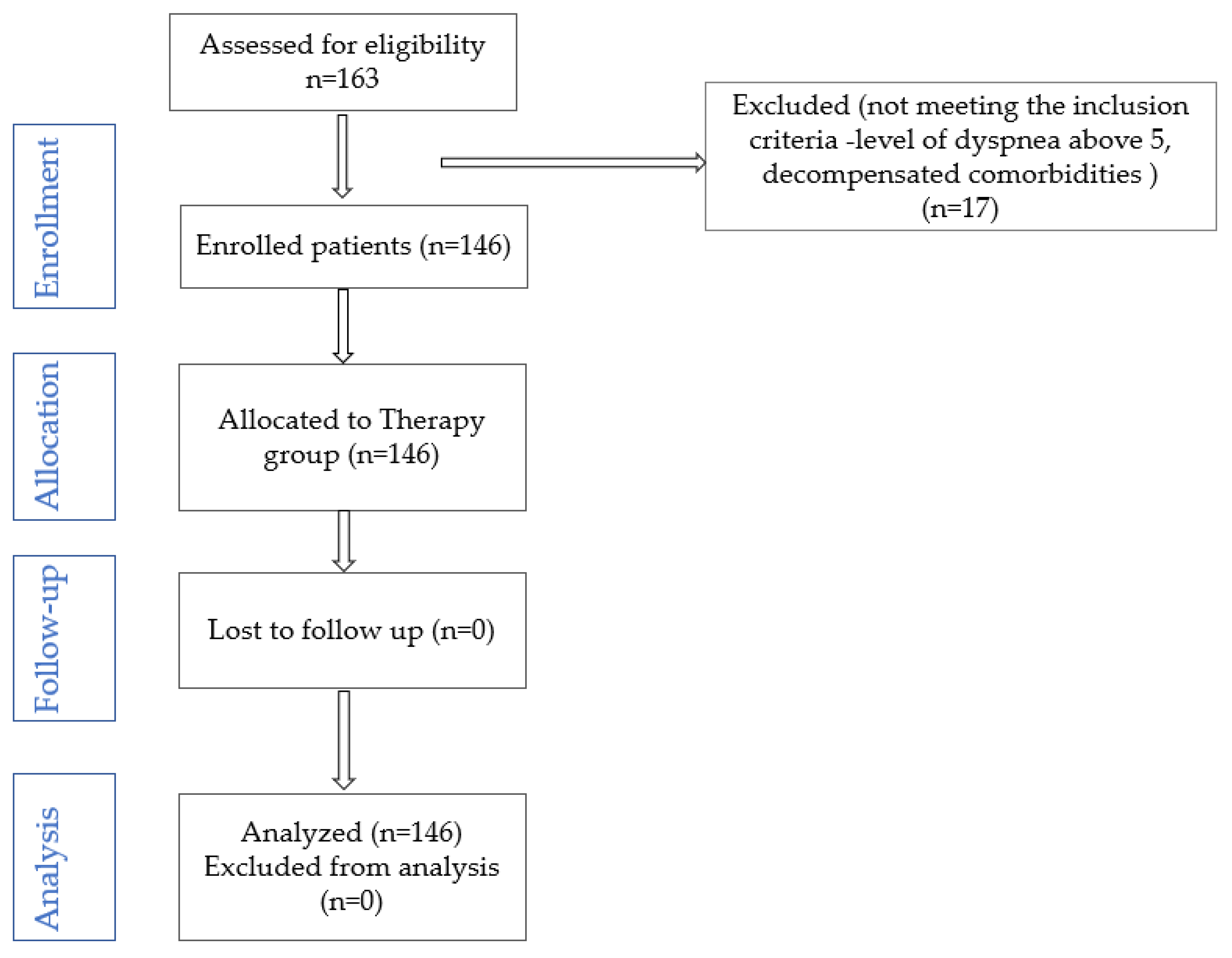
1. Introduction
Dyspnea is a clinical manifestation frequently associated with chronic diseases, with a prevalence of 10% in the general population [
1]. According to some authors, the incidence would be approximately 25% of the patients seen in consultation at the outpatient clinic [
2]. Reflecting a deterioration in cardiorespiratory function, it has a negative impact on tolerance to physical activities, and on the ability to perform professional tasks, ultimately affecting quality of life, both personally and professionally. There is an international concern to ensure the functional autonomy of patients with chronic dyspnea, which could considerably reduce social, economic, and individual costs.
