Associations of Thyroid and Parathyroid Hormones with Arterial Stiffness in Emergency Department Patients: A Prospective Cross-Sectional Study
Abstract
1. Background
2. Methods
2.1. Study Population
2.2. Study-Related Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arter. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andia, J.B.; Roman-Garcia, P.; Hruska, K. The connections between vascular calcification and bone health. Nephrol. Dial. Transplant. 2011, 26, 3429–3436. [Google Scholar] [CrossRef]
- Panizo, S.; Cardus, A.; Encinas, M.; Parisi, E.; Valcheva, P.; López-Ongil, S.; Coll, B.; Fernandez, E.; Valdivielso, J.M. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 2009, 104, 1041–1048. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef]
- Weng, S.; Sprague, J.E.; Oh, J.; Riek, A.E.; Chin, K.; Garcia, M.; Bernal-Mizrachi, C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS ONE 2013, 8, e54625. [Google Scholar] [CrossRef]
- Ellam, T.; Hameed, A.; Haque, R.U.; Muthana, M.; Wilkie, M.; Francis, S.E.; Chico, T.J.A. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PLoS ONE 2014, 9, e88767. [Google Scholar] [CrossRef]
- Carrillo-Sepúlveda, M.A.; Ceravolo, G.S.; Fortes, Z.B.; Carvalho, M.H.; Tostes, R.C.; Laurindo, F.R.; Webb, R.C.; Barreto-Chaves, M.L.M. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc. Res. 2010, 85, 560–570. [Google Scholar] [CrossRef]
- Mizuma, H.; Murakami, M.; Mori, M. Thyroid hormone activation in human vascular smooth muscle cells: Expression of type II iodothyronine deiodinase. Circ. Res. 2001, 88, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, Y.; Kim, H.H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.-H.; Scanlan, T.S.; et al. Rapid nongenomic actions of thyroid hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef]
- Davis, P.J.; Mousa, S.A.; Lin, H.Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Shargorodsky, M.; Serov, S.; Gavish, D.; Leibovitz, E.; Harpaz, D.; Zimlichman, R. Long-term thyrotropin-suppressive therapy with levothyroxine impairs small and large artery elasticity and increases left ventricular mass in patients with thyroid carcinoma. Thyroid 2006, 16, 381–386. [Google Scholar] [CrossRef]
- Lekakis, J.; Papamichael, C.; Alevizaki, M.; Piperingos, G.; Marafelia, P.; Mantzos, J.; Stamatelopoulos, S.; Koutras, D.A. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid 1997, 7, 411–414. [Google Scholar] [CrossRef]
