Clinical Value of Circulating Angiopoietin-like Protein 8/Betatrophin Levels in Patients with Acute Pancreatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Data Collection
| pO2 < 60 mmHg | 1 point |
| Age > 55 years | 1 point |
| Neutrophil count > 15,000 µ/L | 1 point |
| Calcium < 8 mg/dL | 1 point |
| Urea > 16 mmol/L | 1 point |
| LDH > 600 u/L | 1 point |
| AST > 200 u/L | 1 point |
| Albumin < 32 g/L | 1 point |
| Glucose > 180 mg/dL | 1 point |
| Modified Glasgow–Imrie severity criteria for AP. | |
2.3. Collection of Samples
2.4. Measurement of Serum ANGPTL8 Levels
2.5. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chen, H.; Li, H.; Zhang, J.; Gao, Y. Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. Int. J. Mol. Med. 2013, 32, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 10211. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Lee, M.J.; Hwang, S.Y.; Lee, H.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Circulating angiopoietin-like protein 8 (ANGPTL8) and ANGPTL3 concentrations in relation to anthropometric and metabolic profiles in Korean children: A prospective cohort study. Cardiovasc. Diabetol. 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhao, Z.; Deng, X.; Chen, Z.; Tu, Z.; Yuan, G. Regulation of angiopoietin-like protein 8 expression under different nutritional and metabolic status. Endocr. J. 2019, 66, 1039–1046. [Google Scholar] [CrossRef]
- Guo, C.; Wang, C.; Deng, X.; He, J.; Yang, L.; Yuan, G. ANGPTL8 in metabolic homeostasis: More friend than foe? Open Biol. 2021, 11, 210106. [Google Scholar] [CrossRef]
- Jiao, X.; Yang, Y.; Li, L.; Yu, H.; Yang, Y.; Li, J.; Du, Y.; Zhang, J.; Hu, C.; Qin, Y. Angiopoietin-like protein 8 accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis 2020, 307, 63–71. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Pottanat, T.G.; Siegel, R.W.; Ehsani, M.; Qian, Y.W.; Zhen, E.Y.; Regmi, A.; Roell, W.C.; Guo, H.; Luo, M.J.; et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J. Lipid Res. 2020, 61, 1203–1220. [Google Scholar] [CrossRef]
- Ebert, T.; Kralisch, S.; Hoffmann, A.; Bachmann, A.; Lössner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Tönjes, A.; Fasshauer, M. Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2014, 99, E2510–E2517. [Google Scholar] [CrossRef]
- Yi, P.; Park, J.S.; Melton, D.A. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell 2013, 153, 747–758. [Google Scholar] [CrossRef]
