Type 2 Diabetes Mellitus and Osteoporosis: Site-Specific Bone Mineral Density Variations and Metabolic Correlations in Postmenopausal Saudi Women
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Population
- -
- Inclusion Criteria: This study included postmenopausal Saudi women aged ≥ 45 years with at least 12 months of amenorrhea, indicating the onset of menopause. Women diagnosed with type 2 diabetes mellitus (T2DM) were selected based on the American Diabetes Association (ADA) criteria: HbA1c ≥ 6.5% or fasting plasma glucose ≥ 126 mg/dL. Additionally, women without diabetes with normal fasting glucose levels (i.e., <100 mg/dL) were included. All participants had to provide informed consent and express willingness to partake in this study.
- -
- Exclusion Criteria: Women with type 1 diabetes mellitus, gestational diabetes, a history of malignancies, thyroid disorders, chronic kidney disease, or primary/secondary hyperparathyroidism were excluded. Also excluded were women on medications that could influence bone metabolism, such as corticosteroids, bisphosphonates, hormone replacement therapy, or thiazolidinediones, as well as those with metabolic bone diseases or autoimmune disorders affecting bone health. By rigorously selecting participants, this study aimed to minimize confounding factors and ensure that the results accurately reflect the effects of T2DM and menopause on bone health in postmenopausal women.
2.2. Informed Consent
2.3. Ethical Considerations
2.4. Data Collection and Measurements
- -
- Anthropometric and Clinical Assessments
- -
- Bone Mineral Density (BMD) Assessment
- -
- Diagnosis of Type 2 Diabetes Mellitus (T2DM)
- -
- Blood Sample Collection and Storage
- A total of 2 mL in ethylenediaminetetraacetic acid (EDTA) tubes;
- A total of 6 mL in serum separator tubes (SST);
- A total of 2 mL in sodium fluoride tubes for fasting blood glucose measurements.
- Calcitonin Hormone: Measured using a Human Calcitonin (CT) kit, with a normal range of 4.5–40 pg/mL, via the DSX Automated ELISA Processing System.
- Parathyroid Hormone (PTH): Measured using a diagnostic spectrophotometer kit, with a normal range of 15–68.3 pg/mL, on the Abbott Architect C 8000 analyzer.
- Vitamin D: Measured using a diagnostic spectrophotometer kit, with a normal range of 20–30 ng/mL.
- Calcium Levels: Measured using a diagnostic spectrophotometer kit (lot number 44322UN21, Germany), with a normal range of 2.12–2.52 mmol/L.
- Albumin (ALB): Measured using a diagnostic spectrophotometer kit, with a normal range of 34–50 g/L. ALB-corrected calcium was calculated using the formula
