Does the Uterine Injection Site Matter for the Pelvic Sentinel Lymph Node Mapping? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Study Selection and Data Extraction
2.3. Assessment of Risk of Bias
2.4. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
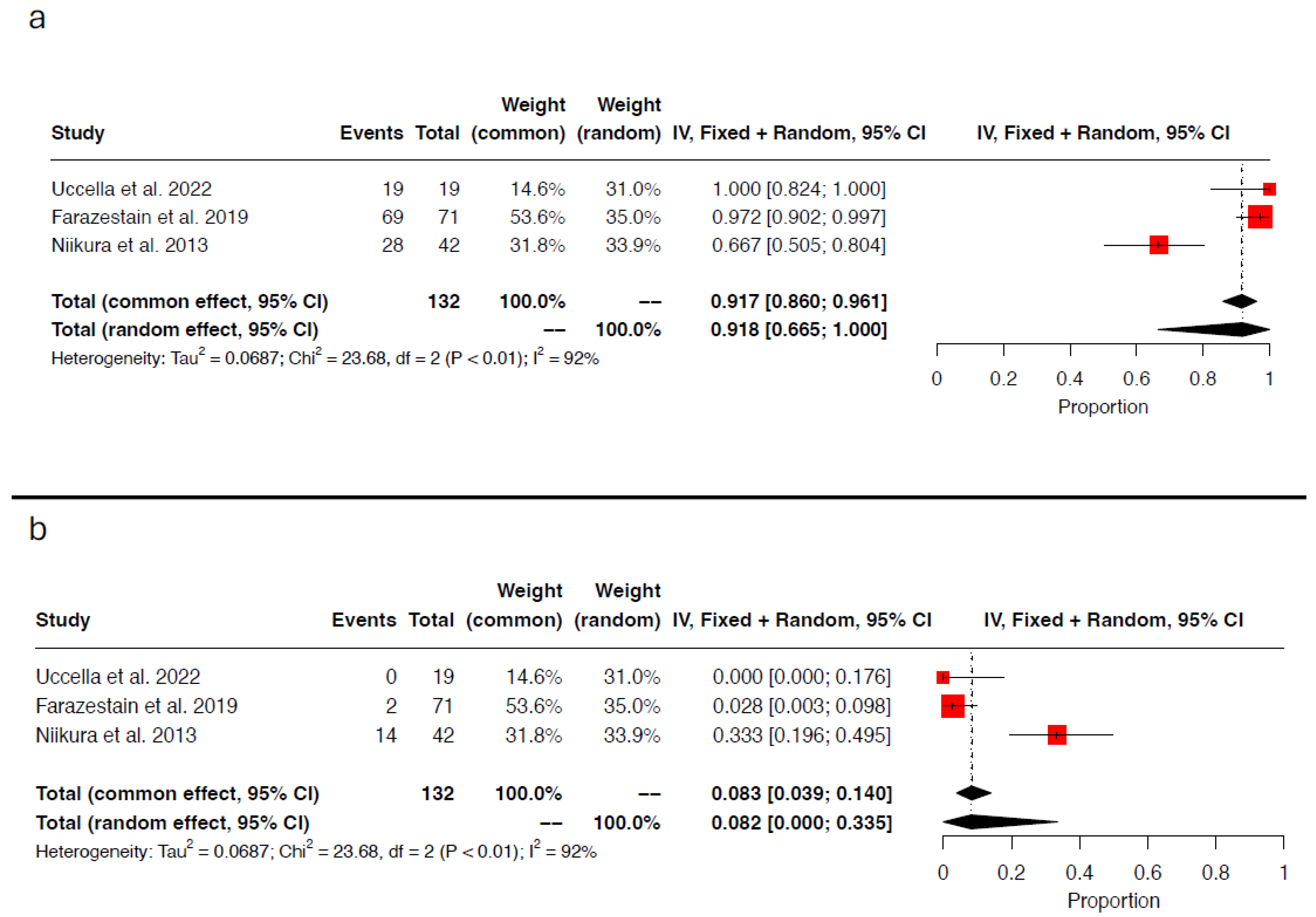
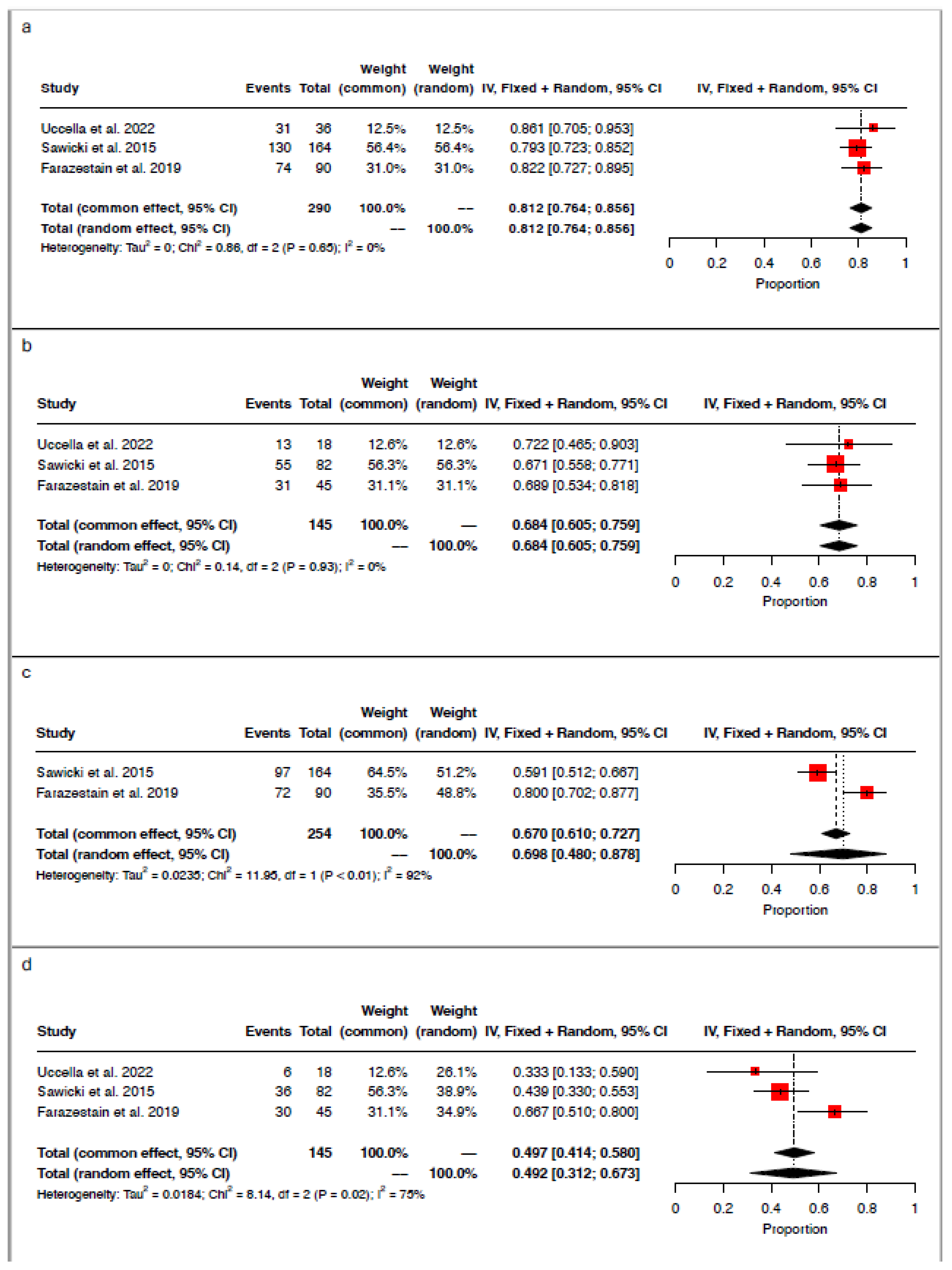
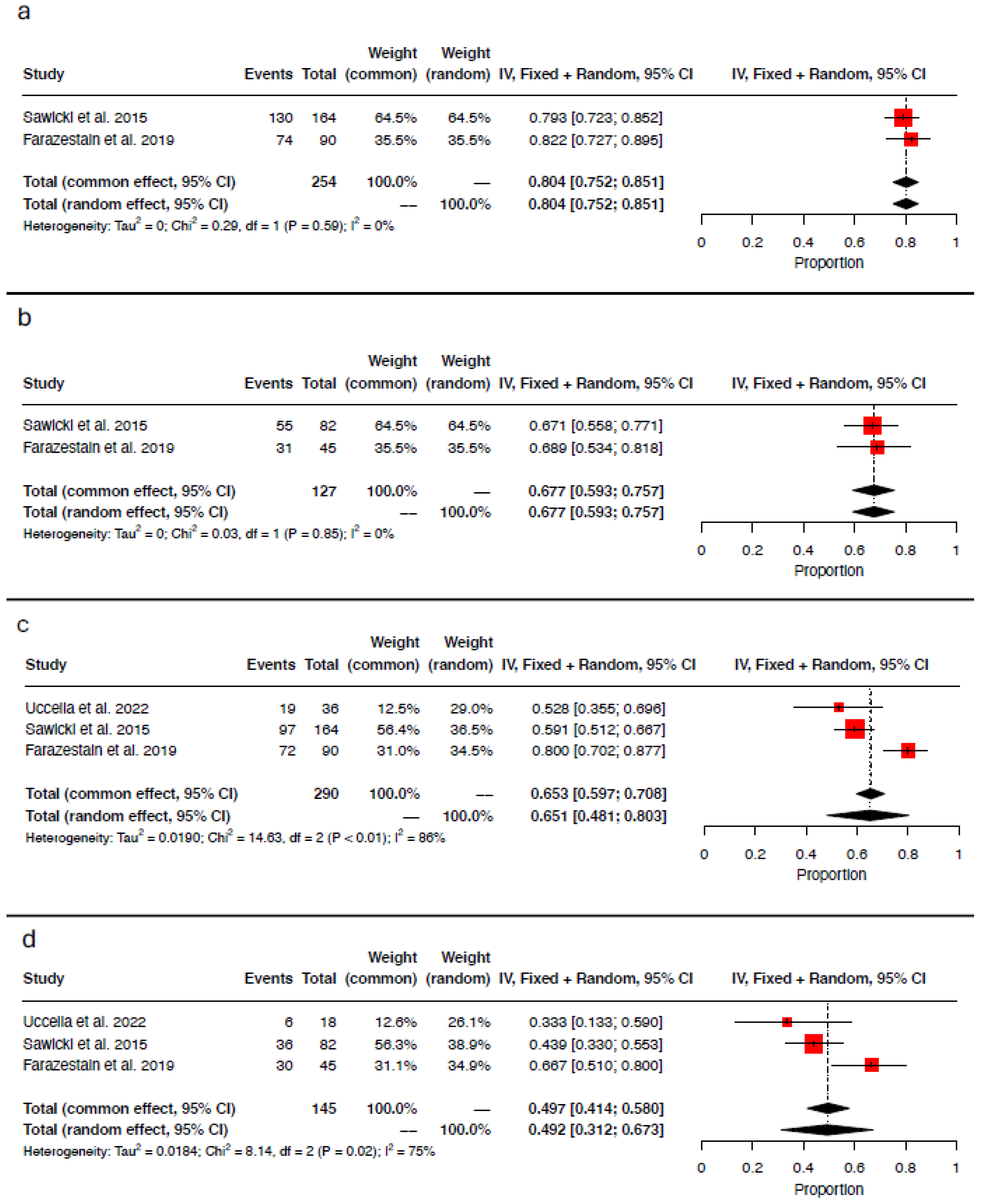
3.4. Concordance and Discordance Rates
3.5. Detection Rate per Injection Site
3.6. Detection Rate per Tracer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer-Update 2023. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Agusti, N.; Viveros-Carreño, D.; Grillo-Ardila, C.; Izquierdo, N.; Paredes, P.; Vidal-Sicart, S.; Torne, A.; Díaz-Feijoo, B. Sentinel Lymph Node Detection in Early-Stage Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Rey, I.; Lago, V.; Arnáez, M.; Bizzarri, N.; Agustí, N.; Nero, C.; Díaz-Feijoo, B.; Padilla-Iserte, P.; Domingo, S. Key