- Zhang, L.; Shannon, C.E.; Bakewell, T.M.; Abdul-Ghani, M.A.; Fourcaudot, M.; Norton, L. Regulation of ANGPTL8 in liver and adipose tissue by nutritional and hormonal signals and its effect on glucose homeostasis in mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E613–E624. [Google Scholar] [CrossRef]
- Catalano-Iniesta, L.; Robledo, V.S.; Iglesias-Osma, M.C.; Albiñana, A.G.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M.; Carretero, J.; García-Barrado, M.J. Evidences for Expression and Location of ANGPTL8 in Human Adipose Tissue. J. Clin. Med. 2020, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Mısırlıoglu, N.F.; Ergun, S.; Kucuk, S.H.; Himmetoglu, S.; Ozen, G.D.; Sayili, U.; Uzun, N.; Uzun, H. The Importance of Resolvin D1, LXA4, and LTB4 in Patients with Acute Pancreatitis Due to Gallstones. Medicina 2025, 61, 239. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, Y.; Ergun, S.; Turgut, B.C.; Dumur, S.; Sayili, U.; Uzun, H.; Pekmezci, S.; Velidedeoglu, M. The Role of Resolvin D1 in the Differential Diagnosis of Pancreatic Ductal Adenocarcinoma and Acute Pancreatitis: A Case-Control Study. Medicina 2025, 61, 168. [Google Scholar] [CrossRef]
- Mititelu, A.; Grama, A.; Colceriu, M.C.; Benţa, G.; Popoviciu, M.S.; Pop, T.L. Role of Interleukin 6 in Acute Pancreatitis: A Possible Marker for Disease Prognosis. Int. J. Mol. Sci. 2024, 25, 8283. [Google Scholar] [CrossRef] [PubMed]
- Efgan, M.G.; Karakaya, Z.; Kanter, E.; Kırık, S.; Tekindal, M.A. Can CONUT and PNI Scores Predict Necrotizing Pancreatitis in Acute Pancreatitis Patients Presenting to the Emergency Department? J. Clin. Med. 2024, 13, 5902. [Google Scholar] [CrossRef]
- Buxbaum, J.; Quezada, M.; Chong, B.; Gupta, N.; Yu, C.Y.; Lane, C.; Da, B.; Leung, K.; Shulman, I.; Pandol, S.; et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: Findings from a prospective cohort study. Am. J. Gastroenterol. 2018, 113, 755–764. [Google Scholar] [CrossRef]
- Feng, P.; He, C.; Liao, G.; Chen, Y. Early enteral nutrition versus delayed enteral nutrition in acute pancreatitis: A PRISMA compliant systematic review and meta-analysis. Medicine 2017, 96, e8648. [Google Scholar] [CrossRef]
- Jung, K.H.; Son, M.K.; Yan, H.H.; Fang, Z.; Kim, J.; Kim, S.J.; Park, J.H.; Lee, J.E.; Yoon, Y.C.; Seo, M.S.; et al. ANGPTL4 exacerbates pancreatitis by augmenting acinar cell injury through upregulation of C5a. EMBO Mol. Med. 2020, 12, e11222. [Google Scholar] [CrossRef]
- Rafaqat, S.; Radoman-Vujačić, I.; Patoulias, D.; Khurshid, H.; Klisić, A. Adipokines and their role in acute pancreatitis. J. Med. Biochem. 2024, 43, 512–527. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Baillie, J.; AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007, 132, 2022–2044. [Google Scholar] [CrossRef]
