Sternal Resections: An Attempt to Find the Ideal Reconstruction Method
Abstract
1. Introduction
2. Materials and Methods
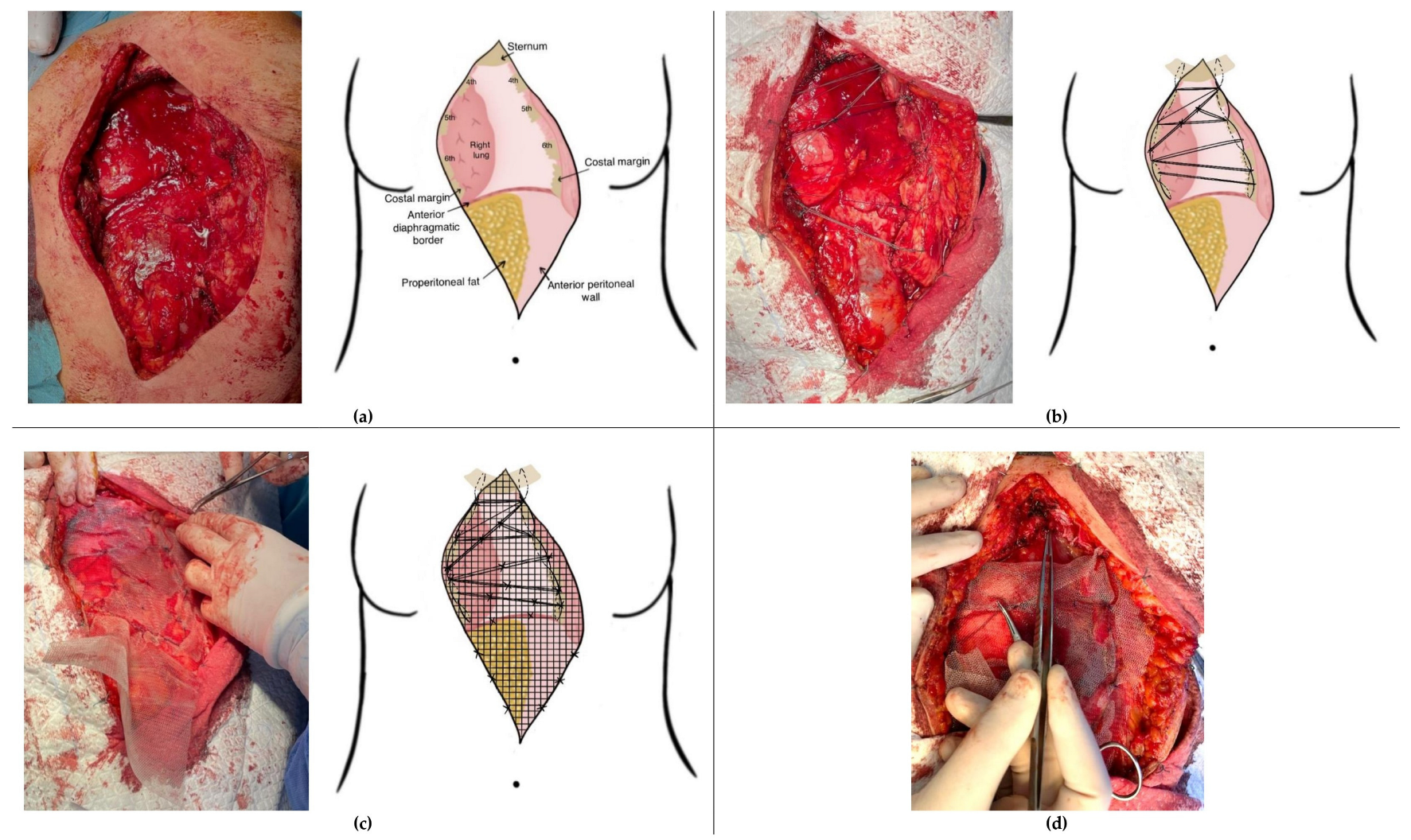
2.1. “Spider Web” Reconstruction Technique Following Sternectomy
2.2. Statistical Analysis
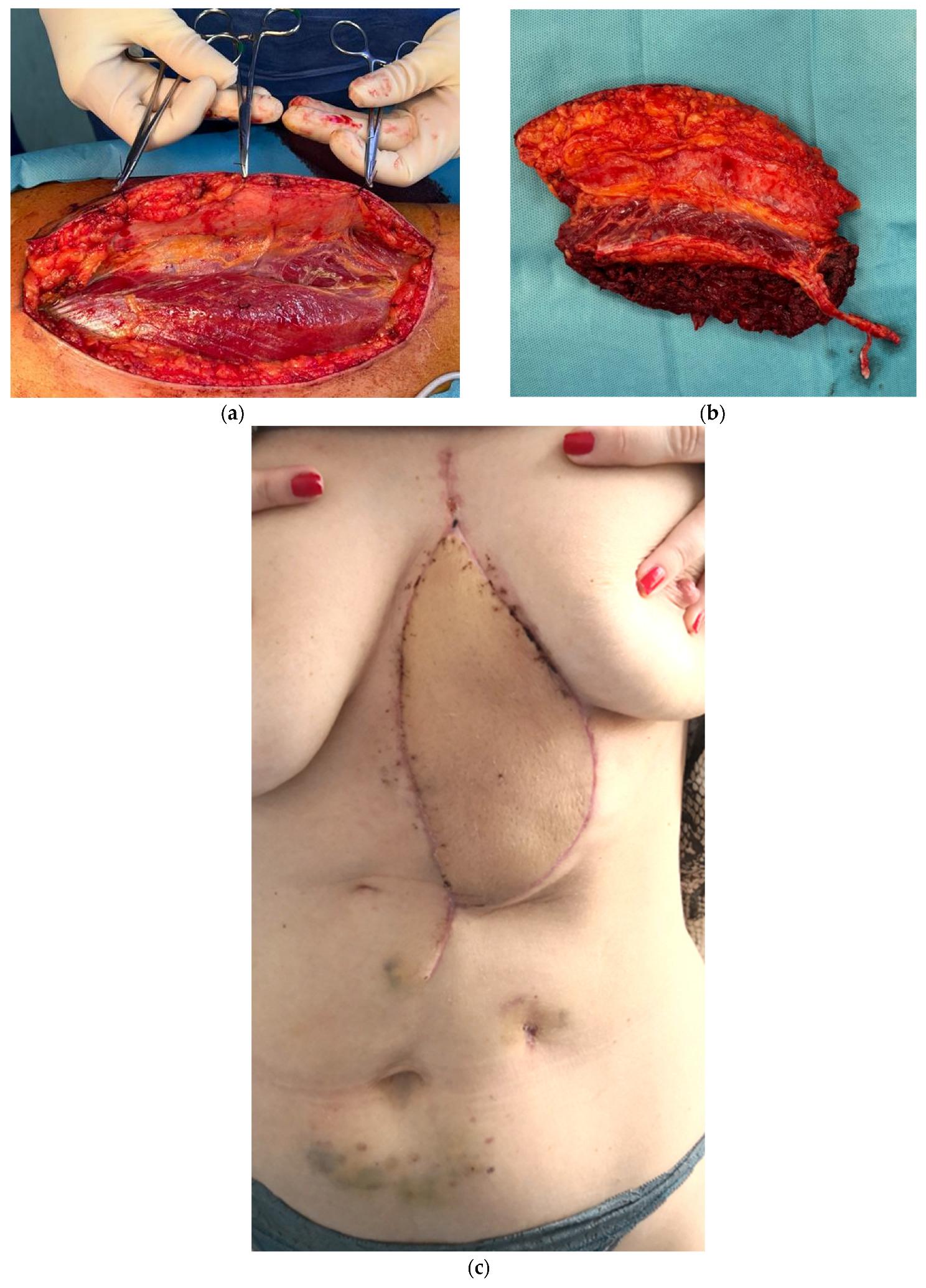
3. Results
3.1. Postoperative Course and Complications
3.2. Case-Specific Surgical Considerations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spicer, J.D.; Shewale, J.B.; Antonoff, M.B.; Correa, A.M.; Hofstetter, W.B.; Rice, D.C.; Vaporciyan, A.A.; Mehran, R.J.; Walsh, G.L.; Roth, J.A.; et al. The Influence of Reconstructive Technique on Perioperative Pulmonary and Infectious Outcomes Following Chest Wall Resection. Ann. Thorac. Surg. 2016, 102, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Joel, R.K.; Benjamin, S.R.; Rao, V.M.; Kodiatte, T.A.; Gnanamuthu, B.R.; Mohammad, A.; Sameer, M.; David, N. Surgical management of sternal tumours-a decade of experience from a tertiary care centre in India. Indian J. Thorac. Cardiovasc. Surg. 2024, 40, 184–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banuelos, J.; Abu-Ghname, A.; Bite, U.; Moran, S.L.; Bakri, K.; Blackmon, S.H.; Shen, R.; Allen, M.S.; Pairolero, P.C.; Arnold, P.G.; et al. Reconstruction of Oncologic Sternectomy Defects: Lessons Learned from 60 Cases at a Single Institution. Plast. Reconstr. Surg. Glob. Open. 2019, 7, e2351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bongiolatti, S.; Voltolini, L.; Borgianni, S.; Borrelli, R.; Innocenti, M.; Menichini, G.; Politi, L.; Tancredi, G.; Viggiano, D.; Gonfiotti, A. Short and long-term results of sternectomy for sternal tumours. J. Thorac. Dis. 2017, 9, 4336–4346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, U.; Yang, H.; Sima, C.; Buitrago, D.H.; Ripley, R.T.; Suzuki, K.; Bains, M.S.; Rizk, N.P.; Rusch, V.W.; Huang, J.; et al. Resection of Primary and Secondary Tumors of the Sternum: An Analysis of Prognostic Variables. Ann. Thorac. Surg. 2015, 100, 215–221; discussion 221–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aranda, J.L.; Gomez, M.T.; Fuentes, M.; Rivas, C.; Forcada, C.; Jimenez, M.F. Sternal resection and reconstruction: A review. J. Thorac. Dis. 2024, 16, 708–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dell’Amore, A.; Kalab, M.; Miller, A.S., 3rd; Dolci, G.; Liparulo, V.; Beigee, F.S.; Rosso, L.; Ferrigno, P.; Pangoni, A.; Schiavon, M.; et al. Indications and Results of Sternal Allograft Transplantation: Learning from a Worldwide Experience. Ann. Thorac. Surg. 2021, 112, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Aramini, B.; Masciale, V.; Radaelli, L.F.Z.; Sgarzani, R.; Dominici, M.; Stella, F. The sternum reconstruction: Present and future perspectives. Front. Oncol. 2022, 12, 975603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Novoa, N.M.; Alcaide, J.L.A.; Hernández, M.T.G.; Fuentes, M.G.; Goñi, E.; Lopez, M.F.J. Chest wall—Reconstruction: Yesterday, today and the future. Shanghai Chest 2019, 3, 15. [Google Scholar] [CrossRef]
