Wu, A.K. Scalloping of placenta-myometrium interface on ultrasound in case with myomectomy scar. Ultrasound Obstet. Gynecol. 2016, 47, 518–522. [Google Scholar] [CrossRef]
- Saleh, M.M.; Mallmann, M.R.; Essakly, A.; Drebber, U.; Kleinert, R.; Kütting, F.; Bratke, G.; Müller, A.M. Placental Invasion into the Small Bowel Intestine Through a Myomectomy Scar: A Case Report With Literature Review. Int. J. Gynecol. Pathol. 2022, 41, 151–156. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsuzaki, S.; Matsuzaki, S.; Kakigano, A.; Kumasawa, K.; Ueda, Y.; Endo, M.; Kimura, T. Placenta accreta following hysteroscopic myomectomy. Clin. Case Rep. 2016, 4, 541–544. [Google Scholar] [CrossRef]
- Takeda, A.; Shibata, M.; Koike, W. Early identification of uterine scar defect by preconception magnetic resonance imaging to achieve successful pregnancy outcome after laparoscopic-assisted myomectomy: Two case reports. Clin. Case Rep. 2022, 10, e05441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, X.; Sun, W.; Qian, L.; Li, S.; Li, D. Myomectomy scar pregnancy: A case report and review of the literature. J. Int. Med. Res. 2020, 48, 300060520924542. [Google Scholar] [CrossRef] [PubMed]
- Park, W.I.; Jeon, Y.M.; Lee, J.Y.; Shin, S.Y. Subserosal pregnancy in a previous myomectomy site: A variant of intramural pregnancy. J. Minim. Invasive Gynecol. 2006, 13, 242–244. [Google Scholar] [CrossRef]
- Wong, K.S.; Tan, J.; Ang, C.; Ngu, A. Myomectomy scar ectopic pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2010, 50, 93–94. [Google Scholar] [CrossRef]
- Ishiguro, T.; Yamawaki, K.; Chihara, M.; Nishikawa, N.; Enomoto, T. Myomectomy scar ectopic pregnancy following a cryopreserved embryo transfer. Reprod. Med. Biol. 2018, 17, 509–513. [Google Scholar] [CrossRef]
- Paul, P.; Mannur, S.; Shintre, H.; Paul, G.; Gulati, G. Myomectomy scar pregnancy: A rare complication of myomectomy. J. Gynecol. Surg. 2018, 34, 53–57. [Google Scholar] [CrossRef]
- Bannon, K.; Fernandez, C.; Rojas, D.; Levine, E.M.; Locher, S. Diagnosis and management of intramural ectopic pregnancy. J. Minim. Invasive Gynecol. 2013, 20, 697–700. [Google Scholar] [CrossRef]
- Vagg, D.; Arsala, L.; Kathurusinghe, S.; Ang, W.C. Intramural Ectopic Pregnancy Following Myomectomy. J. Investig. Med. High. Impact Case Rep. 2018, 6, 2324709618790605. [Google Scholar] [CrossRef]
- Liu, D.; Gu, X.; Liu, F.; Shi, F.; Yang, M.; Wu, Q. Application of contrast-enhanced ultrasound for scar pregnancy cases misdiagnosed as other diseases. Clin. Chim. Acta 2019, 496, 134–139. [Google Scholar] [CrossRef]
- Li, M.; Kailun, C. A Rare Case of Ectopic Pregnancy at a Previous Laparoscopic Adenomyomectomy Scar. J. Minim. Invasive Gynecol. 2019, 26, 384–385. [Google Scholar] [CrossRef]
- Dutta, I.; Haldar, A.; Nath, M. Scar Pregnancy: A Case Series Involving Two Medical College Hospitals in West Bengal. J. South. Asian Feder Obstet. Gynae 2020, 12, 51–58. [Google Scholar] [CrossRef]
- Al-Serehi, A.; Mhoyan, A.; Brown, M.; Benirschke, K.; Hull, A.; Pretorius, D.H. Placenta accreta: An association with fibroids and Asherman syndrome. J. Ultrasound Med. 2008, 27, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Uotani, T.; Ohara, K.; Takai, Y.; Baba, K.; Seki, H. Two cases of placenta accreta identified during pregnancy after laparoscopic myomectomy and resection of adenomyosis. Hypertens. Res. Pregnancy 2015, 3, 38–41. [Google Scholar] [CrossRef]
- Fukutani, R.; Hasegawa, J.; Arakaki, T.; Oba, T.; Nakamura, M.; Sekizawa, A. Silent uterine rupture occluded by intestinal adhesions following laparoscopic myomectomy: A case report. J. Obstet. Gynaecol. Res. 2017, 43, 1209–1211. [Google Scholar] [CrossRef]