- Fu, S.; Chen, W.; Luo, L.; Ye, P. Roles of fasting and postprandial blood glucose in the effect of type 2 diabetes on central arterial stiffness: A 5-year prospective community-based analysis. Diabetol. Metab. Syndr. 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.D.S.; Lelis, D.F.; Cunha, R.S.; Griep, R.H.; Barreto, S.M.; Molina, M.D.C.B.; Schmidt, M.I.; Duncan, B.B.; Bensenor, I.; Lotufo, P.A.; et al. Fasting Glucose, Glycated Hemoglobin, and 2h Post-load Blood Glucose Are Independently Associated with Arterial Stiffness in Diabetes: The ELSA-Brasil Study. Angiology 2023, 23, 33197231166180. [Google Scholar] [CrossRef]
- Foreman, Y.D.; Brouwers, M.C.G.J.; Berendschot, T.T.J.M.; van Dongen, M.C.J.M.; Eussen, S.J.P.M.; van Greevenbroek, M.M.J.; Henry, R.M.A.; Houben, A.J.H.M.; van der Kallen, C.J.H.; Kroon, A.A.; et al. The oral glucose tolerance test-derived incremental glucose peak is associated with greater arterial stiffness and maladaptive arterial remodeling: The Maastricht Study. Cardiovasc. Diabetol. 2019, 18, 152. [Google Scholar] [CrossRef]
- Monteiro, C.I.; Simões, R.P.; Goulart, C.L.; da Silva, C.D.; Borghi-Silva, A.; Mendes, R.G. Arterial stiffness in type 2 diabetes: Determinants and indication of a discriminative value. Clinics 2021, 76, e2172. [Google Scholar] [CrossRef]
- Stone, K.; Fryer, S.; Meyer, M.L.; Kucharska-Newton, A.; Faulkner, J.; Zieff, G.; Paterson, C.; Credeur, D.; Matsushita, K.; Hughes, T.M.; et al. The aortic-femoral arterial stiffness gradient: An atherosclerosis risk in communities (ARIC) study. J. Hypertens. 2021, 39, 1370–1377. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Shang, F.; Wu, H.; Shi, H.; Ren, L.; He, Y.; Zhang, M.; Peng, H. Association Between Glucose Metabolism and Vascular Aging In Chinese Adults: A Cross-Sectional Analysis in The Tianning Cohort Study. Clin. Interv. Aging 2019, 14, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Puzantian, H.; Teff, K.; Townsend, R.R. Investigating the effect of glucose on aortic pulse wave velocity using pancreatic clamping methodology. Biol. Res. Nurs. 2015, 17, 270–275. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Reference Values for Arterial Stiffness’ Collaboration Determinants of pulse wave velocity in healthy people in the presence of cardiovascular risk factors: ‘establishing normal reference values’. Eur. Heart J. 2010, 31, 2338–2350. [CrossRef] [PubMed]