Issues in Diagnostic Accuracy of Sentinel Lymph Node Biopsy in Early-Stage Ovarian Cancer: Systematic Review and Meta-Analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2024, 34, 1787–1794. [Google Scholar] [CrossRef]
- Uccella, S.; Zorzato, P.C.; Lanzo, G.; Fagotti, A.; Cianci, S.; Gallina, D.; Gueli Alletti, S.; Monterossi, G.; Franchi, M.; Ghezzi, F.; et al. The Role of Sentinel Node in Early Ovarian Cancer: A Systematic Review. Minerva Med. 2019, 110, 358–366. [Google Scholar] [CrossRef]
- Kleppe, M.; Kraima, A.C.; Kruitwagen, R.F.P.M.; Van Gorp, T.; Smit, N.N.; Van Munsteren, J.C.; DeRuiter, M.C. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 1405–1414. [Google Scholar] [CrossRef]
- Ercoli, A.; Delmas, V.; Iannone, V.; Fagotti, A.; Fanfani, F.; Corrado, G.; Ferrandina, G.; Scambia, G. The Lymphatic Drainage of the Uterine Cervix in Adult Fresh Cadavers: Anatomy and Surgical Implications. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2010, 36, 298–303. [Google Scholar] [CrossRef]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Arechvo, A.; Persson, J. A Study on Uterine Lymphatic Anatomy for Standardization of Pelvic Sentinel Lymph Node Detection in Endometrial Cancer. Gynecol. Oncol. 2017, 145, 256–261. [Google Scholar] [CrossRef]
- Vanneuville, G.; Mestas, D.; Le Bouedec, G.; Veyre, A.; Dauplat, J.; Escande, G.; Guillot, M. The Lymphatic Drainage of the Human Ovary in Vivo Investigated by Isotopic Lymphography before and after the Menopause. Surg. Radiol. Anat. SRA 1991, 13, 221–226. [Google Scholar] [CrossRef]
- Ditto, A.; Casarin, J.; Pinelli, C.; Perrone, A.M.; Scollo, P.; Martinelli, F.; Bogani, G.; Leone Roberti Maggiore, U.; Signorelli, M.; Chiappa, V.; et al. Hysteroscopic versus Cervical Injection for Sentinel Node Detection in Endometrial Cancer: A Multicenter Prospective Randomised Controlled Trial from the Multicenter Italian Trials in Ovarian Cancer (MITO) Study Group. Eur. J. Cancer 2020, 140, 1–10. [Google Scholar] [CrossRef]
- Nero, C.; Bizzarri, N.; Di Berardino, S.; Sillano, F.; Vizzielli, G.; Cosentino, F.; Vargiu, V.; De Iaco, P.; Perrone, A.M.; Vizza, E.; et al. Sentinel-Node Biopsy in Apparent Early Stage Ovarian Cancer: Final Results of a Prospective Multicentre Study (SELLY). Eur. J. Cancer 2024, 196, 113435. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.; Bossuyt, P.; Leeflang, M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Chung, M.; Chen, M.L.; Trikalinos, T.A.; Kong Win Chang, L. Assessing the Accuracy of Google Translate to Allow Data Extraction From Trials Published in Non-English Languages; AHRQ Methods for Effective Health Care; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013.