- Wang, L.; Yan, X.; Zhao, J.; Chen, C.; Chen, C.; Chen, J.; Chen, K.N.; Cao, T.; Chen, M.W.; Duan, H.; et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl. Lung Cancer Res. 2021, 10, 4057–4083. [Google Scholar] [CrossRef]
- Sanna, S.; Brandolini, J.; Pardolesi, A.; Argnani, D.; Mengozzi, M.; Dell’Amore, A.; Solli, P. Materials and techniques in chest wall reconstruction: A review. J. Vis. Surg. 2017, 3, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marulli, G.; Dell’amore, A.; Calabrese, F.; Schiavon, M.; Daddi, N.; Dolci, G.; Stella, F.; Rea, F. Safety and Effectiveness of Cadaveric Allograft Sternochondral Replacement After Sternectomy: A New Tool for the Reconstruction of Anterior Chest Wall. Ann. Thorac. Surg. 2017, 103, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Seder, C.W.; Rocco, G. Chest wall reconstruction after extended resection. J. Thorac. Dis. 2016, 8 (Suppl. S11), S863–S871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agostini, T.; Lazzeri, D.; Spinelli, G. Anterolateral thigh flap: Systematic literature review of specific donor-site complications and their management. J. Craniomaxillofac. Surg. 2013, 41, 15–21. [Google Scholar] [CrossRef]
- Jo, G.Y.; Ki, S.H. Analysis of the Chest Wall Reconstruction Methods after Malignant Tumor Resection. Arch. Plast. Surg. 2023, 50, 10–16. [Google Scholar] [CrossRef]
- Haraguchi, S.; Hioki, M.; Hisayoshi, T.; Yamashita, K.; Yamashita, Y.; Kawamura, J.; Hirata, T.; Yamagishi, S.; Koizumi, K.; Shimizu, K. Resection of sternal tumors and reconstruction of the thorax: A review of 15 patients. Surg. Today 2006, 36, 225–229. [Google Scholar] [CrossRef]
- Gao, E.; Li, Y.; Zhao, T.; Guo, X.; He, W.; Wu, W.; Zhao, Y.; Yang, Y. Reconstruction of anterior chest wall: A clinical analysis. J. Cardiothorac. Surg. 2018, 13, 124. [Google Scholar] [CrossRef]
- Novoa, N.; Benito, P.; Jiménez, M.F.; de Juan, A.; Luis Aranda, J.; Varela, G. Reconstruction of chest wall defects after resection of large neoplasms: Ten-year experience. Interact. Cardiovasc. Thorac. Surg. 2005, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Wei, F.C. Anterolateral thigh flap. Head Neck. 2010, 32, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C. A simplified classification and economical application of anterolateral thigh flap. J. Plast. Reconstr. Aesthet. Surg. 2024, 88, 153–160. [Google Scholar] [CrossRef] [PubMed]