- Schnaubelt, S.; Oppenauer, J.; Bader, M.; Du, N.; Eibensteiner, F.; Kienbacher, C.L.; Baldi, E.; Mueller, M.; Perkmann, T.; Haslacher, H.; et al. Arterial stiffness in acute coronary syndrome as a potential triage tool: A prospective observational study. Minerva Med. 2023, 114, 1–14. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Oppenauer, J.; Tihanyi, D.; Mueller, M.; Maldonado-Gonzalez, E.; Zejnilovic, S.; Haslacher, H.; Perkmann, T.; Strassl, R.; Anders, S.; et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J. Intern. Med. 2021, 290, 437–443. [Google Scholar] [CrossRef]
- Klinisches Institut für Labormedizin, AKH Wien. Referenzwerte. Available online: https://www.akhwien.at/default.aspx?pid=3986 (accessed on 7 June 2024).
- Schnaubelt, S.; Oppenauer, J.; Kornfehl, A.; Eibensteiner, F.; Veigl, C.; Neymayer, M.; Brock, R.; Du, N.; Wirth, S.; Greisl, N.; et al. Short- and long-term risk stratification in acutely ill medical patients by implementing ankle-brachial index and pulse wave velocity in the emergency setting. Eur. J. Clin. Investig. 2025, e70015. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Carrillo-López, N.; Messina, O.D.; Hamdy, N.A.T.; Panizo, S.; Ferrari, S.L.; on behalf of the International Osteoporosis Foundation Iof Working Group on Bone and Cardiovascular Diseases. Pathophysiology of Vascular Calcification and Bone Loss: Linked Disorders of Ageing? Nutrients 2021, 13, 3835. [Google Scholar] [CrossRef]
- Coen, G.; Ballanti, P.; Mantella, D.; Manni, M.; Lippi, B.; Pierantozzi, A.; Di Giulio, S.; Pellegrino, L.; Romagnoli, A.; Simonetti, G.; et al. Bone turnover, osteopenia and vascular calcifications in hemodialysis patients. A histomorphometric and multislice CT study. Am. J. Nephrol. 2009, 29, 145–152. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Román-García, P.; Rodríguez, I.; Martínez-Salgado, C.; Dusso, A.S.; Naves, M.; Cannata-Andía, J.B. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016, 90, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Graciolli, F.G.; Neves, K.R.; dos Reis, L.M.; Graciolli, R.G.; Noronha, I.L.; Moysés, R.M.A.; Jorgetti, V. Phosphorus overload and PTH induce aortic expression of Runx2 in experimental uraemia. Nephrol. Dial. Transplant. 2009, 24, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Martínez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andía, J.B.; Naves-Díaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 934–941. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.B.; Okazaki, H.; Stinghen, A.E.M.; Drüeke, T.B.; Massy, Z.A.; Jorgetti, V. Vascular calcification in chronic kidney disease: A review. Braz. J. Nephrol. 2013, 35, 147–161. [Google Scholar] [CrossRef]