The etiology of chronic dyspnea is generally multifactorial, with a high prevalence of pulmonary or cardiac causes [
3]. Along with bronchial asthma, heart failure, ischemic heart disease, chronic obstructive pulmonary disease (COPD), pneumonia, and psychogenic conditions, which account for 85–90% of the cases of dyspnea, there are other causes, such as anemia, obesity, physical deconditioning [
4], gastric, neuromuscular disorders, and uncommon conditions [
5]. The etiology of dyspnea has been shown to have several causes in over one-third of patients. The diagnosis is clinical in over 60% of cases. Additional paraclinical investigations are required to support the diagnosis [
5].
Dyspnea is a complex symptom that indicates a potential threat to homeostasis, occurring due to impairment of the cardiovascular and respiratory systems, but also due to metabolic disturbances, neuromuscular disorders, or psychogenic disorders, caused by a mismatch between pulmonary ventilation and the desire to breathe. The dissociation between these two aspects is the result of a mismatch between receptors in the airways, lungs, and chest wall and central respiratory motor activity. Dyspnea is influenced by afferent signals (information about blood gas levels and lung status), efferent signals (commands to respiratory muscles, diaphragm), and central brain processing, which compares these signals. When there is a mismatch between the need for ventilation and the ability to breathe, the intensity of dyspnea increases, and the sensory cortex activates the conscious sensation of exertion and shortness of breath. The psychological component also plays a role, as some people may be aware of their breathing without feeling discomfort [
6]. At the same time as physiopathological changes occur in the lungs, there are also changes in the cardiovascular system. One can notice an increase in right ventricular afterload, followed by right ventricular hypertrophy, and finally, right ventricular failure [
7], which will affect the mass, geometry [
8], and by implication, left ventricular systolic function [
9]. These changes will cause the left heart to be overloaded and exceed its exercise capacity.
Emotion influences the neural processing of respiratory sensations and interacts with non-emotional mental processes such as attention and expectation, affecting aversive experiences such as pain and dyspnea. Attentional resources are allocated according to emotional salience, and focusing on the threat of dyspnea may reduce performance in daily tasks. High dyspnea-related anxiety impairs the ability to allocate attention to cognitive tasks, especially in negative emotional contexts. Although exercise may benefit cognitive function, its impact on dyspnea patients needs to be studied. Attention allocation is crucial in treatment and patients with pulmonary disease are encouraged to focus on breathing. Inter-interoceptive self-awareness may reduce the intensity of dyspnea, but negative emotions may hinder this cognitive manipulation [
10].
The literature specifically emphasizes the benefits of exercise training in the management of pulmonary dyspnea, especially in patients with COPD. Exercise limitation is closely related to disability and is a significant indicator of poor health as well as a risk factor for premature mortality. Factors influencing exercise restriction vary from patient to patient, including respiratory pathologies, associated cardiovascular and neurological conditions, and musculoskeletal dysfunction. The implementation of a rehabilitation program with a multidisciplinary approach for 6 to 8 weeks demonstrated a significant improvement in dyspnea and exercise tolerance. There was also an improvement in mental well-being and a reduction in hospitalizations. However, despite continued support for pulmonary rehabilitation in the literature, debates remain about the specific content of pulmonary rehabilitation and its benefits for the specific patient [
11]. The American Thoracic Society/European Respiratory Society suggests the use of pulmonary rehabilitation (PR) for all patients who require this intervention, as it can bring significant benefits in terms of quality of life. PR applied to patients with respiratory pathology will reduce dyspnea and increase exercise performance, implicitly improving the quality of life. The highest addressability is for patients with COPD. PR programs include assessing the patient’s condition, and implementing physical training, along with nutritional interventions and psychosocial support [
12].
Regardless of the respiratory function, muscle fatigue of the muscles in the lower limbs is frequently associated with patients with cardiorespiratory pathology. In terms of the skeletal muscle fiber, a series of transformations occur, determined by the deconditioning of these patients. An important witness is the strength of the quadriceps, which correlates with the exercise capacity [
11].
The traditional program, used for patients with chronic dyspnea, consists of aerobic exercises and resistance training for the upper and lower limbs, which is associated with walking and cycle ergometer training, instituted progressively to increase muscle conditioning and cardiorespiratory fitness [
11]. This endurance training applied for 3 months reveals proven benefits on exercise capacity, increasing the strength of the quadriceps muscle (known dysfunction in COPD) [
13,
14].
Patients with chronic dyspnea, regardless of etiology, are forced to reduce their daily activities due to chronic shortness of breath [
15], thus creating a vicious circle that promotes physical deconditioning and social isolation [
16]. The London Chest Activity Scale of Daily Living (LCADL) is a questionnaire that measures functional limitation due to dyspnea experienced during activities of daily living, such as personal care, housework, physical exercise, and recreation [
17]. The 6 min walk test (6MWT) is recommended as an effective tool to assess treatment efficacy by examining pulmonary function and exercise capacity [
18]. Because the test incorporates a level of physical activity comparable to that of daily tasks, it can accurately indicate patients’ pulmonary function and exercise capacities during daily routines [
18].