- De Beule, T.; Van Deun, W.; Vranckx, J.; de Dobbelaere, B.; Maleux, G.; Heye, S. Anatomical variations and pre-operative imaging technique concerning the anterolateral thigh flap: Guiding the surgeon. Br. J. Radiol. 2016, 89, 20150920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colella, S.; Brandimarte, A.; Marra, R.; Marinari, S.; D’Incecco, A.; Di Genesio Pagliuca, M.; De Vico, A.; Crisci, R.; Divisi, D. Chest wall reconstruction in benign and malignant tumors with non-rigid materials: An overview. Front. Surg. 2022, 9, 976463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabre, D.; El Batti, S.; Singhal, S.; Mercier, O.; Mussot, S.; Fadel, E.; Kolb, F.; Dartevelle, P.G. A paradigm shift for sternal reconstruction using a novel titanium rib bridge system following oncological resections. Eur. J. Cardiothorac. Surg. 2012, 42, 965–970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Macedo, J.P.C.; Nabuco-de-Araujo, P.H.X.; Bibas, B.J.; de Campos, J.R.M.; Pêgo-Fernandes, P.M.; Terra, R.M. Predictors of postoperative complications after sternectomy on oncologic patients. Clinics 2024, 79, 100468. [Google Scholar] [CrossRef]
- Marulli, G.; Hamad, A.M.; Cogliati, E.; Breda, C.; Zuin, A.; Rea, F. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J. Thorac. Cardiovasc. Surg. 2010, 139, e69–e70. [Google Scholar] [CrossRef]
- Marulli, G.; De Iaco, G.; Ferrigno, P.; De Palma, A.; Quercia, R.; Brascia, D.; Schiavon, M.; Mammana, M.; Rea, F. Sternochondral replacement: Use of cadaveric allograft for the reconstruction of anterior chest wall. J. Thorac. Dis. 2020, 12, 3–9. [Google Scholar] [CrossRef]
- Lampridis, S.; Minervini, F.; Scarci, M. Management of complications after chest wall resection and reconstruction: A narrative review. J. Thorac. Dis. 2024, 16, 737–749. [Google Scholar] [CrossRef]
- Weise, H.; Naros, A.; Blumenstock, G.; Krimmel, M.; Hoefert, S.; Kluba, S.; Hofer, S.; Reinert, S. Donor site morbidity of the anterolateral thigh flap. J. Craniomaxillofac. Surg. 2017, 45, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Townley, W.A.; Royston, E.C.; Karmiris, N.; Crick, A.; Dunn, R.L. Critical assessment of the anterolateral thigh flap donor site. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
| Type of Method | Type of Material | Advantages | Disadvantages |
|---|---|---|---|
| Non-rigid | Braided synthetic meshes | Largely available, reduced costs, easy to handle and store, easy to adapt to defect size and configuration | |
| |||
| Thin, good tissue integration can be used in combination with rigid methods (methyl methacrylate, titanium bars) | Low resistance to infection, poor protection of mediastinal organs, permeable for fluids | |
| Higher rigidity and stability while preserving elasticity and chest wall dynamics, well tolerated, easy to cut and shape, good biocompatability | High costs, radiopacity, attenuation of radiotherapy | |
| Inert, biocompatible, can be used in infected environment | Low stability, poor protection of mediastinal organs | |
| Compact synthetic meshes | Thicker (increased stability and mediastinal organ protection), impermeable for fluids | Limited tissue ingrowth | |