- Kuwata, T.; Matsubara, S.; Usui, R.; Uchida, S.; Sata, N.; Suzuki, M. Intestinal adhesion due to previous uterine surgery as a risk factor for delayed diagnosis of uterine rupture: A case report. J. Med. Case Rep. 2011, 5, 523. [Google Scholar] [CrossRef]
- Bejarano, M.T.; Mora, R.; Vernon, J.; Duncan, K.; Timor-Tritsch, I.E. VP06.05: Myomectomy scar pregnancy: A serious but little appreciated entity. Ultrasound Obstet. Gynecol. 2021, 58, 120. [Google Scholar] [CrossRef]
- Agarwal, M.; Kashyap, M.; Meshram, S. A case of laparoscopically managed myometrial scar ectopic pregnancy. Int. J. Gynecol. Endsc 2017, 1, 28. [Google Scholar] [CrossRef]
- Bouzari, Z.; Keshani, M.; Yazdani, S.; Barat, S.; Zinalzadeh, M. Intramural pregnancy. J. Obstet. Gynaecol. 2010, 30, 195–196. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, H. Laparoscopic Management of Myomectomy Scar Pregnancy after a Cryopreserved Embryo Transfer. J. Minim. Invasive Gynecol. 2021, 28, 1806–1807. [Google Scholar] [CrossRef]
- Kandaswami, D.; Jayapal, K.; Sudhakar, P.; Usman, S. Myomectomy scar ectopic pregnancy: Diagnostic challenges and management conundrum: A case report. Int. J. Reprod. Contracept. Obstet. Gynecol. 2022, 11, 954–956. [Google Scholar] [CrossRef]
- Hudecová, J.; Hudec, A.; Novotný, N.; Slouka, D. Pregnancy in the scar after myomectomy. Clin. Exp. Obstet. Gynecol. 2018, 45, 632–635. [Google Scholar] [CrossRef]
- Sung, S.; Mahdy, H. Cesarean Section; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cobellis, L.; Pecori, E.; Cobellis, G. Comparison of intramural myomectomy scar after laparotomy or laparoscopy. Int. J. Gynaecol. Obstet. 2004, 84, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Zaman, F. Silent Rupture of Previous Myomectomy Scar in Pregnancy At 27 Weeks. J. Med. Case Rep. Case Ser. 2020, 1. [Google Scholar] [CrossRef]
- Rees, M. Publication of medical case reports and the CARE guidelines. Case Rep. Womens Health 2018, 17, 1–2. [Google Scholar] [CrossRef]
- Koo, Y.-J.; Lee, J.-K.; Lee, Y.-K.; Kwak, D.-W.; Lee, I.-H.; Lim, K.-T.; Lee, K.-H.; Kim, T.-J. Pregnancy outcomes and risk factors for uterine rupture after laparoscopic myomectomy: A single-center experience and literature review. J. Minim. Invasive Gynecol. 2015, 22, 1022–1028. [Google Scholar] [CrossRef]
- Lebovitz, O.; Orvieto, R.; James, K.E.; Styer, A.K.; Brown, D.N. Predictors of reproductive outcomes following myomectomy for intramural fibroids. Reprod. Biomed. Online 2019, 39, 484–491. [Google Scholar] [CrossRef]
- Gyamfi-Bannerman, C.; Gilbert, S.; Landon, M.B.; Spong, C.Y.; Rouse, D.J.; Varner, M.W.; Caritis, S.N.; Meis, P.J.; Wapner, R.J.; Sorokin, Y.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Risk of uterine rupture and placenta accreta with prior uterine surgery outside of the lower segment. Obstet. Gynecol. 2012, 120, 1332–1337. [Google Scholar] [CrossRef]
- Shamshirsaz, A.A.; Fox, K.A.; Salmanian, B. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am. J. Obstet. Gynecol. 2015, 212, 2180–2182.18E11. [Google Scholar] [CrossRef]
- Nieto-Calvache, A.J.; Palacios-Jaraquemada, J.M.; Vergara-Galliadi, L.M.; Matera, L.; Sanín-Blair, J.E.; Rivera, E.P.; Rozo-Rangel, A.P.; Burgos-Luna, J.M. Latin American Group for the Study of Placenta Accreta Spectrum. All maternal deaths related to placenta accreta spectrum are preventable: A difficult-to-tell reality. AJOG Glob. Rep. 2021, 1, 100012. [Google Scholar] [CrossRef]