- Alkaline Phosphatase: Measured using a diagnostic spectrophotometer kit, with a normal range of 50–140 U/L.
- Estrogen Hormone: Measured using a diagnostic spectrophotometer kit, with a normal range of 12.5–166 pg/mL.
3. Statistical Analysis
4. Results
4.1. Comparison of Clinical and Laboratory Characteristics Between the Postmenopausal Group with Diabetes and the Control Group (Table 2)
| Parameter Mean ± SD | Diabetes Patients N = 150 (Mean ± SD) | Control N = 100 (Mean ± SD) | p-Value |
|---|---|---|---|
| Duration of menopause (year) | 8.78 ± 6.66 | 7.18 ± 6.01 | 0.054 |
| Age (year) | 58.47 ± 6.07 | 57.13 ± 6.77 | 0.103 |
| HbA1c (%) | 8.81 ± 1.64 | 5.33 ± 0.37 | *** 0.000 |
| Fasting blood glucose (mmol/L) | 9.05 ± 3.56 | 5.13 ± 0.47 | *** 0.000 |
| Vit- D (ng/mL) | 28.05 ± 22.43 | 23.56 ± 11.22 | 0.065 |
| ALP(U/L) | 85.45 ± 21.93 | 92.78 ± 20.27 | ** 0.008 |
| Calcitonin(pg/mL) | 10.8 ± 10 | 8.90 ± 5.161 | 0.063 |
| PTH (pg/mL) | 65.77 ± 26.22 | 68.65 ± 14.77 | 0.320 |
| Estrogen (pg/mL) | 12.47 ± 8.97 | 19.66 ± 17.2 | *** 0.000 |
| ALB (g/L) | 40.81 ± 5.18 | 41.2 ± 2.62 | 0.488 |
| BMI (kg/m2) | 32.97 ± 5.18 | 33.6 ± 24.57 | 0.762 |
| Total Calcium (mmol/L) | 2.2 ± 0.18 | 2.32 ± 0.12 | *** 0.000 |
| T score Lumbar Spine (LS) (g/cm2) | −1.46 ± 1.27 | −1.44 ± 1.04 | 0.918 |
| T score Femoral Neck (FN) (g/cm2) | −0.48 ± 1.07 | −0.91 ± 0.88 | *** 0.001 |
4.2. Bone Mineral Density (BMD) Differences Between the Diabetes Group and the Control Group (Table 3)
| DXA SCAN | Groups | p | ||
|---|---|---|---|---|
| Diabetes Patients | Controls | Chi-Square | ||
| DXA Scan Lumbar Spine | ||||
| NORMAL | 37.3% | 40.0% | NS | |
| OSTEOPENIA | 40.7% | 42.0% | 0.608 | |
| OSTEOPOROSIS | 22.0% | 18.0% | ||
| DXA Scan Femoral Neck | ||||
| NORMAL | 68.7% | 65.0% | NS | |
| OSTEOPENIA | 29.3% | 31.0% | 1.033 | |
| OSTEOPOROSIS | 2.0% | 4.0% | ||
4.3. Clinical and Laboratory Characteristics Based on DXA Scan of FN and LS (Table 4)
| Mean ± SD | DXA Scan Femoral Neck (Diabetes Patients) | p | DXA Scan Femoral Neck (Controls) | p | ||||
|---|---|---|---|---|---|---|---|---|
| NORMAL | OST.ENIA | OST.SIS | NORMAL | OST.ENIA | OST.SIS | |||
| Menopause Duration (Years) | 7.2 ± 5.23 | 11.95 ± 6.97 | 16.33 ± 20.65 | *** <0.001 | 7.35 ± 6.67 | 6.77 ± 4.13 | 7.5 ± 8.5 | NS |
| Age (years) | 57.03 ± 5.42 | 61.23 ± 6.03 | 67.67 ± 8.14 | *** <0.001 | 56.29 ± 6.93 | 58.48 ± 5.1 | 60.25 ± 13.43 | NS |
| HbA1c (%) | 8.93 ± 1.66 | 8.62 ± 1.6 | 7.57 ± 0.63 | NS | 5.3 ± 0.39 | 5.39 ± 0.32 | 5.25 ± 0.53 | NS |
| FBG (mmol/L) | 9.1 ± 3.58 | 9.02 ± 3.64 | 8.01 ± 1.17 | NS | 5.18 ± 0.49 | 5.01 ± 0.43 | 5.13 ± 0.52 | NS |