Although exercise has been shown to have a positive impact on cognitive function, there is a need to investigate whether this effect also applies to patients suffering from dyspnea [
10]. Based on the hypothesis that a progressive physical training program, adapted to cardiopulmonary reserve, reduces functional limitation in patients with chronic dyspnea, this study aims to (1) evaluate and compare the benefit of traditional physical training in patients with chronic dyspnea of different etiologies, by assessing walking distance; (2) compare the evolution of dyspnea grade in ADLs after physical training; (3) identify predictive factors associated with the 6MWT gait test in patients with chronic dyspnea. Also, an original aspect will be followed, namely the effects on each given ADL domain (personal care, domestic chores, exercise, and leisure activities).
4. Discussion
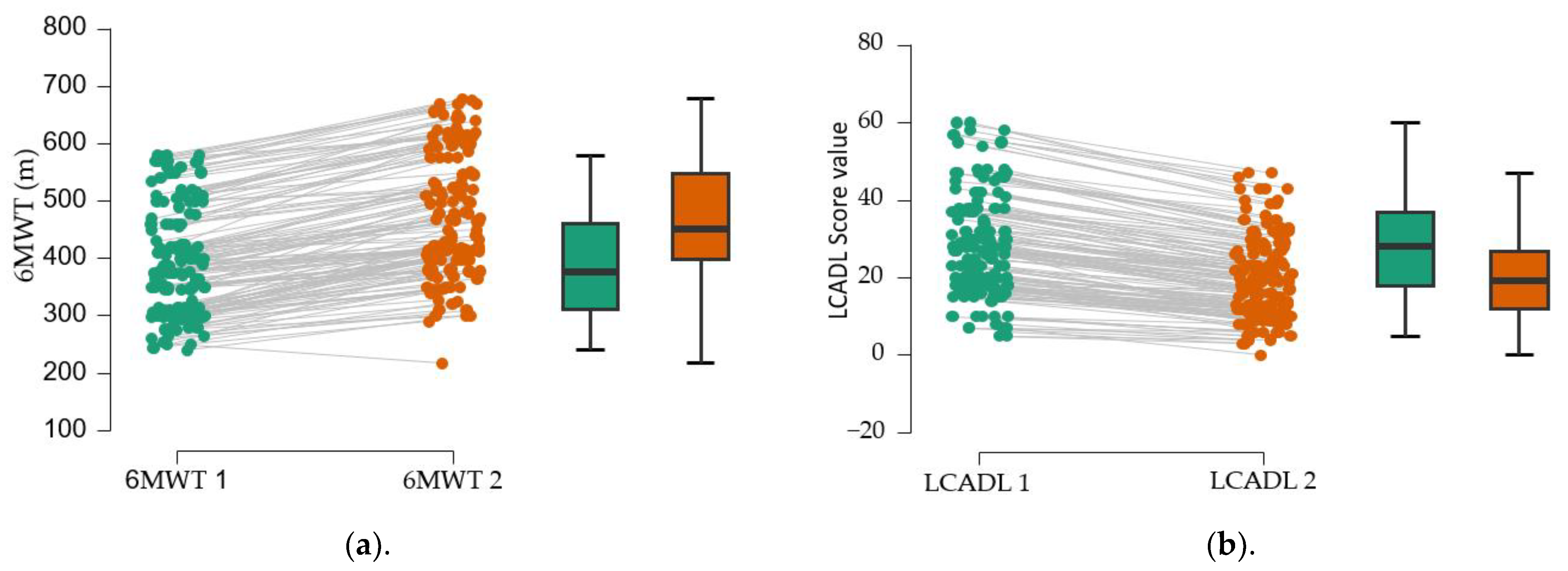
This study aims to fill gaps in the literature on the benefits of physical training in patients with chronic dyspnea. Based on the hypothesis that a progressive physical training program, adapted to cardiopulmonary reserve, reduces functional limitation in patients with chronic dyspnea, the effects of a traditional physical training program on walking distance and daily activities, both globally and by activity domains, were analyzed. The originality of this study lies in the attempt to stratify these benefits according to the etiology of chronic dyspnea. Analysis of the results supports the hypothesis formulated; walking distance traveled significantly increased, regardless of the etiology of dyspnea. In addition, the LCADL score improved at the cohort level, both overall and in all four domains assessed.
