Reduction in Brain Parenchymal Volume Correlates with Depression and Cognitive Decline in HIV-Positive Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuroimaging
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Correlations Between Key Variables
3.3. Correlations Between Volumes and Neurocognitive Tests
3.4. Correlation of Depression Scale with Volumes
3.5. Multiple Regression Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gisslén, M.; Price, R.W.; Nilsson, S. The definition of HIV-associated neurocognitive disorders: Are we overestimating the real prevalence? BMC Infect. Dis. 2011, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Gisslen, M.; Keating, S.M.; Spudich, S.; Arechiga, V.; Stephenson, S.; Zetterberg, H.; Di Germanio, C.; Blennow, K.; Fuchs, D.; Hagberg, L.; et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury, and viral suppression. PLoS ONE 2021, 16, e0247897. [Google Scholar] [CrossRef] [PubMed]
- van Schalkwyk, C.; Mahy, M.; Johnson, L.F.; Imai-Eaton, J.W. Updated data and methods for the 2023 UNAIDS HIV estimates. J. Acquir. Immune Defic. Syndr. 2024, 95, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.A.W.P.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. International research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Adewumi, O.M.; Dukhovlinova, E.; Shehu, N.Y.; Zhou, S.; Council, O.D.; Akanbi, M.O.; Taiwo, B.; Ogunniyi, A.; Robertson, K.; Kanyama, C.; et al. HIV-1 central nervous system compartmentalization and cytokine interplay in non-subtype B HIV-1 infections in Nigeria and Malawi. AIDS Res. Hum. Retroviruses 2020, 36, 490–500. [Google Scholar] [CrossRef]
- Fitri, F.I.; Rambe, A.S.; Fitri, A. Correlation between Lymphocyte CD4 Count, Treatment Duration, Opportunistic Infection and Cognitive Function in Human Immunodeficiency Virus-Acquired Immunodeficiency Syndrome (HIV-AIDS) Patients. Open Access Maced. J. Med. Sci. 2018, 6, 643–647. [Google Scholar] [CrossRef]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef]
- Airoldi, M.; Bandera, A.; Trabattoni, D.; Tagliabue, B.; Arosio, B.; Soria, A.; Rainone, V.; Lapadula, G.; Annoni, G.; Clerici, M.; et al. Neurocognitive impairment in HIV-infected naive patients with advanced disease: The role of virus and intrathecal immune activation. Clin. Dev. Immunol. 2012, 2012, 467154. [Google Scholar] [CrossRef]
- Grant, I. Neurocognitive and neuropsychiatric complications of HIV infection. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 281–300. [Google Scholar]
- Bing, E.G.; Burnam, M.A.; Longshore, D.; Fleishman, J.A.; Sherbourne, C.D.; London, A.S.; Turner, B.J.; Eggan, F.; Beckman, R.; Vitiello, B.; et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry 2001, 58, 721–728. [Google Scholar] [CrossRef]
- Simioni, S.; Cavassini, M.; Annoni, J.-M.; Abraham, A.R.; Bourquin, I.; Schiffer, V.; Calmy, A.; Chave, J.-P.; Giacobini, E.; Hirschel, B.; et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010, 24, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Brew, B.J. HIV associated neurocognitive disorders in the modern antiviral treatment era: Prevalence, characteristics, biomarkers, and effects of treatment. Curr. HIV/AIDS Rep. 2014, 11, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, N.; Fabbiani, M.; Colafigli, M.; Trecarichi, E.M.; Silveri, M.C.; Cauda, R.; Murri, R.; De Luca, A.; Di Giambenedetto, S. Revised central nervous system neuro penetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viremia. Antivir. Ther. 2013, 18, 153–160. [Google Scholar] [CrossRef]
- McArthur, J.C. HIV dementia: An evolving disease. J. Neuroimmunol. 2004, 157, 3–10. [Google Scholar] [CrossRef]
- Rezaei, S.; Ahmadi, S.; Rahmati, J.; Hosseinifard, H.; Dehnad, A.; Aryankhesal, A.; Shabaninejad, H.; Ghasemyani, S.; Alihosseini, S.; Bragazzi, N.L.; et al. Global prevalence of depression in HIV/AIDS: A systematic review and meta-analysis. BMJ Support. Palliat. Care 2019, 9, 404–412. [Google Scholar] [CrossRef]
- Desta, F.; Tasew, A.; Tekalegn, Y.; Zenbaba, D.; Sahiledengle, B.; Assefa, T.; Negash, W.; Tahir, A.; Regasa, T.; Mamo, A.; et al. Prevalence of depression and associated factors among people living with HIV/AIDS in public hospitals of Southeast Ethiopia. BMC Psychiatry 2022, 22, 557. [Google Scholar] [CrossRef]
- Mohammed, M.; Mengistie, B.; Dessie, Y.; Godana, W. Prevalence of depression and associated factors among HIV patients seeking treatments in art clinics at Harar town, eastern Ethiopia. J. AIDS Clin. Res. 2015, 6, 1–6. [Google Scholar]
- Diminić-Lisica, I.; Francisković, T.; Janović, S.; Popović, B.; Klarić, M.; Nemcić-Moro, I. Comorbid chronic diseases in depressed and non-depressed patients in family practice. Psychiatr. Danub 2010, 22, 236–240. [Google Scholar]
- Brucki, S.M.; Nitrini, R.; Caramelli, P.; Bertolucci, P.H.; Okamoto, I.H. Sugestões para o uso do mini-exame do estado mental no Brasil [Suggestions for utilization of the mini-mental state examination in Brazil]. Arq. Neuropsiquiatr. 2003, 61, 777–781. [Google Scholar] [CrossRef]
- Savard, J.; Laberge, B.; Gauthier, J.G.; Bergeron, M.G. Screening Clinical Depression in HIV-Seropositive Patients Using the Hospital Anxiety and Depression Scale. AIDS Behav. 1999, 3, 167–175. [Google Scholar] [CrossRef]
- Shan, L.; Siliciano, R.F. From reactivation of latent HIV-1 to elimination of the latent reservoir: The presence of multiple barriers to viral eradication. Bioessays 2013, 35, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.D.; Vinters, H.V.; Williams, C.K.; Mareninov, S.; Khanlou, N.; Said, J.; Nemanim, N.; Gonzalez, J.; Morales, J.G.; Singer, E.J.; et al. Neuropathologic findings in elderly HIV-positive individuals. J. Neuropathol. Exp. Neurol. 2022, 81, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Damasio, H.; Grabowski, T. Normal neuroanatomical variation in the human brain: An MRI-volumetric study. Am. J. Phys. Anthropol. 2002, 118, 341–358. [Google Scholar] [CrossRef]
- Available online: https://www.batut.org.rs/index.php?content=2918 (accessed on 26 February 2025).