| Vit- D(ng/mL) | 26.9 ± 16.56 | 31.52 ± 32.62 | 16.43 ± 8.6 | NS | 24.28 ± 11.61 | 22.76 ± 10.54 | 17.93 ± 10.28 | ** <0.01 |
| ALP(U/L) | 83.77 ± 21.91 | 87.34 ± 20.83 | 115.67 ± 20.26 | * <0.05 | 94.05 ± 21.43 | 89.0 ± 17.94 | 101.5 ± 16.22 | NS |
| Calcitonin (pg/mL) | 10.91 ± 10.46 | 10.95 ± 9.18 | 4.77 ± 0.71 | NS | 8.680 ± 5.32 | 9.971 ± 5.09 |
10.325
±2.779 | NS |
| PTH (pg/mL) | 65.93 ± 26.02 | 62.84 ± 19.47 | 103.1 ± 79.1 | * <0.05 | 64.97 ± 14.39 | 70.3 ± 18.469 | 76.14 ± 12.4 | ** <0.01 |
| Estrogen (pg/mL) | 14.05 ± 10.0 | 9.05 ± 4.62 | 8.53 ± 6.11 | ** <0.01 | 20.22 ± 18.06 | 19.02 ± 16.29 | 15.5 ± 10.75 | ** <0.01 |
| ALB (g/L) | 42.52 ± 3.65 | 40.23 ± 5.58 | 35.87 ± 1.86 | * <0.05 | 41.31 ± 2.7 | 40.8 ± 2.28 | 42.59 ± 3.84 | NS |
| BMI (kg/m2) | 33.74 ± 5.08 | 31.34 ± 5.07 | 30.28 ± 4.97 | * <0.05 | 35.21 ± 30.27 | 30.96 ± 4.4 | 27.93 ± 3.29 | NS |
| Total Calcium (mmol/L) | 2.22 ± 0.18 | 2.14 ± 0.16 | 2.27 ± 0.32 | * <0.05 | 2.31 ± 0.12 | 2.36 ± 0.12 | 2.32 ± 0.03 | NS |
| T score LS (g/cm2) | −0.99 ± 1.15 | −2.42 ± 0.8 | −3.57 ± 0.49 | *** <0.001 | −1.05 ± 0.76 | −1.78 ± 1.94 | −2.22 ± 0.99 | *** <0.001 |
| DXA Scan Lumbar Spine (diabetes patients) | p | DXA Scan Lumbar Spine (controls) | p | |||||
| Parameter mean ± SD | NORMAL | OST.ENIA | OST.SIS | NORMAL | OST.ENIA | OST.SIS | ||
| Menopause duration (year) | 6.82 ± 5.69 | 8.8 ± 5.86 | 12.06 ± 8.27 |
*** <0.001 | 6.58 ± 6.53 | 7.95 ± 6.2 | 6.72 ± 4.2 | NS |
| Age (years) | 57.32 ± 5.23 | 58.67 ± 6.79 | 60.06 ± 5.76 | NS | 55.88 ± 6.95 | 57.69 ± 6.83 | 58.61 ± 6.04 | NS |
| HbA1c (%) | 9.05 ± 1.72 | 8.73 ± 1.7 | 8.56 ± 1.35 | NS | 5.28 ± 0.37 | 5.42 ± 0.39 | 5.22 ± 0.28 | NS |
| FBG (mmol/L) | 9.15 ± 3.43 | 8.63 ± 3.63 | 9.67 ± 3.63 | NS | 5.16 ± 0.51 | 5.2 ± 0.48 | 4.89 ± 0.29 | NS |
| Vit-D (ng/mL) | 24.92 ± 15.13 | 30.39 ± 26.35 | 29.02 ± 24.87 | NS | 24.55 ± 13.04 | 21.13 ± 9.06 | 17.68 ± 7.35 | * <0.05 |
| ALP(U/L) | 80.07 ± 21.41 | 86.95 ± 21.06 | 91.82 ± 22.86 | * <0.05 | 97.2 ± 18.89 | 89.12 ± 22.79 | 91.5 ± 15.52 | NS |
| Calcitonin (pg/mL) | 10.94 ± 9.79 | 9.7 ± 8.95 | 12.59 ± 12.05 | NS | 7.732 ± 4.137 | 9.819 ± 5.663 | 9.856 ± 5.362 | NS |
| PTH (pg/mL) | 65.19 ± 24.72 | 63.76 ± 21.28 | 70.45 ± 35.66 | NS | 57.92 ± 10.66 | 73.41 ± 13.69 | 81.36 ± 7.71 | *** <0.001 |
| Estrogen (pg/mL) | 15.24 ± 10.94 | 11.82 ± 7.72 | 8.83 ± 5.29 | ** <0.01 | 25.71 ± 23.83 | 19.7 ± 11 | 13.87 ± 7.35 | ** <0.01 |
| ALB (g/L) | 40.5 ± 5.53 | 41.36 ± 4.61 | 40.33 ± 5.61 | NS | 41.46 ± 3.05 | 41.2 ± 2.37 | 40.63 ± 2.17 | NS |