- Tenner, S.; Vege, S.S.; Sheth, S.G.; Sauer, B.; Yang, A.; Conwell, D.L.; Yadlapati, R.H.; Gardner, T.B. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am. J. Gastroenterol. 2024, 119, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Saxena, N.; Kapur, N.; Kardam, D. Comparison of modified Glasgow-IMRIE, Ranson, and Apache II scoring systems in predicting the severity of acute pancreatitis. Pol. J. Surg. 2022, 95, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Yeh, Y.H.; Chen, W.J.; Lin, K.H. Emerging regulation and function of betatrophin. Int. J. Mol. Sci. 2014, 15, 23640–23657. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Pascual, E.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E2004–E2009. [Google Scholar] [CrossRef]
- Maurer, L.; Brachs, S.; Decker, A.-M.; Brachs, M.; Leupelt, V.; von Schwartzenberg, R.J.; Ernert, A.; Bobbert, T.; Krude, H.; Spranger, J.; et al. Weight Loss Partially Restores Glucose-Driven Betatrophin Response in Humans. J. Clin. Endocrinol. Metab. 2016, 101, 4014–4020. [Google Scholar] [CrossRef]
- Gokulakrishnan, K.; Manokaran, K.; Pandey, G.K.; Amutha, A.; Ranjani, H.; Anjana, R.M.; Mohan, V. Relationship of betatrophin with youth onset type 2 diabetes among Asian Indians. Diabetes Res. Clin. Pract. 2015, 109, 71–76. [Google Scholar] [CrossRef]
- Tuhan, H.; Abacı, A.; Anık, A.; Çatlı, G.; Küme, T.; Çalan, Ö.G.; Acar, S.; Böber, E. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Res. Clin. Pract. 2016, 114, 37–42. [Google Scholar] [CrossRef]
- Chen, X.; Lu, P.; He, W.; Zhang, J.; Liu, L.; Yang, Y.; Liu, Z.; Xie, J.; Shao, S.; Du, T.; et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J. Clin. Endocrinol. Metab. 2015, 100, E96–E100. [Google Scholar] [CrossRef]
- Fenzl, A.; Itariu, B.K.; Kosi, L.; Fritzer-Szekeres, M.; Kautzky-Willer, A.; Stulnig, T.M.; Kiefer, F.W. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia 2014, 57, 1204–1208. [Google Scholar] [CrossRef]
- Hu, H.; Sun, W.; Yu, S.; Hong, X.; Qian, W.; Tang, B.; Wang, D.; Yang, L.; Wang, J.; Mao, C.; et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. 2014, 37, 2718–2722. [Google Scholar] [CrossRef]
- Wang, S.; Hong, X.; Tu, Z.; Yuan, G. Angiopoietin-like protein 8: An attractive biomarker for the evaluation of subjects with insulin resistance and related disorders. Diabetes Res. Clin. Pract. 