- Erickson, K.I.; Colcombe, S.J.; Raz, N.; Korol, D.L.; Scalf, P.; Webb, A.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol. Aging 2005, 26, 1205–1213. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P. volBrain: An Online MRI Brain Volumetry System. Front. Neuroinformatics 2016, 10, 30. [Google Scholar] [CrossRef]
- Boban, J.; Thurnher, M.M.; Boban, N.; Law, M.; Jahanshad, N.; Nir, T.M.; Lendak, D.F.; Kozic, D. Gradient Patterns of Age-Related Diffusivity Changes in Cerebral White Matter. Front. Neurol. 2022, 13, 870909. [Google Scholar] [CrossRef]
- Baxter, M.G.; Chiba, A.A. Cognitive functions of the basal forebrain. Curr. Opin. Neurobiol. 1999, 9, 178–183. [Google Scholar] [CrossRef]
- Gollan, J.K.; Dong, H.; Bruno, D.; Nierenberg, J.; Nobrega, J.N.; Grothe, M.J.; Pollock, B.G.; Marmar, C.R.; Teipel, S.; Csernansky, J.G.; et al. Basal forebrain mediated increase in brain CRF is associated with increased cholinergic tone and depression. Psychiatry Res. Neuroimaging 2017, 264, 76–81. [Google Scholar] [CrossRef]
- Jumah, F.R.; Dossani, R.H. Neuroanatomy, Cingulate Cortex; Updated 6 December 2022; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537077 (accessed on 26 February 2025).
- Castrén, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef]
- Lima, V.D.; Geller, J.; Bangsberg, D.R.; Patterson, T.L.; Daniel, M.; Kerr, T.; Montaner, J.S.; Hogg, R.S. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS 2007, 21, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Arnone, D.; Job, D.; Selvaraj, S.; Abe, O.; Amico, F.; Cheng, Y.; Colloby, S.J.; O’Brien, J.T.; Frodl, T.; Gotlib, I.H.; et al. Computational meta-analysis of statistical parametric maps in major depression. Hum. Brain Mapp. 2016, 37, 1393–1404. [Google Scholar] [CrossRef]