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Garzon, S.; Bosco, M.; Porcari, I.; Lanzo, G.; Laganà, A.S.; Chiantera, V.; Cliby, W.A.; Mariani, A.; Franchi, M.; et al. Cervical versus Utero-Ovarian Ligament Injection of the Tracer for the Pelvic Sentinel Lymph Node Mapping in Gynecologic Oncology: A Prospective Observational Study. Gynecol. Obstet. Investig. 2022, 87, 242–247. [Google Scholar] [CrossRef]
- Farazestanian, M.; Yousefi, Z.; Zarifmahmoudi, L.; Hasanzadeh Mofrad, M.; Kadkhodayan, S.; Sadeghi, R. Concordance Between Intracervical and Fundal Injections for Sentinel Node Mapping in Patients With Endometrial Cancer?: A Study Using Intracervical Radiotracer and Fundal Blue Dye Injections. Clin. Nucl. Med. 2019, 44, e123–e127. [Google Scholar] [CrossRef]
- Niikura, H.; Kaiho-Sakuma, M.; Tokunaga, H.; Toyoshima, M.; Utsunomiya, H.; Nagase, S.; Takano, T.; Watanabe, M.; Ito, K.; Yaegashi, N. Tracer Injection Sites and Combinations for Sentinel Lymph Node Detection in Patients with Endometrial Cancer. Gynecol. Oncol. 2013, 131, 299–303. [Google Scholar] [CrossRef]
- Sawicki, S.; Lass, P.; Wydra, D. Sentinel Lymph Node Biopsy in Endometrial Cancer-Comparison of 2 Detection Methods. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 1044–1050. [Google Scholar] [CrossRef]
- Rossi, E.C.; Jackson, A.; Ivanova, A.; Boggess, J.F. Detection of Sentinel Nodes for Endometrial Cancer with Robotic Assisted Fluorescence Imaging: Cervical versus Hysteroscopic Injection. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2013, 23, 1704–1711. [Google Scholar] [CrossRef]
- Gezer, Ş.; Duman Öztürk, S.; Hekimsoy, T.; Vural, Ç.; İşgören, S.; Yücesoy, İ.; Çorakçı, A. Cervical versus Endometrial Injection for Sentinel Lymph Node Detection in Endometrial Cancer: A Randomized Clinical Trial. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 325–331. [Google Scholar] [CrossRef]
- Martinelli, F.; Ditto, A.; Signorelli, M.; Bogani, G.; Chiappa, V.; Lorusso, D.; Scaffa, C.; Recalcati, D.; Perotto, S.; Haeusler, E.; et al. Sentinel Node Mapping in Endometrial Cancer Following Hysteroscopic Injection of Tracers: A Single Center Evaluation over 200 Cases. Gynecol. Oncol. 2017, 146, 525–530. [Google Scholar] [CrossRef]
- Bogani, G.; Murgia, F.; Ditto, A.; Raspagliesi, F. Sentinel Node Mapping vs. Lymphadenectomy in Endometrial Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2019, 153, 676–683. [Google Scholar] [CrossRef] [PubMed]
- About the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Available online: https://www.nccn.org/professionals/default.aspx (accessed on 23 June 2020).
- Capozzi, V.A.; Armano, G.; Maglietta, G.; Rosati, A.; Vargiu, V.; Scarpelli, E.; Sozzi, G.; Chiantera, V.; Cosentino, F.; Gioè, A.; et al. Hysteroscopic Endometrial Tumor Localization and Sentinel Lymph Node Mapping. An Upgrade of the Hysteroscopic Role in Endometrial Cancer Patients. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2023, 49, 106952. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-Infrared Fluorescence for Detection of Sentinel Lymph Nodes in Women with Cervical and Uterine Cancers (FILM): A Randomised, Phase 3, Multicentre, Non-Inferiority Trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, I.; Gasparri, M.L.; Braicu, E.I.; Bellati, F.; Raio, L.; Sehouli, J.; Mueller, M.D.; Panici, P.B.; Papadia, A. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes-A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3749–3756. [Google Scholar] [CrossRef]