2017, 133, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Donelan, W.; Xie, C.; Wang, H.; Wu, Q.; Purich, D.L.; Reeves, W.H.; Tang, D.; Yang, L.J. Angiopoietin-like protein 8 (betatrophin) is a stress-response protein that down-regulates expression of adipocyte triglyceride lipase. Biochim. Biophys. Acta 2016, 1861, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Kim, J.Y.; Smas, C.M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E334–E351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Lipasin, a novel about nutritionally regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012, 424, 786–792. [Google Scholar] [CrossRef]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef]
- Wang, Y.; Quagliarini, F.; Gusarova, V.; Gromada, J.; Valenzuela, D.M.; Cohen, J.C.; Hobbs, H.H. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 16109–16114. [Google Scholar] [CrossRef]
- Verras, G.I.; Mulita, F. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: A prospective single-center study. Front. Surg. 2024, 11, 1379410. [Google Scholar] [CrossRef]
- Klocker, E.V.; Barth, D.A.; Riedl, J.M.; Prinz, F.; Szkandera, J.; Schlick, K.; Kornprat, P.; Lackner, K.; Lindenmann, J.; Stöger, H.; et al. Decreased Activity of Circulating Butyrylcholinesterase in Blood Is an Independent Prognostic Marker in Pancreatic Cancer Patients. Cancers 2020, 12, 1154. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Kavalakatt, S.; Khadir, A.; Behbehani, K.; Alarouj, M.; Bennakhi, A.; et al. Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc. Diabetol. 2016, 15, 25. [Google Scholar] [CrossRef]
- Silbernagel, G.; Chen, Y.Q.; Li, H.; Lemen, D.; Wen, Y.; Zhen, E.Y.; Rief, M.; Kleber, M.E.; Delgado, G.E.; Sarzynski, M.A.; et al. Associations of Circulating ANGPTL3, C-Terminal Domain-Containing ANGPTL4, and ANGPTL3/8 and ANGPTL4/8 Complexes with LPL Activity, Diabetes, Inflammation, and Cardiovascular Mortality. Circulation 2025, 151, 218–234. [Google Scholar] [CrossRef]


| Control | Patient | ||
|---|---|---|---|
| Median [IQR] | Median [IQR] | p | |
| Age (Years) | 50 [43–64] | 58.5 [49–66.3] | 0.066 |
| Gender (Male/Female) (%) | 13/26 (33.3/66.7) | 24/26 (48/52) | 0.164 * |
| BMI (kg/m2) | 26.2 [25.2–27.4] | 26.2 [25.2–27.4] | 0.429 |
| CRP (mg/L) | 3 [2–5] | 88 [31.3–121.3] | <0.001 |
| ALT (U/L) | 19 [14–29] | 59 [18.8–124.5] | <0.001 |
| AST (U/L) | 21 [17–26] | 46.5 [21.5–209.5] | <0.001 |
| Glucose (mg/dL) | 87 [82–93] | 131 [113–192] | <0.001 |
| Urea (mg/dL) | 22 [20–25] | 38.5 [24–52.3] | <0.001 |
| Creatinine (mg/dL) | 0.7 [0.6–0.8] | 0.9 [0.7–1.1] | <0.001 |
| Albumin (g/L) | 41 [40–43] | 42.5 [38–45] | 0.323 |
| Calcium (mg/dL) | 8.9 [8.7–9.1] | 8.9 [8.2–9.3] | 0.627 |
| D.Bilirubin (mg/dL) | 0.7 [0.4–0.8] | 0.3 [0.1–1.1] | 0.072 |