The results are supported by several published studies, which show that the increase in walking distance and the reduction in dyspnea is achieved following appropriate physical training applied to patients with chronic dyspnea [
27,
28], but most studies refer to respiratory pathology (COPD). The physical training used to increase the exercise capacity of patients with chronic dyspnea is diverse, from traditional exercises to Tai Chi, Yoga, Daoyin, and even walking [
14]. In this study, the physical training followed was traditional. Traditional endurance training consists of long sessions of moderate-intensity exercise, such as walking, cycling, or jogging, performed at a consistent pace for an extended duration, typically 30 min or more. This type of training is effective in enhancing aerobic capacity and overall fitness, particularly for individuals with chronic diseases. Long-term exercise leads to a more efficient delivery of oxygen to the muscles and enhances endurance capacity. Additionally, training with heavy weights promotes muscle growth and toning. However, recent studies in high-intensity training with short intervals and low-load exercises—aimed not at increasing muscle size—challenge the traditional view of training specificity. To gain a better understanding of the muscle adaptations resulting from different types of exercise, it is essential to explore the molecular mechanisms that influence changes in muscle phenotype [
29]. High-intensity interval training (HIIT), on the other hand, involves short bursts of intense exercise followed by periods of rest or low-intensity activity. Research on the relationship between chronic obstructive pulmonary disease (COPD) and the effects of HIIT in pulmonary rehabilitation is still limited. However, one study has demonstrated that COPD patients can improve their functional capacity after 12 weeks of high-intensity training [
30]. Another study also reported positive changes in systolic function and cardiovascular values following HIIT and moderate exercise. A systematic review found that patients with moderate to severe COPD can perform HIIT, leading to improvements in ventilatory parameters and a reduction in exercise-associated dyspnea [
31]. HIIT leads to positive changes in ventilatory parameters and a reduction in exertional dyspnea [
32]. The findings of the systematic review published by J. R. Adolfo (2019) suggest that the effects of HIIT are similar to those obtained with continuous exercise in terms of relative VO2max, absolute VO2max, and cardiovascular variables among patients with COPD. Regarding the type and intensity of physical activities recommended in pulmonary rehabilitation programs, research has shown favorable results for both HIIT and continuous moderate-intensity exercise in COPD patients [
31]. The narrative review, published by M Emtner and K Wadell (2016), showed that aerobic and resistance training lead to increased quality of life in patients, as well as decreased dyspnea, anxiety, and depression [
33], results supported by the data obtained in this study.
The analysis of the data suggests that, although according to the average age, the patients included in the study are middle-aged, only about 50% of them have the status of an employee, meaning that chronic dyspnea, regardless of the etiology, significantly limits their ability to carry out a professional activity. Domestic activities can be performed by more than 85% of the recruited patients, but only about 10% of patients are able to practice hobbies prior to the onset of the disease. Pulmonary etiology is present in over 60% of cases (COPD, asthma), and cardiac pathology in about 30% (heart failure). A study conducted on 120 patients with COPD [
34], published by H. Carette (2019), suggests that 53% of the patients treated with inhaled medication have severe chronic dyspnea, and 40% have undergone a pulmonary rehabilitation program. In our study, the incidence of dyspnea level 3 is 37.67%.
Obstructive ventilatory defect is present in 35.61% of recruited cases. Restrictive ventilatory defect, present in 38.35% of cases, was determined by the presence of kyphoscoliosis and associated neurological diseases. In about 8% of cases, chronic pulmonary pathology was associated with kyphoscoliosis or neurological diseases.
The meta-analysis published by Ahmed I. et al. (2022), which monitored the effect of PR on dyspnea, fatigue, and exercise capacity, identified five clinical trials (N = 449 patients, in patients with acute and/or chronic COVID-19) that suggested significant benefits of PR on dyspnea, fatigue, and exercise capacity [
25]. The PR program is structured, similar to the program used in our study, in the three specific stages of a training program (warm-up phase—consisting of active mobilizations, the actual training phase—consisting of strength and gait training; at the end, stretching exercises are performed to allow blood pressure and heart rate to return to normal); the difference is the recommendation to be used 4 times/week compared to our study, in which the recommendation is 5 times/week. In both cases, the benefit of applied physical training was demonstrated.