| Biological meshes: biological collagen matrixes derived from porcine dermis or bovine pericardium | Stable reconstruction, durability (non-absorbable), good tissue integration, allows remodeling (useful in the pediatric population), decreased infection risk | High costs, for more extensve defects coverage with muscle flap still necessary | |
| Rigid | Methylmethacrylate: in general, used in combination with polypropylene mesh (“sandwich” technique) | Stable reconstruction even for large defects, prevents paradoxical movements and chest wall deformities | Excessive chest wall rigidity, increased pain, increased risk of infection |
| Titanium bars | Stable reconstruction, biocompatible, high osseointegration, low optical density, high strength/weight ration, relative resistance to infection, easy to adapt to defect size and shape, good cosmetic results, compatible with MRI | Risk of bar fracture or displacement, higher costs | |
| Silicone molds injected with methylmethacrylate, systems based on rubber or Carbone fibers | Good stability, tailored reconstruction | Very limited published experience | |
| Autologous bone graft: iliac graft, fibula graft, rib graft | Stable reconstruction, good tissue integration | Limited size (unsuitable for large defects), increased operative time, possible donor site complications, have to be fixed with bars resulting in a “rigid plate-effect” | |
| Allografts (human cadaveric) and homografts (porcine): | Stable reconstruction, good tissue integration, easily adjustable to defect size and shape, no donor site complications, can be stored for long periods, reasonable costs | ||
| Need for tissue bank, have to be fixed with bars | ||
| Limited published experience, need for tissue bank, legislatory constraints | ||
| Recent developments and perspective methods | Three-dimensional printing (titanium, polyether-ether-ketone) | Theoretical advantages: more precise adaptation to defect, better fixation systems, shorter operative time, less pain, improved aesthetics | Very limited published clinical experience |
| Tissue regeneration using mesenchymal stem cells (MSCs) |
| Type of Soft Tissue Substitute | Type of Flap | Variants | Advantages | Disadvantages |
|---|---|---|---|---|
| Pectoralis major flap | Local advancement muscle or myocutaneous flap | Pedicled on thoracoacromial trunk | Optimal coverage of upper 1/2 of sternum defects Good blood supply (<3% failure) | Insufficient for lower 1/3 of sternum defects |
| Turn-over flap (IMAP–internal mammary artery perforator flap) | Perforators from internal mammary artery | Alternative for midline defects if thoracoacromial vessels are compromised | ||
| Rectus abdominus flaps | Pedicled myocutaneous flaps based on superior epigastric vessels | Vertical rectus abdominus myocutaneous flap (VRAM) | Can cover large defects after sternal resections | High incidence (13%) of abdominal hernias |
| Transverse rectus abdominus myocutaneous flap (TRAM) | Can cover large defects of the anterolateral chest wall Poorer blood supply of the skin island as VRAM | |||