- Schermuly, I.; Wolf, D.; Lieb, K.; Stoeter, P.; Fellgiebel, A. State-dependent posterior hippocampal volume increases in patients with major depressive disorder. J. Affect. Disord. 2011, 135, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bronshteyn, M.; Yang, F.N.; Shattuck, K.F.; Dawson, M.; Kumar, P.; Moore, D.J.; Ellis, R.J.; Jiang, X. Depression is associated with hippocampal volume loss in adults with HIV. Hum. Brain Mapp. 2021, 42, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Peric, R.; Romčević, I.; Mastilović, M.; Starčević, I.; Boban, J. Age-related volume decrease in subcortical gray matter is a part of healthy brain aging in men. Ir. J. Med. Sci. 2024, 194, 339–345, Erratum in Ir. J. Med. Sci. 2025, 194, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Convery, R.S.; Neason, M.R.; Cash, D.M.; Cardoso, M.J.; Modat, M.; Ourselin, S.; Warren, J.D.; Rohrer, J.D.; Bocchetta, M. Basal forebrain atrophy in frontotemporal dementia. Neuroimage Clin. 2020, 26, 102210. [Google Scholar] [CrossRef]
- Kilimann, I.; Grothe, M.; Heinsen, H.; Alho, E.J.; Grinberg, L.; Amaro, E., Jr.; Dos Santos, G.A.; da Silva, R.E.; Mitchell, A.J.; Frisoni, G.B.; et al. Subregional basal forebrain atrophy in Alzheimer’s disease: A multicenter study. J. Alzheimers Dis. 2014, 40, 687–700. [Google Scholar] [CrossRef]