| BMI(kg/m2) | 34.54 ± 4.24 | 32.44 ± 5.3 | 31.29 ± 5.78 | ** <0.01 | 30.96 ± 4.67 | 37.37 ± 37.49 | 30.64 ± 3.9 | NS |
| Total Calcium (mmol/L) | 2.22 ± 0.2 | 2.19 ± 0.17 | 2.17 ± 0.16 | NS | 2.31 ± 0.09 | 2.32 ± 0.14 | 2.36 ± 0.09 | NS |
| T score FN (g/cm2 ) | 0.3 ± 0.94 | −0.65 ± 0.76 | −1.49 ± 0.71 | *** <0.001 | 6.58 ± 6.53 | 7.95 ± 6.2 | 6.72 ± 4.2 | NS |
4.4. Factors Associated with BMD in DXA Scan of FN and LS (Table 5)
| DXA Scan Lumbar Spine. a | B | Sig. | Exp(B) | 95% CI Exp(B) | |||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Diabetes patients | OSTEOPENIA | Intercept | 0.152 | 0.722 | |||
| Duration of menopause | 0.060 | 0.081 | 1.062 | 0.993 | 1.135 | ||
| Estrogen | −0.039 | 0.064 | 0.961 | 0.922 | 1.002 | ||
| OSTEOPOROSIS | Intercept | −0.335 | 0.564 | ||||
| Duration of menopause | 0.117 | 0.003 | 1.124 | 1.042 | 1.212 | ||
| Estrogen | −0.113 | 0.006 | 0.893 | 0.825 | 0.968 | ||
| Controls | OSTEOPENIA | Intercept | −4.127 | 0.286 | |||
| FBG | −0.388 | 0.555 | 0.679 | 0.187 | 2.461 | ||
| Vit-D | −0.042 | 0.144 | 0.959 | 0.907 | 1.014 | ||
| Calcitonin | 1.021 | 0.006 | 2.775 | 1.347 | 5.717 | ||
| PTH | 0.108 | 0.000 | 1.114 | 1.057 | 1.174 | ||
| Estrogen | −0.139 | 0.003 | 0.870 | 0.794 | 0.953 | ||
| OSTEOPOROSIS | Intercept | 1.334 | 0.835 | ||||
| FBG | −2.856 | 0.012 | 0.058 | 0.006 | 0.529 | ||
| Vit-D | −0.140 | 0.009 | 0.870 | 0.783 | 0.966 | ||
| Calcitonin | 1.341 | 0.002 | 3.823 | 1.620 | 9.020 | ||
| PTH | 0.185 | 0.000 | 1.204 | 1.101 | 1.316 | ||
| Estrogen | −0.073 | 0.161 | 0.929 | 0.839 | 1.029 | ||
| DXA Scan Femoral Neck. a | B | Sig. | Exp(B) | 95% CI Exp(B) | |||
| Lower Bound | Upper Bound | ||||||
| Diabetes patients | OSTEOPENIA | Duration of menopause | 0.117 | 0.000 | 1.125 | 1.058 | 1.196 |
| Age | 0.126 | 0.000 | 1.134 | 1.062 | 1.212 | ||
| Estrogen | −0.098 | 0.003 | 0.907 | 0.850 | 0.967 | ||
| ALB | 0.109 | 0.016 | 1.115 | 1.021 | 1.218 | ||
| BMI | −0.100 | 0.011 | 0.905 | 0.837 | 0.978 | ||
| Total calcium | −2.860 | 0.016 | 0.057 | 0.006 | 0.583 | ||
| OSTEOPOROSIS | Duration of menopause | 0.174 | 0.009 | 1.191 | 1.044 | 1.358 | |
| Age | 0.296 | 0.007 | 1.345 | 1.086 | 1.665 | ||
| ALP | 0.065 | 0.034 | 1.067 | 1.005 | 1.134 | ||
| PTH | 0.029 | 0.039 | 1.029 | 1.001 | 1.057 | ||
| Controls | OSTEOPENIA | PTH | 0.061 | 0.001 | 1.063 | 1.025 | 1.103 |
| OSTEOPOROSIS | Calcitonin | 0.923 | 0.021 | 2.517 | 1.151 | 5.505 | |
4.5. Correlation Between BMD (LS and FN T-Score) and HbA1c in Postmenopausal Women with and Without Diabetes (Figure 1 and Figure 2)
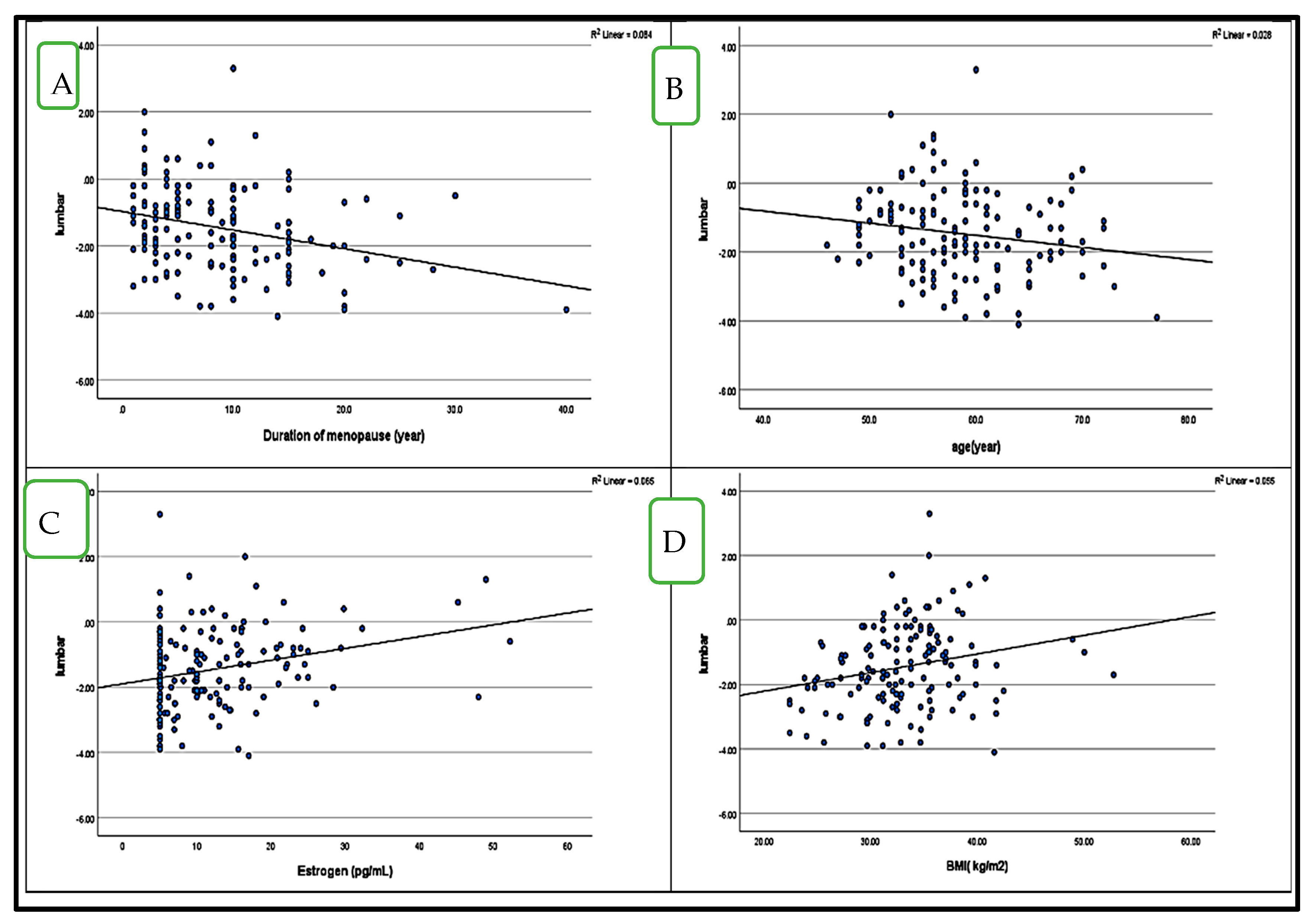
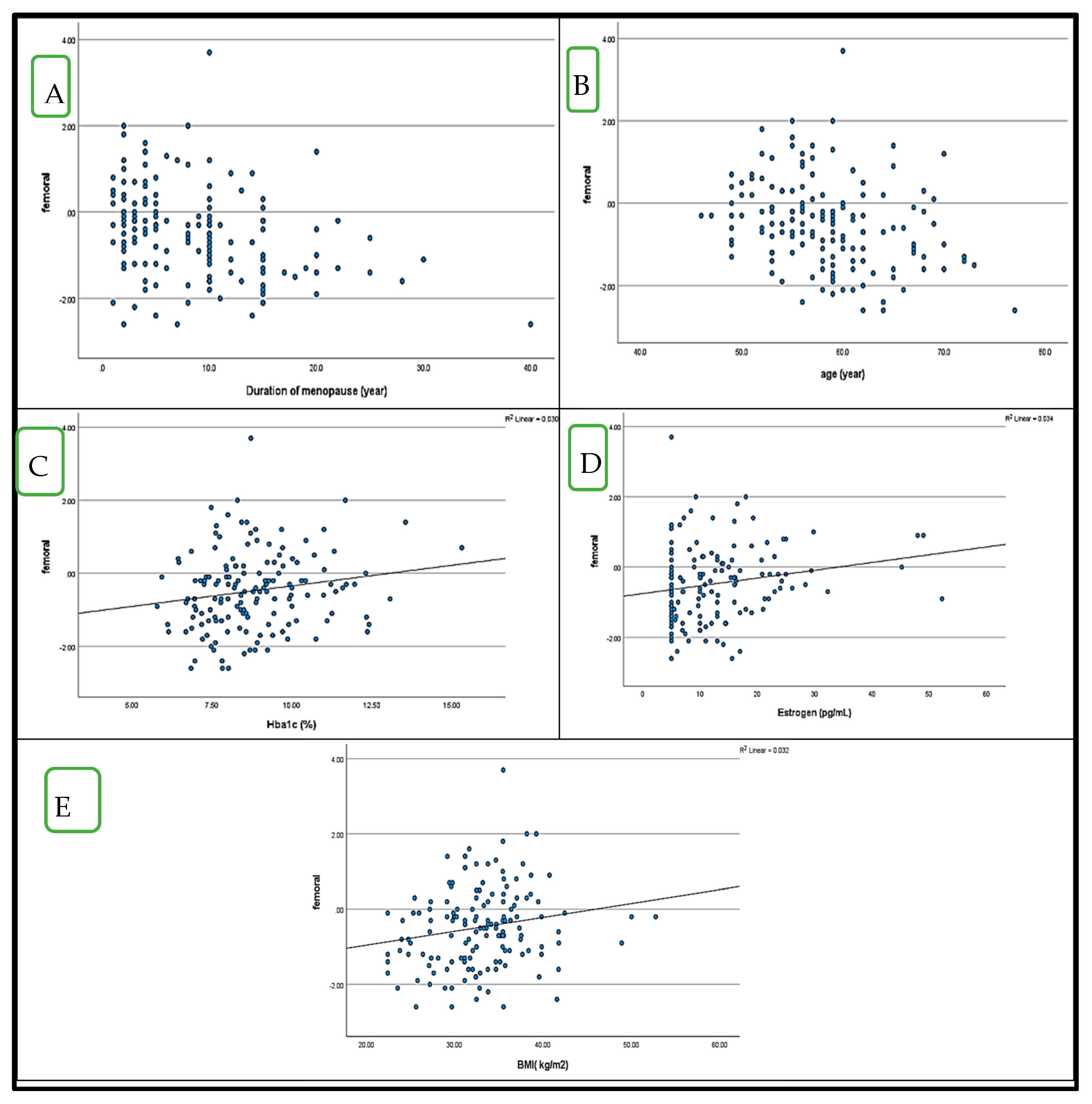