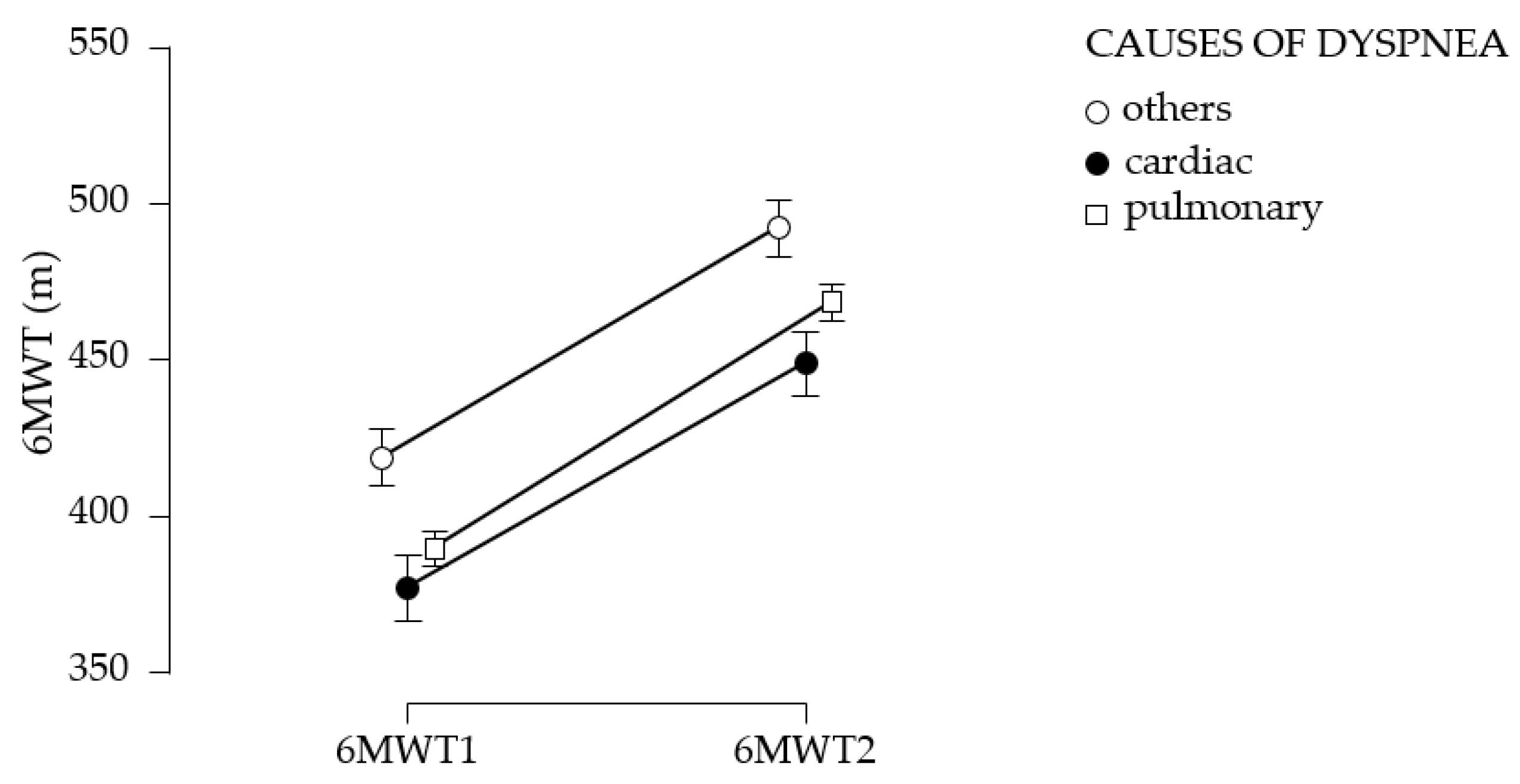
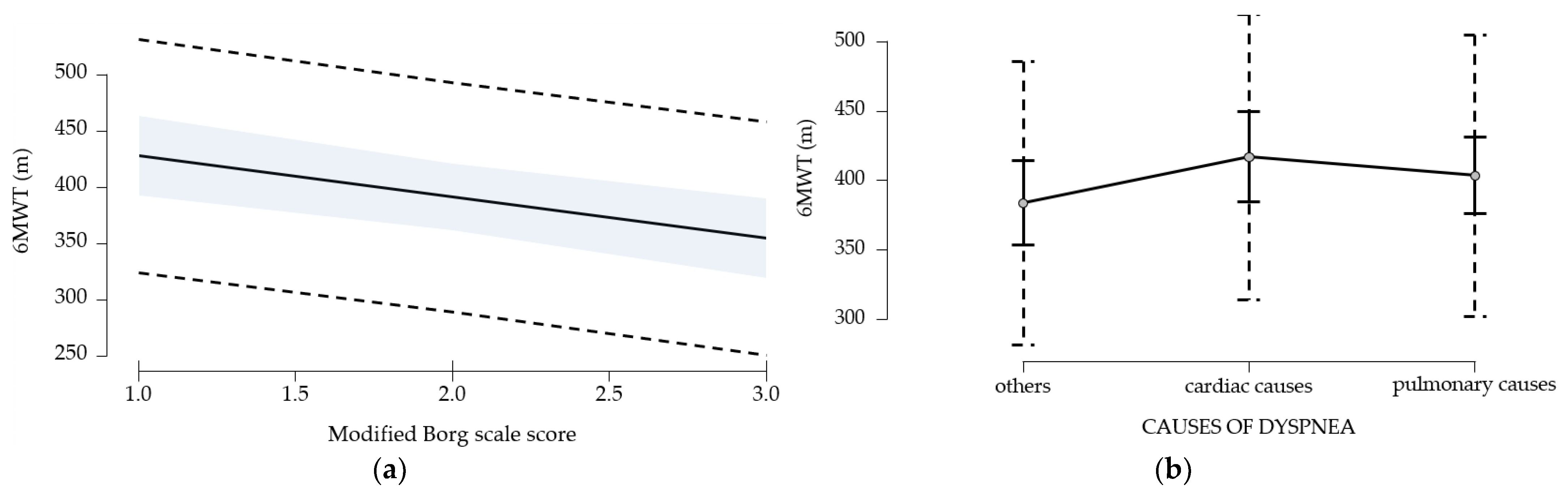
The assessment of functional limitation, as determined by dyspnea experienced during daily activities with the LCADL test, suggests a 38% limitation at first determination. Following the establishment of a pulmonary rehabilitation program, followed intensively for 10 days, then 3 times a week for 3 months, an 11.5% improvement in functional capacity was observed. This improvement is measured by the 6MWT gait test; walking distance increases by 76.58 m at 3 months after the training. The study suggests the improvement of dyspnea, regardless of the cause; the best effects, in terms of increased walking distance, were obtained in patients with dyspnea of etiology other than cardiac or pulmonary, followed by patients with pulmonary involvement; patients with cardiac involvement have the poorest results. A study published by C. B. Moreno et al. (2018) suggested increased physiological trainability in patients with COPD (N = 193), as evidenced by the 6MWT gait test; exercise tolerance improved and walking distance increased by 20.76 m [
28]. In our study, the walking distance increased significantly (76.58 m) compared to the results of the study published by C. B. Moreno et al. because 55 of the patients in the study only underwent an educational program of disease awareness, healthy habit discussions (nutrition, physical training, physical activity in daily life), exact-herbs awareness, and vaccination. Another explanation would be that our study did not exclude patients with severe dyspnea, above level 5 on the modified Borg scale, and the group of patients was inhomogeneous in terms of dyspnea etiology.