- Papadia, A.; Zapardiel, I.; Bussi, B.; Ghezzi, F.; Ceccaroni, M.; De Ponti, E.; Elisei, F.; Imboden, S.; de la Noval, B.D.; Gasparri, M.L.; et al. Sentinel Lymph Node Mapping in Patients with Stage I Endometrial Carcinoma: A Focus on Bilateral Mapping Identification by Comparing Radiotracer Tc99m with Blue Dye versus Indocyanine Green Fluorescent Dye. J. Cancer Res. Clin. Oncol. 2017, 143, 475–480. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel Lymph Node Detection in High-Risk Endometrial Cancer (SHREC-Trial)-the Final Step towards a Paradigm Shift in Surgical Staging. Eur. J. Cancer Oxf. Engl. 1990 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Uccella, S.; Nero, C.; Vizza, E.; Vargiu, V.; Corrado, G.; Bizzarri, N.; Ghezzi, F.; Cosentino, F.; Turco, L.C.; Fagotti, A.; et al. Sentinel-Node Biopsy in Early-Stage Ovarian Cancer: Preliminary Results of a Prospective Multicentre Study (SELLY). Am. J. Obstet. Gynecol. 2019, 221, 324.e1–324.e10. [Google Scholar] [CrossRef]
- Laven, P.; Kruitwagen, R.; Zusterzeel, P.; Slangen, B.; Van Gorp, T.; Van Der Pol, J.; Lambrechts, S. Sentinel Lymph Node Identification in Early Stage Ovarian Cancer: Is It Still Possible after Prior Tumor Resection? J. Ovarian Res. 2021, 14, 132. [Google Scholar] [CrossRef]
- Lago, V.; Bello, P.; Montero, B.; Matute, L.; Padilla-Iserte, P.; Lopez, S.; Marina, T.; Agudelo, M.; Domingo, S. Sentinel Lymph Node Technique in Early-Stage Ovarian Cancer (SENTOV): A Phase II Clinical Trial. Int. J. Gynecol. Cancer 2020, 30, 1390–1396. [Google Scholar] [CrossRef]
| Author, Year (Country) | Design | N of Patients | Histotype | Stage (FIGO) | Lymphovascular Invasion | Surgical Approach | Site of Injection | Tracer | Technique | Time Tracer Injection—SLN Evaluation |
|---|---|---|---|---|---|---|---|---|---|---|
| Niikura et al., 2013 [18] (Japan) | Prospective | 27 | NA | NA | NA | Open Surgery | Cervix | 99mTc | Pre-operative lymphoscintigraphy by injecting Tc99m-labeled phytate in the cervix at 3, 6, 9, and 12 o’clock positions one day before surgery. At the time of surgery, a gamma-detecting probe was used to locate radioactive SLN. | NA |
| Corpus | MB | Injection into five different sites of the subserosal endometrium. | ||||||||
| Sawicki et al., 2015 [19] (Poland) | Retrospective | 82 | 96.4% endometrioid 1.2% clear cell 2.4% serous | IA: 47.6% IB: 23.2% II: 17.1% IIIA: 2.4% IIIB: 2.4% IIIC1: 7.3% | Yes: 12.2% No: 87.8% | Open Surgery | Cervix | 99mTc | Injection of the cervix with Tc99m-labeled nanocolloid and the SLN was subsequently located using a handheld gamma probe. | NA |
| Corpus | MB | 4 mL in the uterine fundus. | ||||||||
| Farazestanian et al., 2019 [17] (Iran) | Prospective | 45 | 86.7% endometrioid 8.9% clear cell 4.4% serous | NA | Yes: 15.6% No: 84.4% | Open Surgery | Cervix | 99mTc | Two injections of Tc99m-phytate into the cervix at the 6- and 12-o’clock positions the day before surgery. Planar lymphoscintigraphy was conducted for each patient 8 to 18 h after injections, and surgery was scheduled 18 to 24 h after the radiotracer injection. Hot SLN were identified using a handheld gamma probe. | 15 min |