5. Discussion
Limitations and Recommendation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A.; Harvey, N.C.; McCloskey, E.; Bruyère, O.; Veronese, N.; Lorentzon, M.; Cooper, C.; Rizzoli, R.; Adib, G.; Al-Daghri, N.; et al. Advances in the diagnosis and management of osteoporosis. Lancet Diabetes Endocrinol. 2022, 10, 567–583. [Google Scholar]
- Ilany, J.; Liu, J.; Welsch, C.; Reznik-Wolf, H.; Levy-Lahad, E.; Auchus, R.J. Osteoporosis management in postmenopausal women: Recent advances. J. Clin. Endocrinol. Metab. 2021, 106, 1234–1250. [Google Scholar] [CrossRef]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; Jan de Beur, S.M.; Khosla, S.; Lane, N.E.; et al. The evolving role of bone mineral density in assessing fracture risk. Nat. Rev. Endocrinol. 2022, 18, 327–341. [Google Scholar]
- Schwartz, A.V.; Schafer, A.L.; Vittinghoff, E. Diabetes, bone, and fracture risk: Current perspectives. J. Bone Miner. Res. 2023, 38, 45–61. [Google Scholar]
- Napoli, N.; Chandran, M.; Pierroz, D.D. Type 2 diabetes and bone fractures: An unresolved puzzle. Endocr. Rev. 2021, 42, 269–289. [Google Scholar]
- Mahmoud, M.A.; Alshammari, S.A.; Alswat, K.A. Epidemiology and burden of osteoporosis in Saudi Arabia. Osteoporos. Int. 2023, 34, 789–801. [Google Scholar]
- Auler, A.; Maraschin, C.K.; Medeiros, B.M.; Passaglia, L.; Polanczyk, C.; Gossenheimer, A.N.; Cureau, F.V.; Schaan, B.D. The prevalence and risk factors of type 2 diabetes in the Middle East: A Saudi perspective. Diabetes Res. Clin. Pract. 2022, 186, 109247. [Google Scholar] [CrossRef]
- Farr, J.N.; Khosla, S.; Almeida, M. Advanced glycation end products and their impact on bone fragility. Bone Rep. 2023, 17, 101641. [Google Scholar]
- Janghorbani, M.; Feskanich, D.; Willett, W.C. Impact of hyperglycemia on bone microarchitecture and fracture risk. Curr. Osteoporos. Rep. 2022, 20, 112–126. [Google Scholar]
- Sánchez-Rodríguez, D.; García-Martínez, O.; Reyes-Torres, M.D. Estrogen and bone metabolism: A mechanistic review. Trends Endocrinol. Metab. 2023, 34, 212–229. [Google Scholar]
- Al-Daghri, N.M.; Mohammed, A.K.; Bukhari, I.; Rikli, M.; Abdi, S.; Ansari, M.G.A.; Sabico, S.; Hussain, S.D.; Alenad, A.; Al-Saleh, Y.; et al. Genetic and environmental influences on osteoporosis risk in Saudi women. Front. Endocrinol. 2022, 13, 889456. [Google Scholar]
- Karimifar, M.; Pasha, M.A.P.; Salari, A.; Zamani, A.; Salesi, M.; Motaghi, P. Evaluation of bone loss in diabetic postmenopausal women. J. Res. Med. Sci. 2012, 17, 1033–1038. [Google Scholar] [PubMed] [PubMed Central]