The study conducted on 18 patients with COPD published by N Miyahara et al. (2000) supports the benefits of physical training (gymnastics to tone the respiratory muscles, the skeletal muscles, and ergometer cycling) on dyspnea, fatigue, and emotional condition for 3 weeks [
35]. Another study conducted on 30 patients (15 patients—control group), published by M. S. Ali (2014), suggests the benefits of physical training (20 min of walking, ergometer cycling, and resistance exercises, three sessions/week, three consecutive weeks) on 6MWT and exercise capacity [
36].
Patients experiencing dyspnea of cardiac origin, particularly those with heart failure (HF), often face limitations in their exercise capacity. Regarding the improvement of dyspnea and exercise capacity in patients with HF, published studies highlight the critical role of exercise [
37,
38,
39]. For instance, a study by Donna M. Mancini et al. (1995) involving 14 HF patients undergoing specific respiratory muscle training for 3 months (90 min, three times a week) indicated an increase in exercise capacity as measured by the 6-minute walk test (6MWT) [
38]. Similar findings were reported by B. Pozehl et al. (2008) [
40], where 15 HF patients participated in aerobic and endurance training three times a week for six months, demonstrating notable improvements in dyspnea and fatigue compared to a control group without rehabilitation. Furthermore, the study by Alexis L. Beatty et al. (2012, n = 556) [
41] found that the distance achieved in the 6MWT can predict subsequent cardiovascular events. The repeated-measures ANOVA method, applied to the data collected in our study, suggests the benefits of physical training on chronic dyspnea, highlighting the differences obtained at 3-month vs. first assessment. Moreover, statistical analysis of the data suggests that the mean walking distance obtained in the initial and final 6MWT test did not differ according to the etiology of dyspnea (
p > 0.05).