- Grothe, M.J.; Heinsen, H.; Amaro, E., Jr.; Grinberg, L.T.; Teipel, S.J. Cognitive Correlates of Basal Forebrain Atrophy and Associated Cortical Hypometabolism in Mild Cognitive Impairment. Cereb. Cortex. 2016, 26, 2411–2426. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Mesulam, M.M. The cholinergic innervation of the human cerebral cortex. Prog. Brain Res. 2004, 145, 67–78. [Google Scholar] [CrossRef]
- Davidson, R.J. Toward a biology of personality and emotion. Ann. N. Y. Acad. Sci. 2002, 935, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Kallianpur, K.J.; Jahanshad, N.; Sailasuta, N.; Benjapornpong, K.; Chan, P.; Pothisri, M.; Dumrongpisutikul, N.; Laws, E.; Ndhlovu, L.C.; Clifford, K.M.; et al. Regional brain volumetric changes despite 2 years of treatment initiated during acute HIV infection. AIDS 2020, 34, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Sun, G.; Ji, J.; Li, Z.; Chen, X.; Zhang, X.; Ma, Y.; Zhang, Y.; Zhang, T.; Zhang, Y. Brain function abnormalities and neuroinflammation in people living with HIV-associated anxiety disorders. Front. Psychiatry 2024, 15, 1336233. [Google Scholar] [CrossRef] [PubMed]
- Philippi, C.L.; Reyna, L.; Nedderman, L.; Chan, P.; Samboju, V.; Chang, K.; Phanuphak, N.; Ratnaratorn, N.; Hellmuth, J.; Benjapornpong, K.; et al. Resting-state neural signatures of depressive symptoms in acute HIV. J. Neurovirol. 2020, 26, 226–240. [Google Scholar] [CrossRef]
- Anthony, I.C.; Bell, J.E. Neuropathological findings associated with long-term human immunodeficiency virus infection. Curr. Opin. Neurol. 2008, 21, 695–700. [Google Scholar]
| Characteristics | Number of Participants | Sex | Age (Years) | MMSE Score | Education Level (Years) | BDI Score | cART |
|---|---|---|---|---|---|---|---|
| Mean ± SD | 48 | Males | 39.26 ± 10.66 | 28.10 ± 1.43 | 12.54 ± 2.70 | 9.24 ± 6.78 | All participants |