- Al-Homood, I.A.; Sheshah, I.; Mohammed, A.G.; Gasim, G.I. The prevalence and risk factors of osteoporosis among a Saudi female diabetic population. Open Access Maced. J. Med. Sci. 2017, 5, 177–181. [Google Scholar] [CrossRef]
- Al-Maatouq, M.A.; I El-Desouki, M.; A Othman, S.; Mattar, E.H.; A Babay, Z.; Addar, M. Prevalence of osteoporosis among postmenopausal females with diabetes mellitus. Saudi Med. J. 2004, 25, 1423–1427. [Google Scholar] [PubMed]
- Zeid, A.F.; Ahmed, A.S.; Shohdy, M.T.; Asssy, M.M. Evaluation of bone mineral density among type 2 diabetes mellitus patients in Zagazig University Hospitals. Egypt J. Hosp. Med. 2020, 80, 599–607. [Google Scholar] [CrossRef]
- Roomi, A.B.; Salih, A.-H.M.; Noori, S.D.; Nori, W.; Tariq, S. Evaluation of bone mineral density, serum osteocalcin, and osteopontin levels in postmenopausal women with type 2 diabetes mellitus, with/without osteoporosis. J. Osteoporos. 2022, 2022, 1437061. [Google Scholar] [CrossRef]
- Liu, D.; Bai, J.-J.; Yao, J.-J.; Wang, Y.-B.; Chen, T.; Xing, Q.; Bai, R. Association of insulin glargine treatment with bone mineral density in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2021, 14, 1909–1916. [Google Scholar] [CrossRef]
- Roomi, A.B.; AL-Salih, R.M.; Ali, S.A. Impact of metformin and insulin therapy on parathyroid hormone and 25(OH) vitamin D in diabetic postmenopausal Iraqi women. J. Phys. Conf. Ser. 2019, 1279, 012008. [Google Scholar] [CrossRef]
- Roomi, A.B.; Nori, W.; Al-Badry, S.H. The value of serum adiponectin in osteoporotic women: Does weight have an effect? J. Obes. 2021, 2021, 5325813. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Shao, X. Association analysis of insulin resistance and osteoporosis risk in Chinese patients with T2DM. Ther. Clin. Risk Manag. 2021, 17, 909–916. [Google Scholar] [CrossRef]
- Paul, J.; Devarapalli, V.; Johnson, J.T.; Cherian, K.E.; Jebasingh, F.K.; Asha, H.S.; Kapoor, N.; Thomas, N.; Paul, T.V. Do proximal hip geometry, trabecular microarchitecture, and prevalent vertebral fractures differ in postmenopausal women with type 2 diabetes mellitus? Osteoporos. Int. 2021, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Leidig-Bruckner, G.; Grobholz, S.; Bruckner, T.; Scheidt-Nave, C.; Nawroth, P.; Schneider, J.G. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr. Disord. 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Majima, T.; Komatsu, Y.; Yamada, T.; Koike, Y.; Shigemoto, M.; Takagi, C.; Hatanaka, I.; Nakao, K. Decreased bone mineral density at the distal radius, but not at the lumbar spine or femoral neck, in Japanese type 2 diabetic patients. Osteoporos. Int. 2005, 16, 907–913. [Google Scholar] [CrossRef]