| Latissimus dorsi flap | Pedicled muscle or myocutaneous flap | Pedicled based on the thoracodorsal vessels | Can cover large soft tissue defects Can reach every region of the anterolateral and posterior ipsilateral chest wall Can be used pedicled or as free flap | High incidence (79%) of seroma Large scar Temporary reduction in arm strength Need for patient repositioning on the operating table |
| Omental flap | Pedicled omentum flap | Pedicled on the right or left gastroepiploic vessels | Very useful in infected areas or reconstructions for radionecrosis Can cover large sternal defects | Requires laparatomy or laparoscopy Offers insufficient stability (combination with meshes necessary) Additional free skin graft necessary |
| Chimeric flap of latissimus dorsi and serratus anterior muscles | Chimeric flaps based on subscapular trunk | Good coverage of large defects | Relative high incidence of seroma due to large undermining | |
| Anterolateral thigh (ALT) flap | Free flap based on perforators from the descending branch of the lateral circumflex femoral vessels | Can provide muscle, fascia, skin, or a combination of any of these | Relatively constant vascular supply Widely used microsurgical flap Good coverage of large defects Minimal donor site complications Two-team approach to minimize operative time possible | Require microsurgical skills Possible donor site complications (bleeding, hematoma, seroma, infection) |
| Type A | manubrial resection (preservation of corpus sterni) | 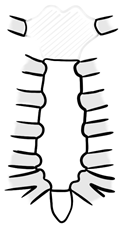 |
| Type B | resection of corpus sterni (preservation of manubrium) | 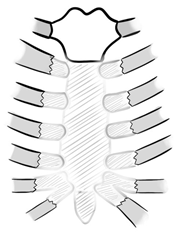 |
| Type C | total sternal resection | 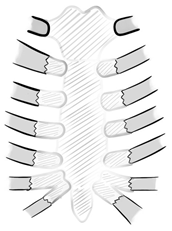 |
| ALT Type | Number of Perforators | Source Vessel |
|---|---|---|
| I | Single | Transverse branch |
| II | Single | Descending branch |
| III | Multiple | Transverse branch |
| IV | Multiple | Descending branch |
| V | Multiple | Both branches |
| No. | Sex | Age (Years) | Diagnosis | Medical History/Comorbidities | Tumor Size AP/CC/LL (mm) | Tumor Location and Extent | Type of Resection | Type of Reconstruction | Type of Soft Tissue Reconstruction | Surgery Duration (min) | Blood Transfusion | Postoperative LOS (Days) | Resection Margin Status | 30-Day Morbidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | F | 57 | Breast cancer metastasis |
Breast cancer (invasive lobular carcinoma of the left breast, 2016) Grade 3 mitral regurgitation Grade 1 tricuspid regurgitation Osteopenia | 34/70/52 | Sternal body |
Sternal body (type B sternal resection) | Spider-Web + polypropylene mesh | ALT flap (right side) | 370 | 1 packed red blood cells | 9 | R0 | No complications |