| Triglyceride (mg/dL) | 104 [97–132] | 100 [80.5–130.5] | 0.331 |
| LDL (mg/dL) | 99 [89–109] | 100 [88.5–111] | 0.882 |
| Leukocyte (103 µ/L) | 5500 [4700–7300] | 13,800 [11,150–16,825] | <0.001 |
| Hemoglobin (g/L) | 13.4 [12.3–14.2] | 14 [12.8–15.1] | 0.129 |
| Platelet (103 µ/L) | 318,000 [246,000–376,000] | 235,000 [194,500–313,250] | 0.011 |
| ANGPTL8 (ng/L) | 285.9 [202–540] | 110 [74–163] | <0.001 |
| ANGPTL8 | Mean ± SD | Median [IQR] | Min–Max | p |
|---|---|---|---|---|
| GI score < 3 | 149.05 ± 80.5 | 127.7 [100.2–174.4] | 75.52–490 | 0.003 |
| GI score ≥ 3 | 104.49 ± 89.74 | 86.1 [48.93–127.75] | 21.51–472 |
| Cut-Off | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | p | |
|---|---|---|---|---|---|
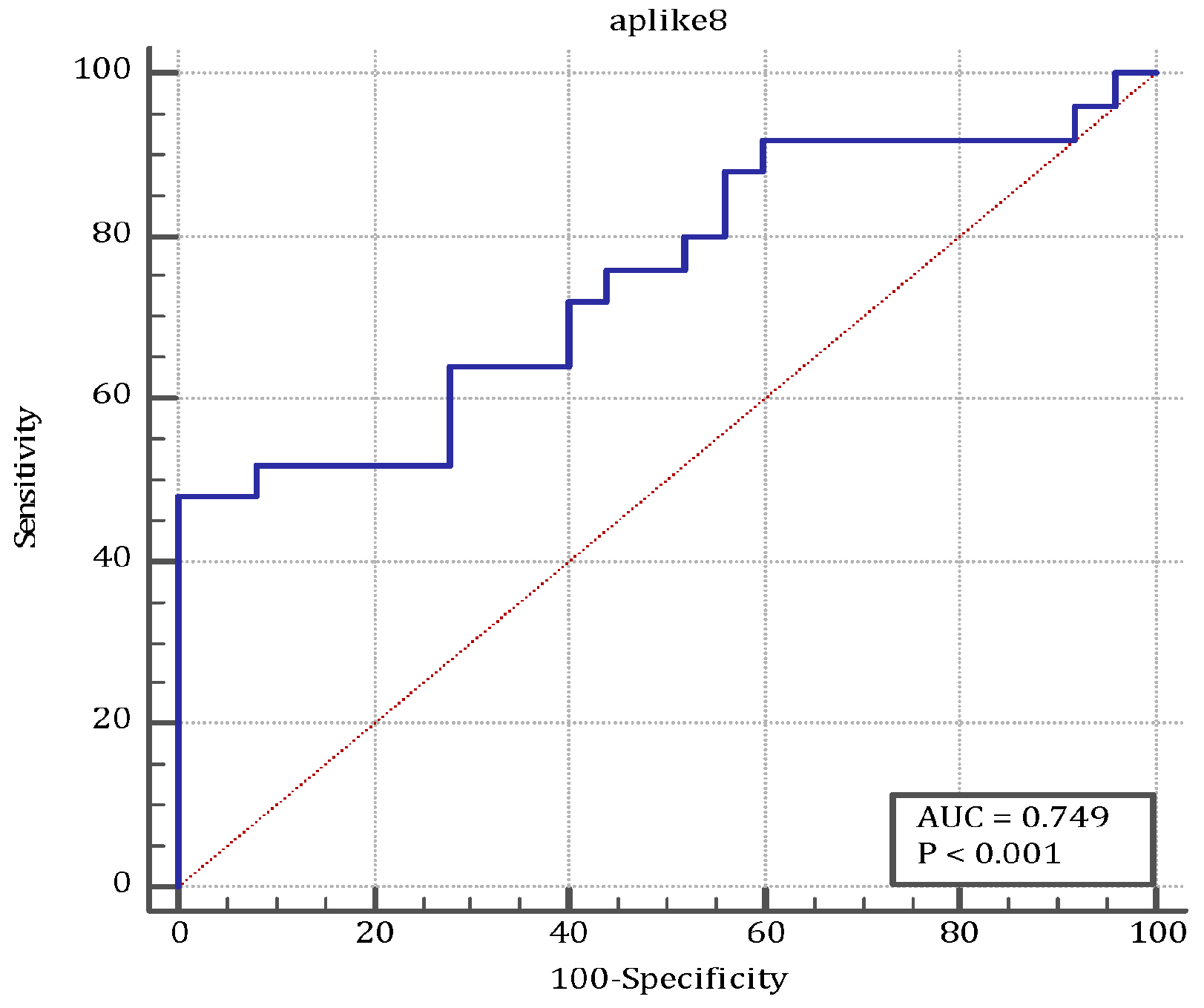
| ANGPTL8 | ≤70.9 | 0.749 (0.606–0.861) | 48.0 (27.8–68.7) | 100.0 (86.3–100.0) | <0.001 |
| Cut-Off | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | p | |
|---|---|---|---|---|---|
| ANGPTL8 | ≤179.2 | 0.936 (0.864–0.977) | 88.00 (75.7–95.5) | 89.74 (75.8–97.1) | <0.001 |
| Cut-Off | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | p | |
|---|---|---|---|---|---|
| CRP | >11 | 0.999 (0.957–1.00) | 100 (92.9–100.0) | 97.4 (86.5–99.9) | <0.001 |
| WBC | >9900 | 0.967 (0.905–0.993) | 82.0 (68.6–91.4) | 100 (91.0–100) | <0.001 |
| Control | Patient | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median [IQR] | Min–Max | Mean ± SD | Median [IQR] | p | p | padj | |
| ANGPTL8 | 476.46 ± 461.48 | 285.9 [201.9–539.6] | 139.4–2400 | 126.77 ± 87.32 | 109.45 [74.37–162.95] | 21.51–490.2 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumuskaya, P.O.; Yildirim, E.; Altun, O.; Uzun, H. Clinical Value of Circulating Angiopoietin-like Protein 8/Betatrophin Levels in Patients with Acute Pancreatitis. Medicina 2025, 61, 708. https://doi.org/10.3390/medicina61040708
Gumuskaya PO, Yildirim E, Altun O, Uzun H. Clinical Value of Circulating Angiopoietin-like Protein 8/Betatrophin Levels in Patients with Acute Pancreatitis. Medicina. 2025; 61(4):708. https://doi.org/10.3390/medicina61040708
Chicago/Turabian StyleGumuskaya, Perihan Ozkan, Emine Yildirim, Ozgur Altun, and Hafize Uzun. 2025. "Clinical Value of Circulating Angiopoietin-like Protein 8/Betatrophin Levels in Patients with Acute Pancreatitis" Medicina 61, no. 4: 708. https://doi.org/10.3390/medicina61040708
APA StyleGumuskaya, P. O., Yildirim, E., Altun, O., & Uzun, H. (2025). Clinical Value of Circulating Angiopoietin-like Protein 8/Betatrophin Levels in Patients with Acute Pancreatitis. Medicina, 61(4), 708. https://doi.org/10.3390/medicina61040708