- Malheiros-Souza, D.; Gaia, L.F.P.; Sousa, F.F.d.A.; Favaro, P.I.F.; Rodrigues, V.; Rodrigues, D.B.R. Evaluation of hormonal influence in patients with fractures attributed to osteoporosis. Rev. Bras. Ortop. 2022, 56, 804–808. [Google Scholar] [CrossRef]
- El-Maksoud, N.M.A.; Abulsoud, A.; Abulsoud, M.; Elshaer, S. Association between vitamin D receptor gene polymorphism and osteoporotic fractures. Azhar Int. J. Pharm. Med. Sci. 2022, 2, 12–19. [Google Scholar] [CrossRef]
- Ran, C.; Xiaojuan, X.; Wenxue, G.; Zhaoliang, F.; Hui, S.; Shen, Q. Sexual dimorphism in the relation between sex hormones and osteoporosis in patients with type 2 diabetes mellitus. J. Bone Miner. Metab. 2022, 40, 460–467. [Google Scholar] [CrossRef]
- Kotwal, S.D.; Bhat, A.N.; Yograj, S.; Kotwal, S. Evaluation of vitamin D status in premenopausal and postmenopausal type 2 diabetic women and its relation to glycemic control. Int. J. Res. Med. Sci. 2020, 8, 1292. [Google Scholar] [CrossRef]
- López-Baena, M.T.; Pérez-Roncero, G.R.; Pérez-López, F.R.; Mezones-Holguín, E.; Chedraui, P. Vitamin D, menopause, and aging: Quo vadis? Climacteric 2020, 23, 123–129. [Google Scholar] [CrossRef]
- Sharifi, F.; Ahmadimoghadam, N.; Mousavinasab, N. The relationship between type 2 diabetes mellitus and bone density in postmenopausal women. Int. J. Endocrinol. Metab. 2006, 3, 117–122. [Google Scholar]
- Hyassat, D.; Alyan, T.; Jaddou, H.; Ajlouni, K.M. Prevalence and risk factors of osteoporosis among Jordanian postmenopausal women attending the National Center for Diabetes, Endocrinology and Genetics in Jordan. Biores Open Access 2017, 6, 85–93. [Google Scholar] [CrossRef]
| Diabetes Patients N = 150 | Control N = 100 | p- Value | ||
|---|---|---|---|---|
| 1 | Marital status: | |||
| Widower | 46 (30.67%) | 21 (21%) | ||
| Married | 100 (66.67%) | 76 (76%) | NS | |
| Divorced | 4 (2.67%) | 3 (3%) | ||
| 2 | Educational level: | |||
| No education | 88 (58.67%) | 39 (39%) | ||
| Primary | 41 (27.33%) | 27 (27%) | ||
| Middle education | 12 (8%) | 13 (13%) | NS | |
| Secondary | 4 (2.67%) | 10 (10%) | ||
| University and above | 5 (3.33%) | 11 (11%) | ||
| 4 | Do you have any medical history? | |||
| No | 132 (88%) | 93 (93%) | NS | |
| Yes | 18 (12%) | 7 (7%) | ||
| 5 | Do you have any smoking history? | |||
| No | 150 (100%) | 99 (99%) | NS | |
| Yes | 0 (0%) | 1 (1%) | ||
| 6 | Do you practice a physical activity? | |||
| No | 43 (28.67%) | 24 (24%) | NS | |
| Walking sport | 107 (71.33%) | 76 (76%) | ||
| 7 | Do you consume calcium daily? | |||
| No | 28 (18.67%) | 14 (14%) | NS | |