| Structures | MMSE | |
|---|---|---|
| Accumbens total | Pearson correlation coefficient (r) | 0.090 |
| P | 0.566 | |
| N | 48 | |
| Accumbens right | Pearson correlation coefficient (r) | 0.014 |
| P | 0.928 | |
| N | 48 | |
| Accumbens left | Pearson correlation coefficient (r) | 0.165 |
| P | 0.289 | |
| N | 48 | |
| Corpus amygdaloideum total | Pearson correlation coefficient (r) | 0.119 |
| P | 0.443 | |
| N | 48 | |
| Corpus amygdaloideum right | Pearson correlation coefficient (r) | 0.060 |
| P | 0.703 | |
| N | 48 | |
| Corpus amygdaloideum left | Pearson correlation coefficient (r) | 0.175 |
| P | 0.262 | |
| N | 48 | |
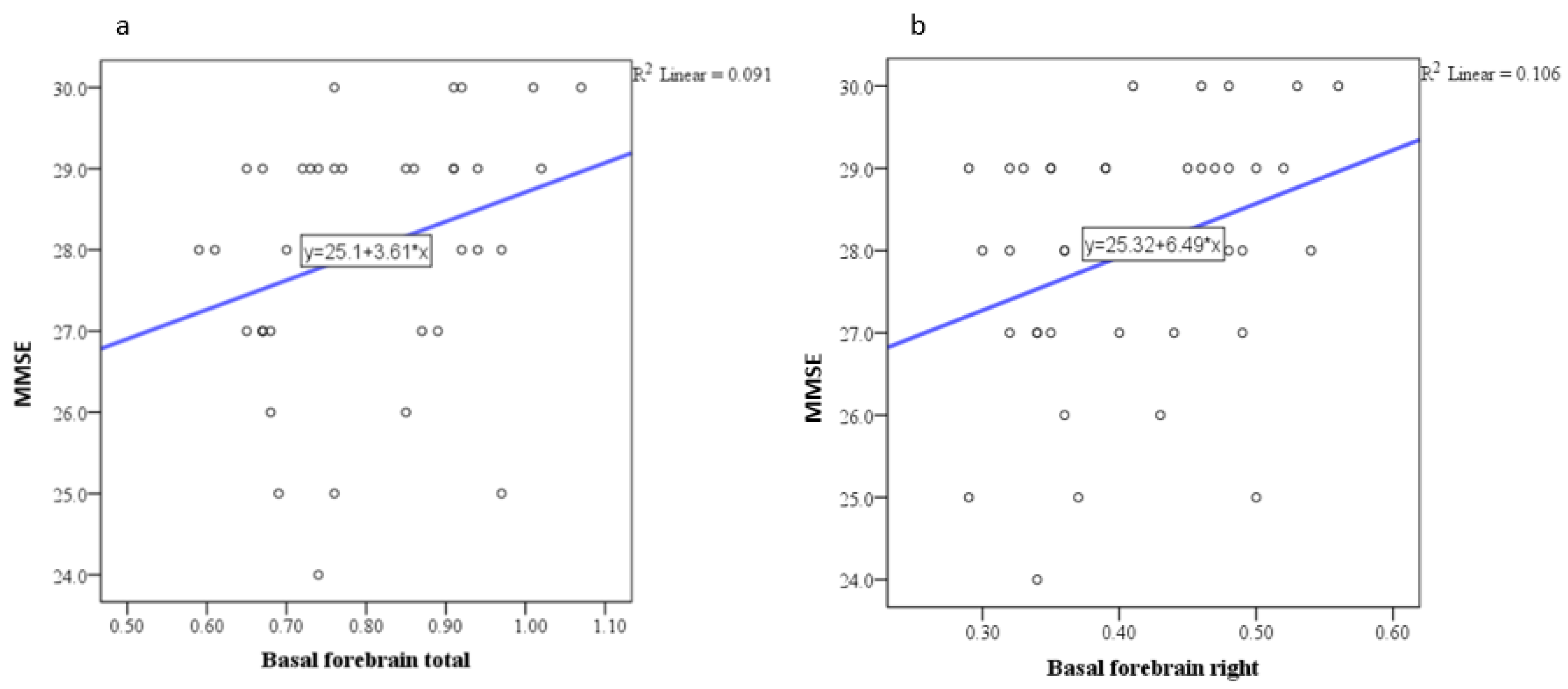
| Basal forebrain | Pearson correlation coefficient (r) | 0.304 |
| P | 0.047 | |
| N | 48 | |
| Basal forebrain right | Pearson correlation coefficient (r) | 0.326 |
| P | 0.033 | |
| N | 48 | |
| Basal forebrain left | Pearson correlation coefficient (r) | 0.214 |
| P | 0.169 | |
| N | 48 | |
| Nucleus caudatus total | Pearson correlation coefficient (r) | 0.186 |
| P | 0.233 | |
| N | 48 | |
| Nucleus caudatus right | Pearson correlation coefficient (r) | 0.230 |
| P | 0.138 | |
| N | 48 | |
| Nucleus caudatus left | Pearson correlation coefficient (r) | 0.142 |
| P | 0.365 | |
| N | 48 | |
| Hippocampus total | Pearson correlation coefficient (r) | 0.173 |
| P | 0.266 | |
| N | 48 | |
| Hippocampus right | Pearson correlation coefficient (r) | 0.138 |
| P | 0.379 | |
| N | 48 | |
| Hippocampus left | Pearson correlation coefficient (r) | 0.202 |
| P | 0.194 | |
| N | 48 | |