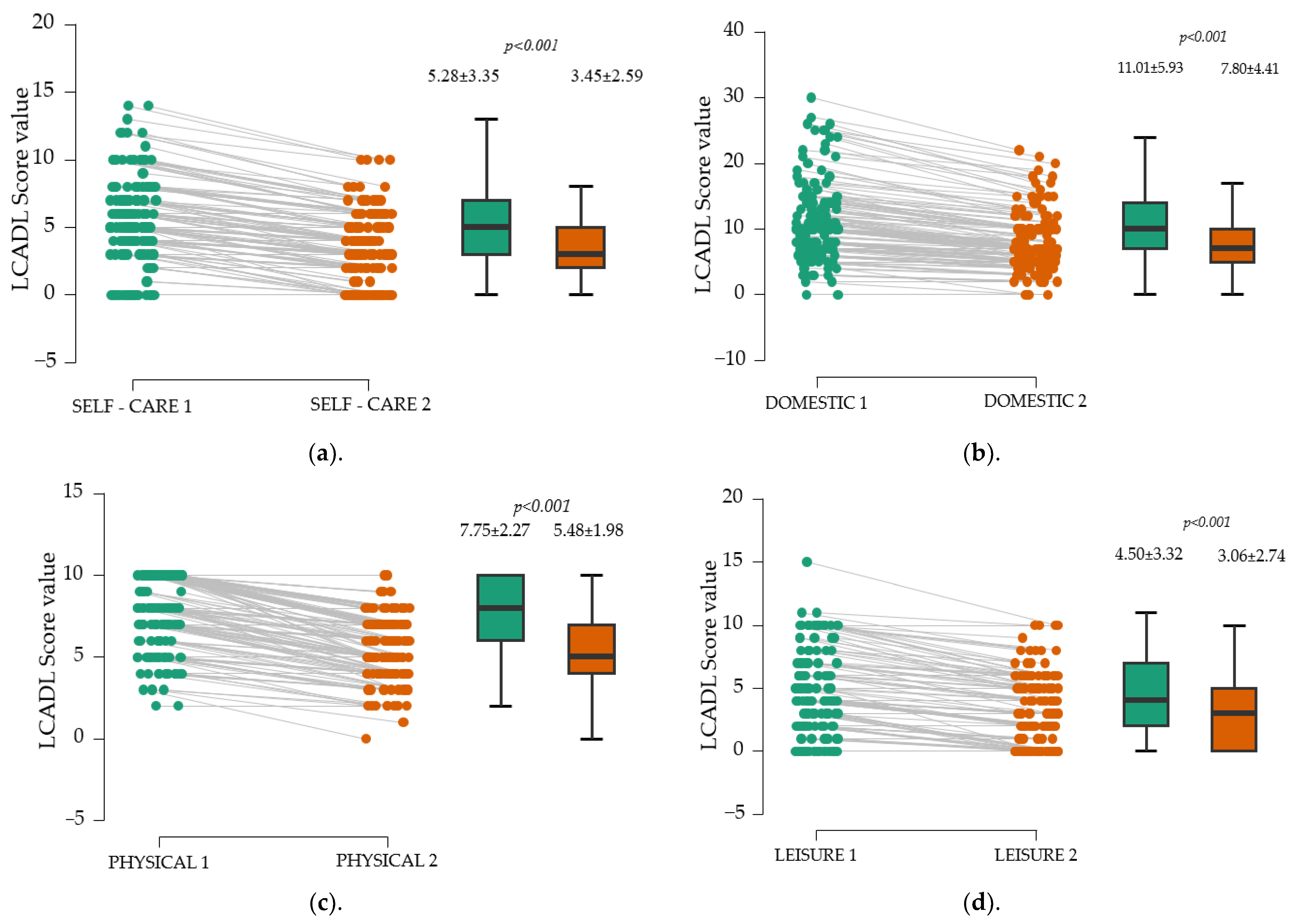
Besides enhancing walking distance, a secondary objective of our study was to evaluate the LACDL score across different domains. After three months, significant improvements were observed in all four assessed areas of the LACDL score—personal care, domestic chores, exercise, and leisure activities (p < 0.001).