| Yes | 122 (81.33%) | 86 (86%) | ||
| 8 | Are you exposed to sunlight? | |||
| No | 52 (34.67%) | 36 (36%) | NS | |
| Yes | 98 (65.33%) | 64 (64%) | ||
| 9 | Do you drink caffeine? | |||
| No | 4 (2.67%) | 7 (7%) | NS | |
| Yes | 146 (97.33%) | 93 (93%) | ||
| 10 | Does family history include osteoporosis or fragility fracture? | |||
| No | 137 (91.33%) | 80 (80%) | NS | |
| Yes | 13 (8.67%) | 20 (20%) | ||
| 11 | Do you have osteoporosis? | |||
| No | 126 (84%) | 94 (94%) | NS | |
| Yes | 24 (16%) | 6 (6%) | ||
| 12 | Breastfeeding | |||
| No | 6 (4%) | 5 (5%) | NS | |
| Yes | 144 (96%) | 95 (95%) | ||
| 13 | Diabetes status | |||
| Gestational diabetes | 37 (24.67%) | 0 (0%) | ||
| Diabetes | 113 (75.33%) | 0 (0%) | NS | |
| Nothing | 0 (0%) | 100 (100%) | ||
| 14 | Duration of Diabetes status (years) | 15.06 ± 7.84 | 0 | |
| 15 | Hypertension status | |||
| Hypertension | 70 (46.67%) | 18 (18%) | ||
| Hypotension | 0 (0%) | 1 (1%) | NS | |
| Nothing | 80 (53.33%) | 81 (81%) | ||
| 16 | Use of statin or vitamin D3 supplementation: | |||
| Every week | 32 (21.33%) | 15 (15%) | ||
| Per month | 3 (2%) | 2 (2%) | NS | |
| Every day | 10 (6.67%) | 3 (3%) | ||
| Nothing | 105 (70%) | 80 (80%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhowiti, N.M.; Mackawy, A.M.H.; Al Wanian, W.M.; Alshebremi, M.; Allemailem, K.S.; Alharbi, H.O.A. Type 2 Diabetes Mellitus and Osteoporosis: Site-Specific Bone Mineral Density Variations and Metabolic Correlations in Postmenopausal Saudi Women. Medicina 2025, 61, 789. https://doi.org/10.3390/medicina61050789
Alhowiti NM, Mackawy AMH, Al Wanian WM, Alshebremi M, Allemailem KS, Alharbi HOA. Type 2 Diabetes Mellitus and Osteoporosis: Site-Specific Bone Mineral Density Variations and Metabolic Correlations in Postmenopausal Saudi Women. Medicina. 2025; 61(5):789. https://doi.org/10.3390/medicina61050789
Chicago/Turabian StyleAlhowiti, Nogood Mashahi, Amal M. H. Mackawy, Wanian Mohammed Al Wanian, Mohammad Alshebremi, Khaled S. Allemailem, and Hajed Obaid Abdullah Alharbi. 2025. "Type 2 Diabetes Mellitus and Osteoporosis: Site-Specific Bone Mineral Density Variations and Metabolic Correlations in Postmenopausal Saudi Women" Medicina 61, no. 5: 789. https://doi.org/10.3390/medicina61050789
APA StyleAlhowiti, N. M., Mackawy, A. M. H., Al Wanian, W. M., Alshebremi, M., Allemailem, K. S., & Alharbi, H. O. A. (2025). Type 2 Diabetes Mellitus and Osteoporosis: Site-Specific Bone Mineral Density Variations and Metabolic Correlations in Postmenopausal Saudi Women. Medicina, 61(5), 789. https://doi.org/10.3390/medicina61050789