- Fortier, C.; Mac-Way, F.; De Serres, S.A.; Marquis, K.; Douville, P.; Desmeules, S.; Larivière, R.; Agharazii, M. Active vitamin D and accelerated progression of aortic stiffness in hemodialysis patients: A longitudinal observational study. Am. J. Hypertens. 2014, 27, 1346–1354. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Bobryshev, Y.V.; Lartaud-Idjouadiene, I.; Giummelly, P.; Atkinson, J. Aortic calcification produced by vitamin D3 plus nicotine. J. Vasc. Res. 1997, 34, 386–398. [Google Scholar] [CrossRef]
- Watson, K.E.; Abrolat, M.L.; Malone, L.L.; Hoeg, J.M.; Doherty, T.; Detrano, R.; Demer, L.L. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 1997, 96, 1755–1760. [Google Scholar] [CrossRef]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Schwarz, P.; Heegaard, A.M.; Lind, B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: The CopD study. J. Clin. Endocrinol. Metab. 2012, 97, 2644–2652. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Pandak, W.M.; Heuman, D.M.; Redford, K.; Stravitz, R.T.; Chiang, J.Y.; Hylemon, P.B.; Vlahcevic, Z.R. Hormonal regulation of cholesterol 7alpha-hydroxylase specific activity, mRNA levels, and transcriptional activity in vivo in the rat. J. Lipid Res. 1997, 38, 2483–2491. [Google Scholar] [CrossRef]
- Duntas, L.H. Thyroid disease and lipids. Thyroid 2002, 12, 287–293. [Google Scholar] [CrossRef]
- Mousa, S.; Hemeda, A.; Ghorab, H.; Abdelhamid, A.; Saif, A. Arterial wall stiffness and the risk of atherosclerosis in Egyptian patients with overt and subclinical hypothyroidism. Endocr. Pract. 2020, 26, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; López-Bermejo, A.; Castro, A.; Casamitjana, R.; Ricart, W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J. Clin. Endocrinol. Metab. 2006, 91, 3337–3343. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Bakker, S.J.L.; Links, T.P.; Gans, R.O.B.; Wolffenbuttel, B.H.R. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J. Clin. Endocrinol. Metab. 2007, 92, 491–496. [Google Scholar] [CrossRef]