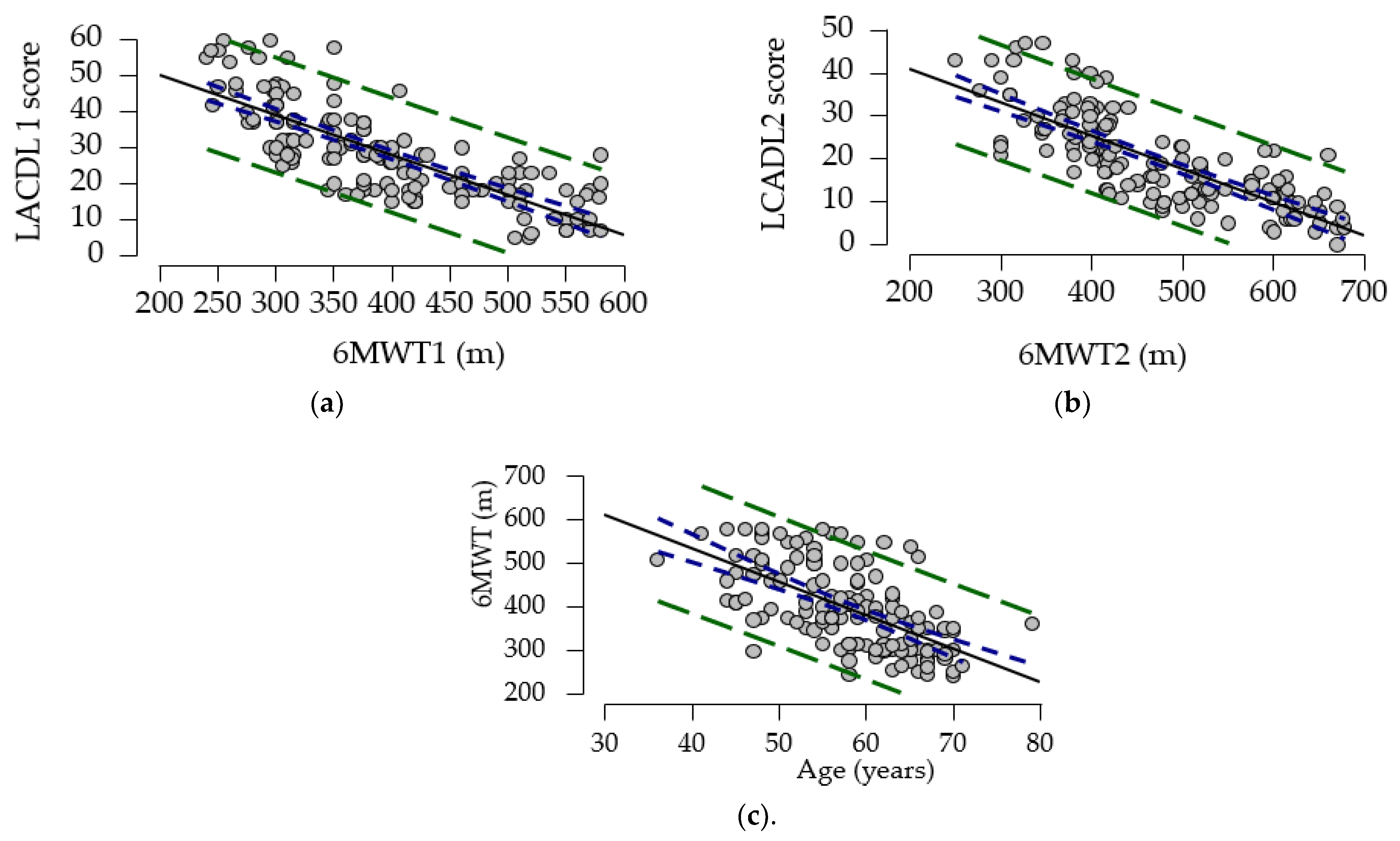
To explore the relationship between LCADL 1, 6MWT, and age, we calculated the correlation coefficient. Our study showed a strong negative association between the gait test and LCADL score compared to the results of a cross-sectional study (N = 44) by Ozsoy I. et al. (2019), who reported a low to moderate association [
42]. To further elucidate the relationships between these variables, a multivariate regression model was employed. This model identified significant predictors for the 6MWT, including age, LCADL score, dyspnea score, and cardiac etiology. Similar associations between the 6MWT and LCADL were noted in the research conducted by Ozsoy I. et al. [
42]. Moreover, in a study by Y. Al Chikhanie et al. (2021), age and dyspnea levels were also identified as predictors for the 6MWT in patients with COPD [
43]. These findings collectively emphasize the importance of targeted exercise interventions in improving outcomes for patients with heart failure and related conditions.
Strengths and Limitations of the Study
This study is among the few studies that assess functional limitation by domains—personal care, domestic chores, exercise, and leisure activities. It is also the first study in Romania that aims to identify the predictive factors that limit the 6 min walk test. The main limitations of the study are primarily due to the inhomogeneity of the study group, from an etiological point of view, the lack of determination of the grip strength of the hands and the strength of the quadriceps muscle. Monitoring adherence to physical training at home is a limitation of the study, but to reduce this, patients were encouraged to keep a diary or purchase a fitness bracelet. The exclusion of patients with high effort scores (Borg score > 5) may introduce a selection bias, causing the results to be influenced by the characteristics of the patients included in the study. Thus, an improvement in outcomes may be observed that is not representative of the entire patient population. The study findings may apply only to a small subset of patients.