| Corpus | MB | 2 mL at subserosal fundal midline locations | ||||||||
| Uccella et al., 2022 [16] (Italy) | Prospective | 18 | 94.5% endometrioid 5.5% non endometrioid | IA: 38.8% IB: 44.4% II: 11.1% IIIC: 5.6% | Yes: 55.5% No: 44.5% | Conventional Laparoscopy | Cervix | ICG | 2 mL ICG at 3 and 9 o’clock positions, 1 mL deeply at 1.5–2.5 cm into the stroma, and 1 mL superficially into the submucosal tissue. | 15 min |
| UOL | MB | 2 mL bilateral injections into the utero-ovarian ligaments trans-abdominally under laparoscopic vision. Nodes were detected using a near-infrared high-intensity light source. |
| Author, Year | N of Patients/Hemipelvis | Site of Injection | Tracer | Detection Rate | Hemipelvis with Double Mapping | Concordance Rate | Discordance Rate | ||
|---|---|---|---|---|---|---|---|---|---|
| Mapped Hemipelvis | Patients with at Least One Mapped Hemipelvis | Patients with Bilateral Pelvic Mapping | |||||||
| Niikura, 2013 [18] | 27/54 | Cervix | 99mTc | NA | NA | NA | 42 | 28/42 (66.7%) | 14/42 (33.3%) |
| Corpus | MB | NA | NA | NA | |||||
| Cervix + Corpus | 42/54 (77.8%) | 27/27 (100%) | 26/27 (96.3%) | ||||||
| Sawicki, 2015 [19] | 82/164 | Cervix | 99mTc | 130/164 (79.3%) | 75/82 (91.5%) | 55/82 (67.1%) | NA | NA | NA |
| Corpus | MB | 97/164 (59.1%) | 61/82 (74.4%) | 36/82 (43.9%) | |||||
| Cervix + Corpus | 140/164 (85.4%) | 78/82 (95.1%) | 62/82 (75.6%) | ||||||
| Farazestanian, 2019 [17] | 45/90 | Cervix | 99mTc | 74/90 (82.2%) | 42/45 (93.3%) | 31/45 (68.8%) | 71 | 69/71 (97.2%) | 2/71 (2.8%) |
| Corpus | MB | 72/90 (80.0%) | 42/45 (93.3%) | 30/45 (66.6%) | |||||
| Cervix + Corpus | 76/90 (84.4%) | 42/45 (93.3%) | 43/45 (95.5%) | ||||||
| Uccella, 2022 [16] | 18/36 | Cervix | ICG | 31/36 (86.1%) | 18/18 (100%) | 13/18 (72.2%) | 19 | 19/19 (100%) | 0/19 (0%) |
| UOL | MB | 19/36 (52.8%) | 13/18 (72.2%) | 6/18 (33.3%) | |||||
| Cervix + UOL | 31/36 (86.1%) | 18/10 (100%) | 13/18 (72.2%) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorzato, P.C.; Garzon, S.; Bosco, M.; Ferrari, F.; Magni, F.; Laterza, R.M.; Laganà, A.S.; Fanfani, F.; Uccella, S. Does the Uterine Injection Site Matter for the Pelvic Sentinel Lymph Node Mapping? A Systematic Review and Meta-Analysis. Medicina 2025, 61, 699. https://doi.org/10.3390/medicina61040699
Zorzato PC, Garzon S, Bosco M, Ferrari F, Magni F, Laterza RM, Laganà AS, Fanfani F, Uccella S. Does the Uterine Injection Site Matter for the Pelvic Sentinel Lymph Node Mapping? A Systematic Review and Meta-Analysis. Medicina. 2025; 61(4):699. https://doi.org/10.3390/medicina61040699
Chicago/Turabian StyleZorzato, Pier Carlo, Simone Garzon, Mariachiara Bosco, Filippo Ferrari, Francesca Magni, Rosa Maria Laterza, Antonio Simone Laganà, Francesco Fanfani, and Stefano Uccella. 2025. "Does the Uterine Injection Site Matter for the Pelvic Sentinel Lymph Node Mapping? A Systematic Review and Meta-Analysis" Medicina 61, no. 4: 699. https://doi.org/10.3390/medicina61040699
APA StyleZorzato, P. C., Garzon, S., Bosco, M., Ferrari, F., Magni, F., Laterza, R. M., Laganà, A. S., Fanfani, F., & Uccella, S. (2025). Does the Uterine Injection Site Matter for the Pelvic Sentinel Lymph Node Mapping? A Systematic Review and Meta-Analysis. Medicina, 61(4), 699. https://doi.org/10.3390/medicina61040699