- Gumieniak, O.; Perlstein, T.S.; Hopkins, P.N.; Brown, N.J.; Murphey, L.J.; Jeunemaitre, X.; Hollenberg, N.K.; Williams, G.H. Thyroid function and blood pressure homeostasis in euthyroid subjects. J. Clin. Endocrinol. Metab. 2004, 89, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, T.Y.; Koh, J.M.; Kim, H.K.; Park, J.Y.; Lee, K.U.; Shong, Y.K.; Kim, W.B. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin. Endocrinol. 2009, 70, 152–160. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Sun, M.; Wang, Z.; Fu, Q.; Shi, Y.; Cao, M.; Zhu, Z.; Meng, C.; Mao, J.; et al. Low serum free thyroxine concentrations associate with increased arterial stiffness in euthyroid subjects: A population-based cross-sectional study. Endocrine 2015, 50, 465–473. [Google Scholar] [CrossRef]
- Wang, P.; Du, R.; Lin, L.; Ding, L.; Peng, K.; Xu, Y.; Xu, M.; Bi, Y.F.; Wang, W.Q.; Ning, G.; et al. Association between Free Triiodothyronine Levels and Peripheral Arterial Disease in Euthyroid Participants. Biomed. Environ. Sci. BES 2017, 30, 128–133. [Google Scholar]
- Grove-Laugesen, D.; Malmstroem, S.; Ebbehoj, E.; Riis, A.L.; Watt, T.; Rejnmark, L.; Würgler Hansen, K. Arterial Stiffness and Blood Pressure in Patients Newly Diagnosed with Graves’ Disease Compared with Euthyroid Controls. Eur. Thyroid. J. 2020, 9, 148–156. [Google Scholar] [CrossRef]
- Yildiz, C.; Altay, M.; Yildiz, S.; Çağir, Y.; Akkan, T.; Ünsal, Y.A.; Beyan, E. Arterial stiffness in hyperthyroid patients is deteriorated due to thyroid hormones. Arch. Endocrinol. Metab. 2019, 63, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Grillo, A.; Antonello, R.M.; Cola, M.F.; Dobrinja, C.; Fabris, B.; Giudici, F. Meta-analysis on the Association Between Thyroid Hormone Disorders and Arterial Stiffness. J. Endocr. Soc. 2022, 6, bvac016. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Swoboda, P.P.; Erhayiem, B.; Kan, R.; McDiarmid, A.K.; Garg, P.; Musa, T.A.; Dobson, L.E.; Witte, K.K.; Kearney, M.T.; Barth, J.H.; et al. Cardiovascular magnetic resonance measures of aortic stiffness in asymptomatic patients with type 2 diabetes: Association with glycaemic control and clinical outcomes. Cardiovasc. Diabetol. 2018, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, C.D.A. Microvascular Dysfunction and Hyperglycemia: A Vicious Cycle with Widespread Consequences. Diabetes 2018, 67, 1729–1741. [Google Scholar] [CrossRef]
- Stehouwer, C.D.A.; Henry, R.M.A.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardiovascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Henry, R.M.A.; Dekker, J.M.; Nijpels, G.; Unger, T.; Schram, M.T.; Stehouwer, C.D.A. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: The Hoorn study. Hypertension 2014, 64, 1299–1305. [Google Scholar] [CrossRef]

| cfPWV < 8.4 m/s (n = 280) | cfPWV 8.4–10.9 m/s (n = 275) | cfPWV > 10.9 m/s (n = 272) | Overall (n = 827) | p-Value | |