| 2. | F | 40 | Recurrence of a desmoid tumor | Desmoid tumor in the left anterior chest wall, excised 2021 | 49/112/76 | Sternal body with extension in the left anterior chest wall and left rectus abdomini muscle | Sternal body (type B sternal resection) + left anterior chest wall from 4th rib downwards and lateral to midclavicular line + superior portion of left rectus abdomini muscle up to left semilunar line | Spider-Web + polypropylene mesh | ALT flap (left side) | 400 | 1 packed red blood cells | 11 | R0 | Thrombosis of venous anastomosis, reintervention with desobstruction and microsurgical vascular anastomosis |
| 3. | F | 20 | Hodgkin lymphoma | No | 37/77/46 | Large lesion originating in the sternal body and extending into manubrium sterni | Total sternectomy (type C sternal resection) | Titanium bars and polypropylene mesh | ALT flap (right side) | 435 | No | 11 | R0 | No complications |
| 4. | F | 47 | Breast cancer metastasis |
Breast cancer (left invasive carcinoma) Sinus tachycardia Mild mitral insufficiency Silent ischemic heart disease Smoker | 45/137/62 | Extension into the chondrocostal cartilages of the right anterior 3rd rib, the 4th rib on both the left and right sides, the right 5th rib, and the manubrium | Total sternectomy (type C sternal resection) | Spider-Web + polypropylene mesh | ALT flap (right side) | 330 | No | 10 | R0 | Pneumonia |
| 5. | F | 64 | Sternal metastasis from poorly differentiated thyroid carcinoma |
Poorly differentiated thyroid carcinoma pT4aNxL1V1R1 treated with total thyroidectomy and resection of the sternal manubrium, right sternoclavicular joint and first right chondrosternal joint, followed by adjuvant radiotherapy (2022) Iatrogenic hypothyroidism Class 1 obesity | 4/4/2 cm | Extension into adjacent soft tissues, invades the presternalsubcutaneous tissue, | Total sternectomy (type C sternal resection) | Spider-Web + polypropylene mesh | ALT flap (right side) | 390 | 1 packed red blood cells | 14 | R | Hematoma at the donor site (right thigh), surgically evacuated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palade, E.; Titu, I.-M.; Fodor, L.; Ciorba, I.M.; Jentimir, I.; Teterea, F.; Mlesnite, M.; Tichil, I. Sternal Resections: An Attempt to Find the Ideal Reconstruction Method. Medicina 2025, 61, 763. https://doi.org/10.3390/medicina61040763
Palade E, Titu I-M, Fodor L, Ciorba IM, Jentimir I, Teterea F, Mlesnite M, Tichil I. Sternal Resections: An Attempt to Find the Ideal Reconstruction Method. Medicina. 2025; 61(4):763. https://doi.org/10.3390/medicina61040763
Chicago/Turabian StylePalade, Emanuel, Ioana-Medeea Titu, Lucian Fodor, Ion Mircea Ciorba, Ion Jentimir, Florin Teterea, Monica Mlesnite, and Ioana Tichil. 2025. "Sternal Resections: An Attempt to Find the Ideal Reconstruction Method" Medicina 61, no. 4: 763. https://doi.org/10.3390/medicina61040763
APA StylePalade, E., Titu, I.-M., Fodor, L., Ciorba, I. M., Jentimir, I., Teterea, F., Mlesnite, M., & Tichil, I. (2025). Sternal Resections: An Attempt to Find the Ideal Reconstruction Method. Medicina, 61(4), 763. https://doi.org/10.3390/medicina61040763