- Sorrentino, F.; De Feo, V.; Stabile, G.; Tinelli, R.; D’Alterio, M.N.; Ricci, G.; Angioni, S.; Nappi, L. Cesarean Scar Pregnancy Treated by Artery Embolization Combined with Diode Laser: A Novel Approach for a Rare Disease. Medicina 2021, 57, 411. [Google Scholar] [CrossRef]
- Rotas, M.A.; Haberman, S.; Levgur, M. Cesarean scar ectopic pregnancies: Etiology, diagnosis and management. Obstet. Gynecol. 2006, 107, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Katano, K.; Ikuta, K.; Matsubara Het, a.l. A case of successful conservative chemotherapy for intramural pregnancy. Fertil. Steril. 1999, 72, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Marchiole, P.; Gorelero, F.; de Caro Get, a.l. Intramural pregnancy embedded in a previous Cesarean section scar treated conservatively. Ultrasound Obstet. Gynecol. 2004, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Etrusco, A.; Laganà, A.S.; Chiantera, V.; Vitagliano, A.; Cicinelli, E.; Mikuš, M.; Šprem Goldštajn, M.; Ferrari, F.; Uccella, S.; Garzon, S.; et al. Feasibility and Surgical Outcomes of Hysteroscopic Myomectomy of FIGO Type 3 Myoma: A Systematic Review. J. Clin. Med. 2023, 12, 4953. [Google Scholar] [CrossRef]
- Favilli, A.; Mazzon, I.; Etrusco, A.; Dellino, M.; Laganà, A.S.; Tinelli, A.; Chiantera, V.; Cicinelli, E.; Gerli, S.; Vitagliano, A. The challenge of FIGO type 3 leiomyomas and infertility: Exploring therapeutic alternatives amidst limited scientific certainties. Int. J. Gynaecol. Obstet. 2024, 165, 975–987. [Google Scholar] [CrossRef]

| Author | Year | Country | Study Design | Period Considered | GA at Diagnosis | Intervention | Cases (n) | Preview Myomectomy Surgical Procedures | Age | Diagnostic Exam | Spontaneous Pregnancy or ART |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ballas [14] | 2012 | USA | retrospective | 2004–2011 | 35 w; 40 w | CS + hysterectomy | 2 | A: HSC; B: HSC | na | na | na |
| Kasuga [15] | 2020 | Japan | retrospective | 2012–2019 | 20 | observation | 14 | 14 HSC | 36 (28–41) | 14 TA US | 3 ART 11 spontaneous |
| Kocher [16] | 2017 | Carolina | case report | 2017 | 20 w | CS + hysterectomy | 1 | na | 37 | US + MRI | ART |
| Matheisen [17] | 2015 | USA | case report | 2013 | 26 w | CS + hysterectomy | 1 | HSC | 34 | MRI | spontaneous |
| Lo [18] | 2015 | Hong Kong | case report | 2015 | 20 w | CS | 1 | LPS | 41 | US | spontaneous |
| Saleh [19] | 2022 | Germany | case report | 2022 | 27 w + 4 | CS + hysterectomy + bowel resection | 1 | na | 47 | MRI | na |
| Tanaka [20] | 2016 | Japan | case report | 2016 | 39 w | hysterectomy | 1 | HSC | 48 | US | ART |
| Takeda [21] | 2022 | Japan | case report | 2021 | 18 w, 14 w | CS + hysterectomy | 2 | 2 LPS | 37; 38 | 2 MRI | ART |
| Zhu [22] | 2020 | China | case report | 2019 | 6 w | LPT surgical removal + metotrexate | 1 | LPS | 26 | MRI | spontaneous |
| Park [23] | 2006 | Korea | case report | 2006 | 7 w | LPS surgical removal | 1 | LPT | 35 | US | ART |
| Wong [24] | 2010 | Australia | case report | 2003 | na | metotrexate | 1 | na | 33 | US | spontaneous |
| Ishiguro [25] | 2018 | Japan | case report | 2018 | 8 w | LPS surgical removal | 1 | LPS | 41 | US | ART |
| Paul [26] | 2018 | India | case report | 2018 | 9 w | LPS surgical removal | 1 | LPS | 31 | US | spontaneous |
| Bannon [27] | 2013 | USA | case report | 2013 | 10 w | LPS surgical removal + metotrexate | 1 | LPT | 27 | US + TC | spontaneous |
| Vagg [28] | 2018 | Australia | case report | 2018 | 12 w | hysterectomy | 1 | LPT | 34 | US + MRI | spontaneous |
| Liu [29] | 2019 | China | retrospective | 2018 | na | HSC + LPS | 1 | na | 34 | CEUS | na |
| Li [30] | 2018 | Singapore | case report | 2018 | 9 w | LPS surgical removal | 1 | LPS | 40 | US | na |