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| Mean (SD) | 47.6 (16.5) | 57.8 (13.6) | 71.7 (12.1) | 58.9 (17.3) | |
| Median (Q1, Q3) | 46.0 (36.0, 58.3) | 58.0 (49.0, 67.0) | 73.5 (65.0, 80.0) | 60.0 (47.0, 72.0) | |
| Sex | 0.727 | ||||
| female | 124 (44.3%) | 113 (41.1%) | 116 (42.6%) | 353 (42.7%) | |
| male | 155 (55.4%) | 162 (58.9%) | 156 (57.4%) | 473 (57.2%) | |
| Missing | 1 (0.4%) | 0 (0%) | 0 (0%) | 1 (0.1%) | |
| BMI | 0.004 | ||||
| Mean (SD) | 27.1 (5.74) | 28.2 (5.33) | 26.7 (4.75) | 27.3 (5.32) | |
| Median (Q1, Q3) | 26.3 (22.9, 30.4) | 27.3 (24.5, 31.2) | 26.0 (23.2, 29.4) | 26.6 (23.5, 30.4) | |
| Missing | 0 (0%) | 0 (0%) | 2 (0.7%) | 2 (0.2%) | |
| iPTH | <0.001 | ||||
| Mean (SD) | 45.4 (27.9) | 62.9 (148) | 63.8 (56.7) | 57.1 (93.0) | |
| Median (Q1, Q3) | 39.8 (29.5, 51.6) | 40.9 (29.9, 60.7) | 47.4 (35.2, 69.8) | 42.2 (30.8, 60.4) | |
| Missing | 107 (38.2%) | 109 (39.6%) | 111 (40.8%) | 327 (39.5%) | |
| 25(OH)D3 | 0.3 | ||||
| Mean (SD) | 55.5 (34.9) | 61.4 (39.3) | 61.7 (43.7) | 59.5 (39.4) | |
| Median (Q1, Q3) | 52.4 (31.5, 70.4) | 56.2 (33.5, 79.8) | 57.0 (29.0, 81.3) | 54.6 (31.7, 77.1) | |
| Missing | 100 (35.7%) | 103 (37.5%) | 99 (36.4%) | 302 (36.5%) | |
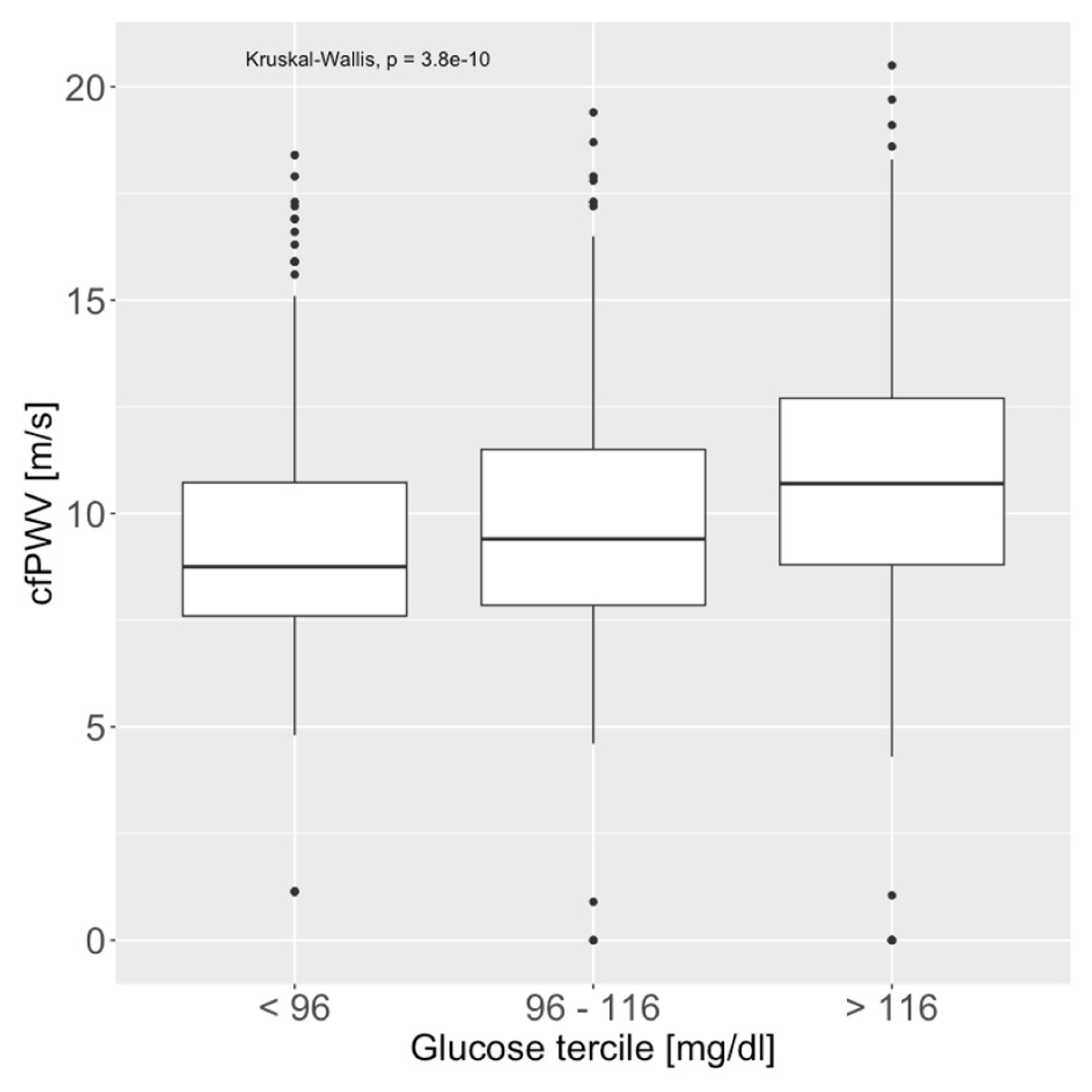
| Glucose | <0.001 | ||||
| Mean (SD) | 103 (30.0) | 113 (36.8) | 132 (61.0) | 116 (46.1) | |
| Median (Q1, Q3) | 97.0 (88.3, 110) | 103 (92.0, 121) | 111 (96.0, 148) | 102 (91.0, 124) | |
| Missing | 26 (9.3%) | 18 (6.5%) | 20 (7.4%) | 64 (7.7%) | |
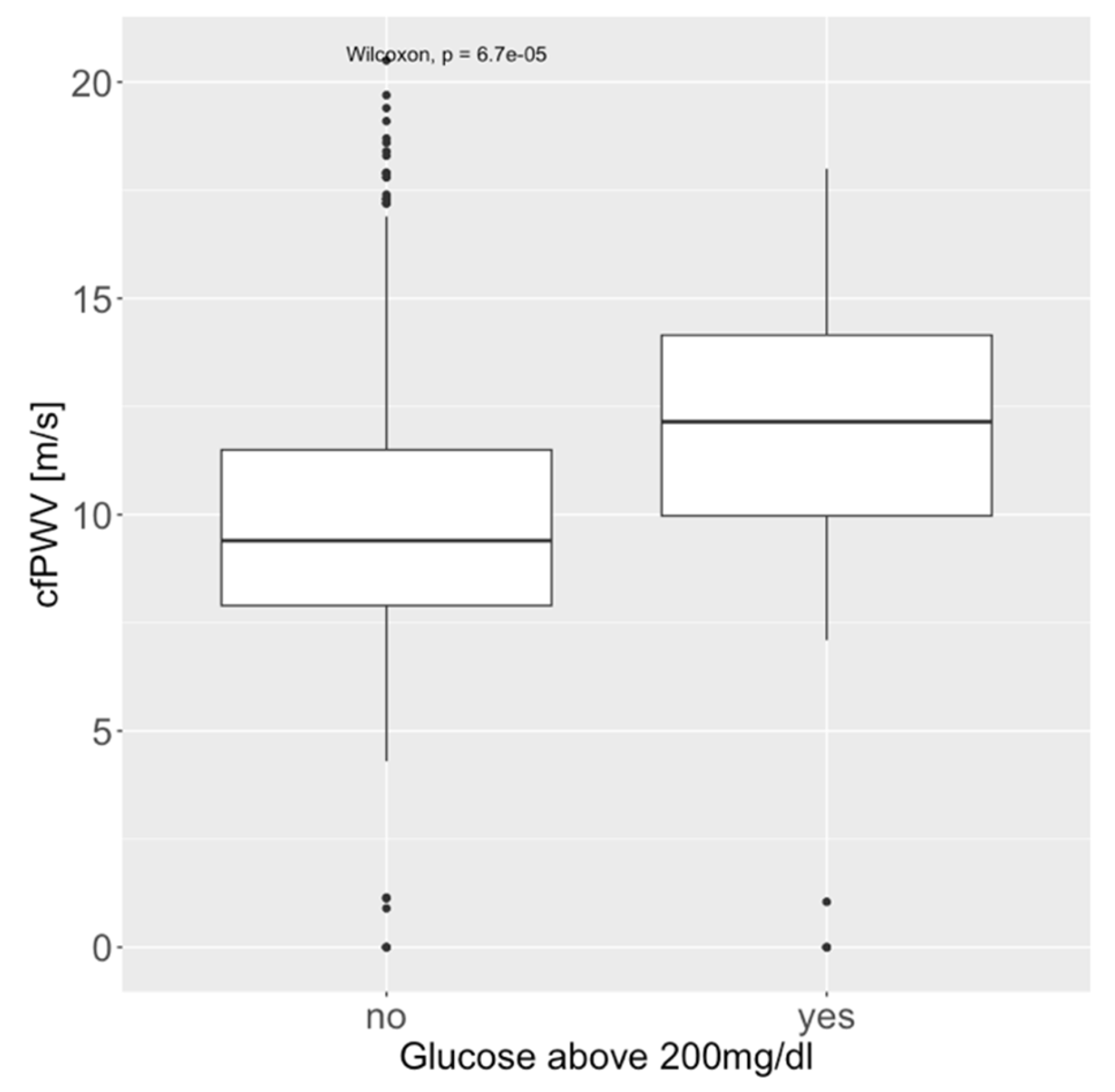
| Glucose above 200 mg/dL | 5 (1.8%) | 6 (2.2%) | 25 (9.2%) | 36 (4.4%) | <0.001 |
| Comorbidities | |||||
| Diabetes mellitus type 2 | 21 (7.5%) | 46 (16.7%) | 83 (30.5%) | 150 (18.1%) | <0.001 |
| Coronary artery disease | 25 (8.9%) | 55 (20.0%) | 76 (27.9%) | 156 (18.9%) | <0.001 |
| Cerebrovascular disease | 6 (2.1%) | 8 (2.9%) | 25 (9.2%) | 39 (4.7%) | <0.001 |
| Peripheral artery disease | 6 (2.1%) | 8 (2.9%) | 22 (8.1%) | 36 (4.4%) | <0.001 |
| Chronic kidney disease | 12 (4.3%) | 18 (6.5%) | 38 (14.0%) | 68 (8.2%) | <0.001 |
| Dyslipidemia | 52 (18.6%) | 80 (29.1%) | 109 (40.1%) | 241 (29.1%) | <0.001 |
| Hypertension | 83 (29.6%) | 138 (50.2%) | 187 (68.8%) | 408 (49.3%) | <0.001 |
| Age Group (Years) | 18–53 (n = 315) | 53–70 (n = 310) | 70–100 (n = 293) | Overall (n = 918) |
|---|---|---|---|---|
| cfPWV | ||||
| Mean (SD) | 8.26 (1.97) | 10.1 (2.49) | 12.1 (3.57) | 9.99 (3.10) |