| Globus pallidus total | Pearson correlation coefficient (r) | 0.023 |
| P | 0.883 | |
| N | 48 | |
| Globus pallidus right | Pearson correlation coefficient (r) | 0.015 |
| P | 0.923 | |
| N | 48 | |
| Globus pallidus left | Pearson correlation coefficient (r) | 0.027 |
| P | 0.864 | |
| N | 48 | |
| Putamen total | Pearson correlation coefficient (r) | 0.227 |
| P | 0.144 | |
| N | 48 | |
| Putamen right | Pearson correlation coefficient (r) | 0.200 |
| P | 0.199 | |
| N | 48 | |
| Putamen left | Pearson correlation coefficient (r) | 0.251 |
| P | 0.104 | |
| N | 48 | |
| Thalamus total | Pearson correlation coefficient (r) | 0.198 |
| P | 0.209 | |
| N | 42 | |
| Thalamus right | Pearson correlation coefficient (r) | 0.193 |
| P | 0.215 | |
| N | 48 | |
| Thalamus left | Pearson correlation coefficient (r) | 0.203 |
| P | 0.191 | |
| N | 48 | |
| Ventral diencephalon total | Pearson correlation coefficient (r) | 0.174 |
| P | 0.265 | |
| N | 48 | |
| Ventral diencephalon right | Pearson correlation coefficient (r) | 0.179 |
| P | 0.251 | |
| N | 48 | |
| Ventral diencephalon left | Pearson correlation coefficient (r) | 0.155 |
| P | 0.322 | |
| N | 48 | |
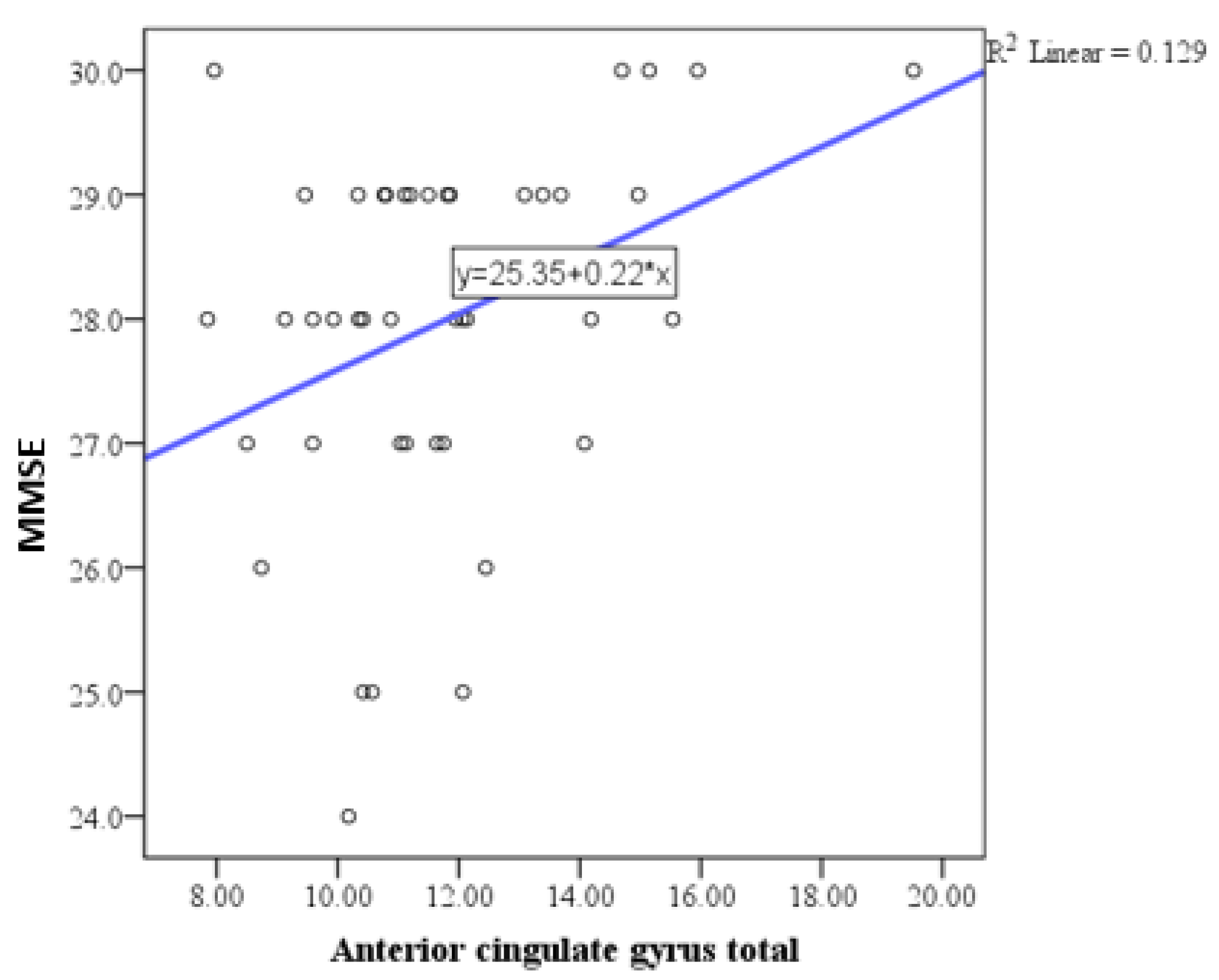
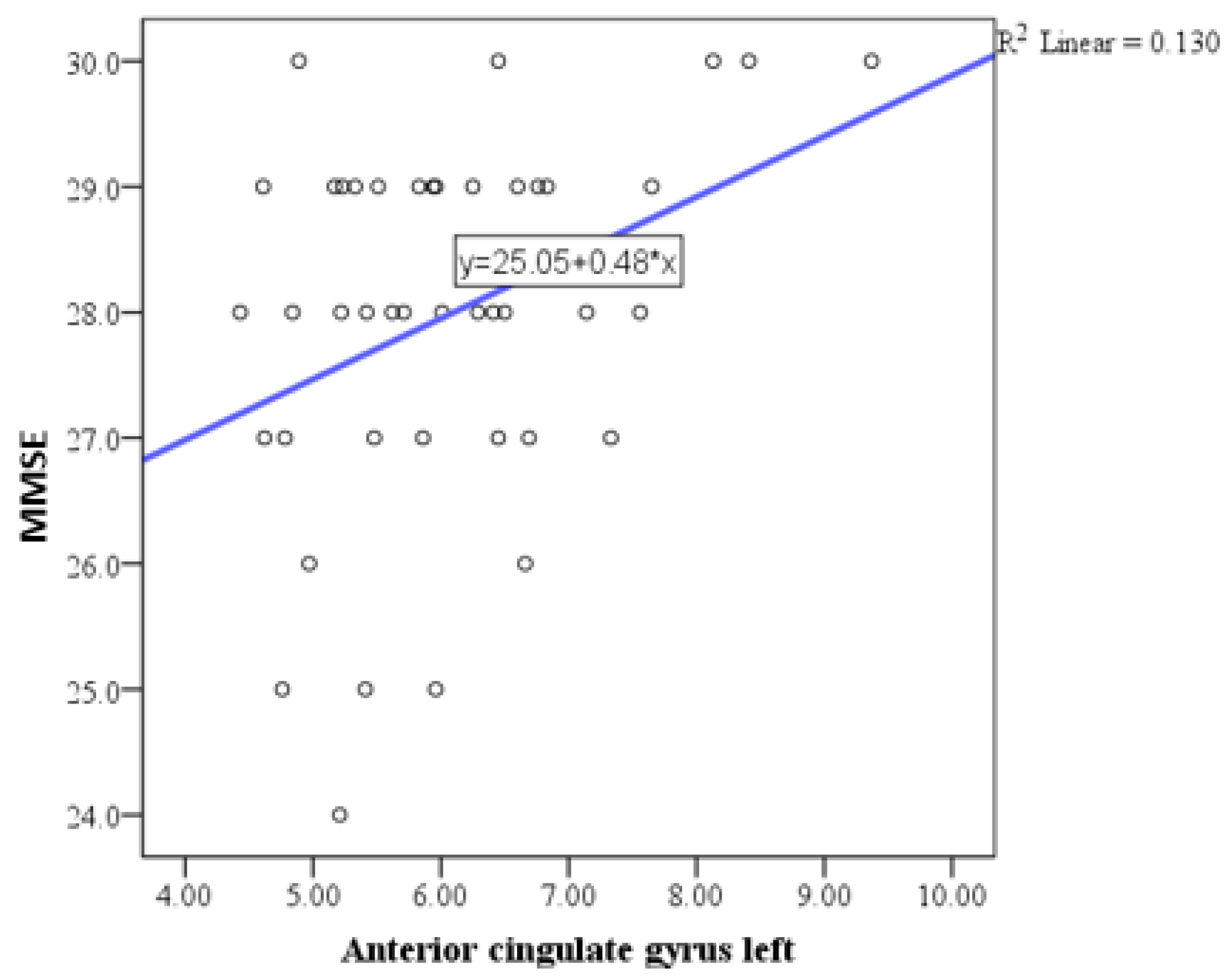
| Anterior cingulate gyrus total | Pearson correlation coefficient (r) | 0.359 |
| P | 0.018 | |
| N | 48 | |
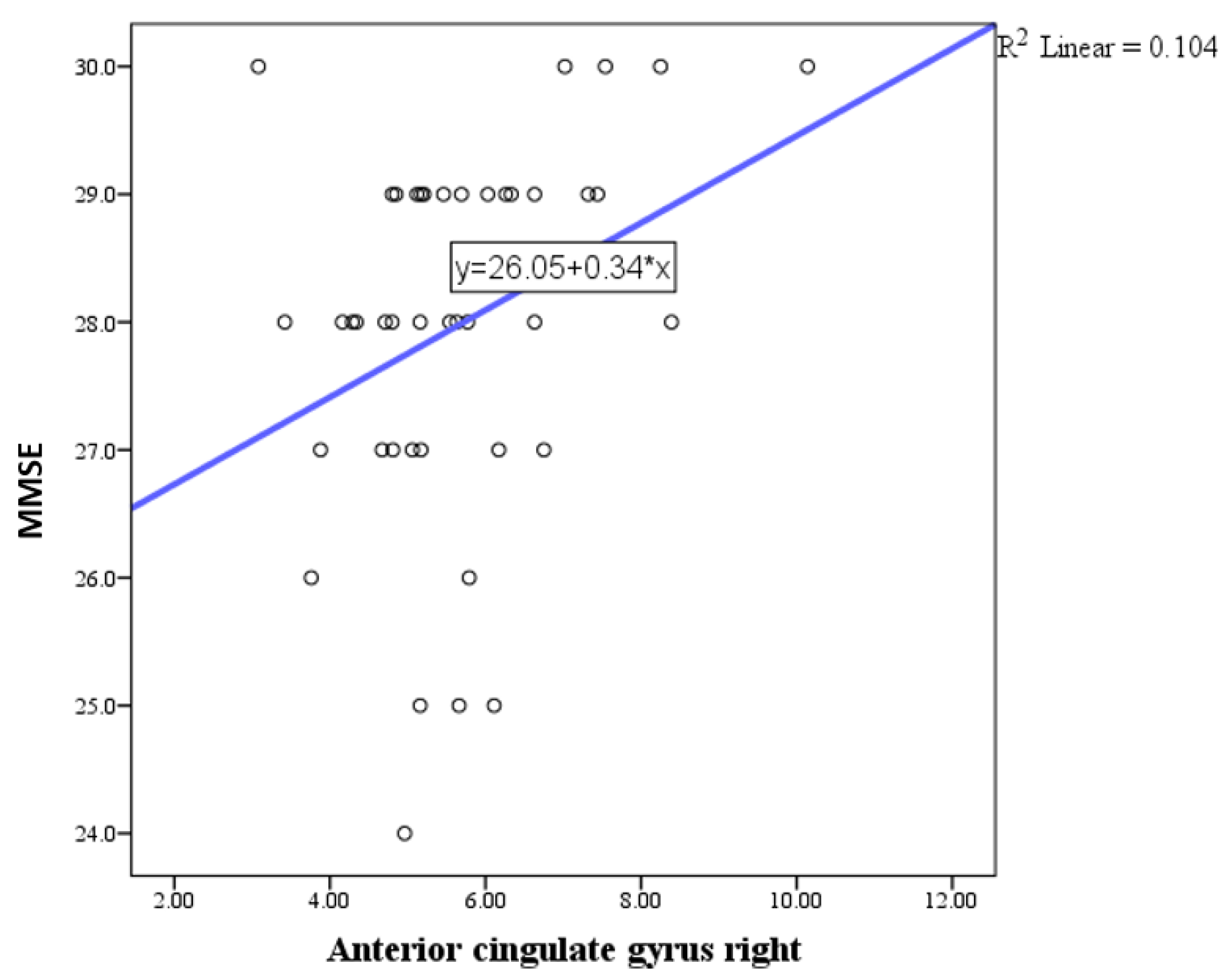
| Anterior cingulate gyrus right | Pearson correlation coefficient (r) | 0.323 |
| P | 0.035 | |
| N | 48 | |
| Anterior cingulate gyrus left | Pearson correlation coefficient (r) | 0.361 |
| P | 0.017 | |
| N | 48 | |
| Middle cingulate gyrus total | Pearson correlation coefficient (r) | 0.287 |