| Dutta [31] | 2020 | India | retrospective | 2012–2019 | na | LPT surgical removal | 1 | na | 19 | na | spontaneous |
| Al-Serehi [32] | 2008 | Canada | case report | 2008 | 34 w | CS + hysterectomy | 1 | na | 48 | na | ART |
| Matsunaga [33] | 2015 | Japan | case report | 2015 | 33 w; 37 wks | CS + myometral repair | 2 | 2 LPS | 39; 37 | 2 US | spontaneous |
| Fukutani [34] | 2017 | Japan | case report | 2017 | 33 w | CS + myometral repair | 1 | LPS | 35 | MRI | ART |
| Kuwata [35] | 2011 | Japan | case report | 2011 | 34 w | CS + myometral repair | 1 | LPT | 38 | TC | spontaneous |
| Bejarano [36] | 2021 | USA | case report | 2019 | 37 w | CS + myometral repair + uterine artery embolization + HSC + D&C | 1 | LPS | 41 | TV US | ART |
| Agarwal [37] | 2017 | India | case report | 2017 | 9 | D&C + LPS | 1 | LPS | 28 | TV US + MRI | ART |
| Bouzari [38] | 2010 | Iran | case report | 2010 | 26 | LPT + myometral repair | 1 | na | 28 | US | spontaneous |
| Zhang H [39] | 2021 | China | case report | 2021 | 4 wks | LPT surgical removal | 1 | HSC | 38 | US + MRI | ART |
| Kandaswami [40] | 2022 | India | case report | 2015 | 4 wks | metotrexate + LPS | 1 | LPS | 45 | US | ART |
| Hudecova [41] | 2018 | Czech Republic | Case report | 2017 | 12 wks | LPT | 1 | LPS | 33 | US + MRI | spontaneous |
| Total Cases | Cases (%) | |
|---|---|---|
| Study design | 44 | |
| - Retrospective; | 18 (40.9%) | |
| - Case report. | 26 (59.1%) | |
| Age (mean ± SD) | 36 ± 6.7 (range 19–48 years) | |
| Diagnostic exam | 40 | |
| - US; | 27 (67.5%) | |
| - MRI; | 11 (27.5%) | |
| - CT. | 2 (5%) | |
| Pregnancy | 39 | |
| - Spontaneous; | 24 (61.5%) | |
| - ART. | 15 (38.5%) | |
| Myomectomy surgical procedures | 37 | |
| - Hysteroscopy; | 19 (51.3%) | |
| - Laparotomy; | 4 (10.8%) | |
| - Laparoscopy. | 14 (37.9%) |
| Intervention Type | Number of Cases (n) | Percentage (%) |
|---|---|---|
| Cesarean section + uterine repair | 10 | 22.7 |
| Vaginal delivery | 9 | 20.5 |
| Hysterectomy | 10 | 22.7 |
| Laparotomy + uterine repair | 4 | 9.1 |
| Laparoscopy + uterine repair | 4 | 9.1 |
| Methotrexate (medical treatment only) | 4 | 9.1 |
| Methotrexate + surgical approach | 3 | 6.8 |
| Combined therapies | 3 | 6.8 |
| Cases | % | |
|---|---|---|
| Previous Cesarean section | 4 | 9% |
| Localization of myoma | 21 Submucosal | 47% |
| 8 Intramural | 18% | |
| 2 cervical | 4.5% | |
| Myomas size | Mean 6 cm | Median 6.6 cm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, F.; Vasciaveo, L.; Greco, F.; Giansiracusa, E.; D’Antonio, F.; Lucidi, A.; Etrusco, A.; Laganà, A.S.; Stabile, G.; Nappi, L. Management of Myomectomy Scar Pregnancy: A Scoping Review. Medicina 2025, 61, 817. https://doi.org/10.3390/medicina61050817
Sorrentino F, Vasciaveo L, Greco F, Giansiracusa E, D’Antonio F, Lucidi A, Etrusco A, Laganà AS, Stabile G, Nappi L. Management of Myomectomy Scar Pregnancy: A Scoping Review. Medicina. 2025; 61(5):817. https://doi.org/10.3390/medicina61050817
Chicago/Turabian StyleSorrentino, Felice, Lorenzo Vasciaveo, Francesca Greco, Elisa Giansiracusa, Francesco D’Antonio, Alessandro Lucidi, Andrea Etrusco, Antonio Simone Laganà, Guglielmo Stabile, and Luigi Nappi. 2025. "Management of Myomectomy Scar Pregnancy: A Scoping Review" Medicina 61, no. 5: 817. https://doi.org/10.3390/medicina61050817
APA StyleSorrentino, F., Vasciaveo, L., Greco, F., Giansiracusa, E., D’Antonio, F., Lucidi, A., Etrusco, A., Laganà, A. S., Stabile, G., & Nappi, L. (2025). Management of Myomectomy Scar Pregnancy: A Scoping Review. Medicina, 61(5), 817. https://doi.org/10.3390/medicina61050817