| Median (Q1, Q3) | 8.00 (7.10, 9.18) | 9.70 (8.50, 11.3) | 11.9 (10.4, 14.1) | 9.40 (7.90, 11.8) |
| Missing | 9 (2.9%) | 26 (8.4%) | 56 (19.1%) | 91 (9.9%) |
| TSH | ||||
| Mean (SD) | 1.48 (1.10) | 2.00 (3.41) | 2.04 (3.43) | 1.83 (2.85) |
| Median (Q1, Q3) | 1.25 (0.818, 1.85) | 1.40 (0.860, 2.07) | 1.29 (0.840, 2.14) | 1.28 (0.835, 2.00) |
| Missing | 87 (27.6%) | 97 (31.3%) | 71 (24.2%) | 255 (27.8%) |
| fT3 | ||||
| Mean (SD) | 3.01 (1.18) | 4.64 (26.2) | 2.47 (0.783) | 3.34 (14.7) |
| Median (Q1, Q3) | 2.90 (2.57, 3.31) | 2.77 (2.42, 3.09) | 2.45 (2.05, 2.81) | 2.73 (2.32, 3.10) |
| Missing | 101 (32.1%) | 118 (38.1%) | 87 (29.7%) | 306 (33.3%) |
| fT4 | ||||
| Mean (SD) | 1.20 (0.220) | 2.11 (12.1) | 1.43 (0.705) | 1.57 (6.86) |
| Median (Q1, Q3) | 1.20 (1.06, 1.33) | 1.24 (1.12, 1.43) | 1.33 (1.19, 1.54) | 1.25 (1.11, 1.43) |
| Missing | 88 (27.9%) | 98 (31.6%) | 73 (24.9%) | 259 (28.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brock, R.; Kornfehl, A.; Oppenauer, J.; Eibensteiner, F.; Neymayer, M.; Veigl, C.; Cuhaj, C.; Erbes, O.; Wirth, S.; Perkmann, T.; et al. Associations of Thyroid and Parathyroid Hormones with Arterial Stiffness in Emergency Department Patients: A Prospective Cross-Sectional Study. Medicina 2025, 61, 812. https://doi.org/10.3390/medicina61050812
Brock R, Kornfehl A, Oppenauer J, Eibensteiner F, Neymayer M, Veigl C, Cuhaj C, Erbes O, Wirth S, Perkmann T, et al. Associations of Thyroid and Parathyroid Hormones with Arterial Stiffness in Emergency Department Patients: A Prospective Cross-Sectional Study. Medicina. 2025; 61(5):812. https://doi.org/10.3390/medicina61050812
Chicago/Turabian StyleBrock, Roman, Andrea Kornfehl, Julia Oppenauer, Felix Eibensteiner, Marco Neymayer, Christoph Veigl, Carina Cuhaj, Oliver Erbes, Sophia Wirth, Thomas Perkmann, and et al. 2025. "Associations of Thyroid and Parathyroid Hormones with Arterial Stiffness in Emergency Department Patients: A Prospective Cross-Sectional Study" Medicina 61, no. 5: 812. https://doi.org/10.3390/medicina61050812
APA StyleBrock, R., Kornfehl, A., Oppenauer, J., Eibensteiner, F., Neymayer, M., Veigl, C., Cuhaj, C., Erbes, O., Wirth, S., Perkmann, T., Haslacher, H., Müller, M., Schlager, O., Wolf, P., & Schnaubelt, S. (2025). Associations of Thyroid and Parathyroid Hormones with Arterial Stiffness in Emergency Department Patients: A Prospective Cross-Sectional Study. Medicina, 61(5), 812. https://doi.org/10.3390/medicina61050812