| P | 0.063 | |
| N | 48 | |
| Middle cingulate gyrus right | Pearson correlation coefficient (r) | 0.206 |
| P | 0.184 | |
| N | 48 | |
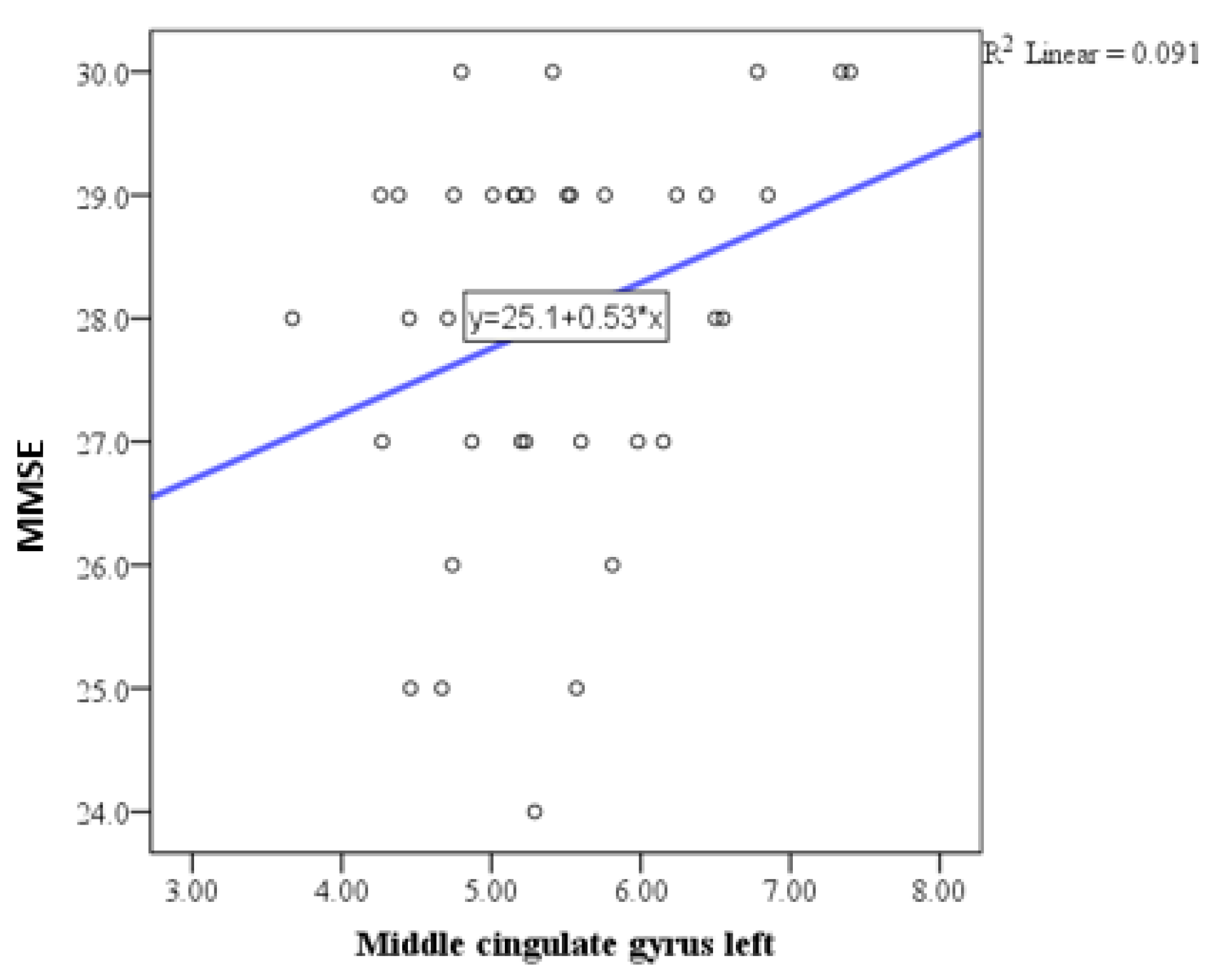
| Middle cingulate gyrus left | Pearson correlation coefficient (r) | 0.301 |
| P | 0.050 | |
| N | 48 | |
| Posterior cingulate gyrus total | Pearson correlation coefficient (r) | 0.264 |
| P | 0.087 | |
| N | 48 | |
| Posterior cingulate gyrus right | Pearson correlation coefficient (r) | 0.218 |
| P | 0.159 | |
| N | 48 | |
| Posterior cingulate gyrus left | Pearson correlation coefficient (r) | 0.275 |
| P | 0.075 | |
| N | 48 | |
| Structures | BDI Depression Scale | |
|---|---|---|
| Nucleus accumbens total | Pearson correlation coefficient (r) | 0.019 |
| P | 0.915 | |
| N | 35 | |
| Nucleus accumbens right | Pearson correlation coefficient (r) | −0.028 |
| P | 0.875 | |
| N | 35 | |
| Nucleus accumbens left | Pearson correlation coefficient (r) | 0.064 |
| P | 0.714 | |
| N | 35 | |
| Corpus amygdaloideum total | Pearson correlation coefficient (r) | −0.020 |
| P | 0.909 | |
| N | 35 | |
| Corpus amygdaloideum right | Pearson correlation coefficient (r) | 0.039 |
| P | 0.824 | |
| N | 35 | |
| Corpus amygdaloideum left | Pearson correlation coefficient (r) | −0.093 |
| P | 0.596 | |
| N | 35 | |
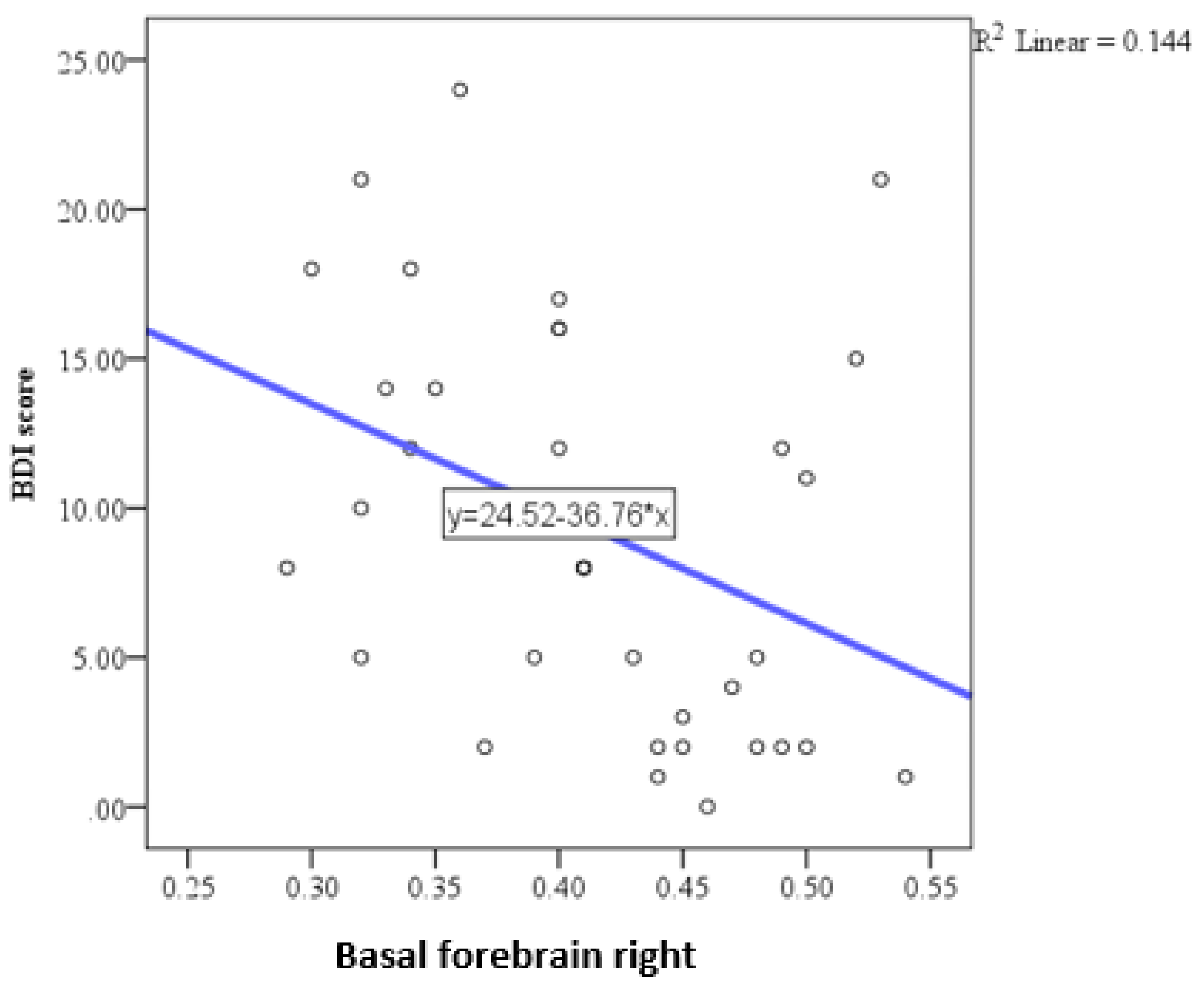
| Basal forebrain total | Pearson correlation coefficient (r) | −0.323 |
| P | 0.059 | |
| N | 35 | |
| Basal forebrain right | Pearson correlation coefficient (r) | −0.379 |
| P | 0.025 | |
| N | 35 | |
| Basal forebrain left | Pearson correlation coefficient (r) | −0.204 |
| P | 0.241 | |
| N | 35 | |
| Nucleus caudatus total | Pearson correlation coefficient (r) | 0.042 |
| P | 0.809 | |
| N | 35 | |
| Nucleus caudatus right | Pearson correlation coefficient (r) | 0.037 |
| P | 0.833 | |
| N | 35 | |
| Nucleus caudatus left | Pearson correlation coefficient (r) | 0.047 |
| P | 0.790 | |
| N | 35 | |
| Hippocampus total | Pearson correlation coefficient (r) | −0.213 |
| P | 0.219 | |
| N | 35 | |
| Hippocampus right | Pearson correlation coefficient (r) | −0.182 |
| P | 0.294 | |
| N | 35 | |
| Hippocampus left | Pearson correlation coefficient (r) | −0.245 |
| P | 0.156 | |
| N | 35 | |
| Globus pallidus total | Pearson correlation coefficient (r) | 0.037 |
| P | 0.833 | |
| N | 35 | |
| Globus pallidus right | Pearson correlation coefficient (r) | 0.018 |
| P | 0.917 | |
| N | 35 | |
| Globus pallidus left | Pearson correlation coefficient (r) | 0.048 |
| P | 0.783 | |
| N | 35 | |
| Putamen total | Pearson correlation coefficient (r) | −0.122 |
| P | 0.487 | |
| N | 35 | |
| Putamen right | Pearson correlation coefficient (r) | −0.113 |
| P | 0.516 | |
| N | 35 | |
| Putamen left | Pearson correlation coefficient (r) | −0.126 |
| P | 0.471 | |
| N | 35 | |
| Thalamus total | Pearson correlation coefficient (r) | 0.012 |
| P | 0.947 | |
| N | 35 | |
| Thalamus right | Pearson correlation coefficient (r) | 0.023 |
| P | 0.897 | |
| N | 35 | |
| Thalamus left | Pearson correlation coefficient (r) | −0.003 |
| P | 0.985 | |
| N | 35 | |
| Ventral DC total | Pearson correlation coefficient (r) | −0.085 |
| P | 0.626 | |
| N | 35 | |
| Ventral DC right | Pearson correlation coefficient (r) | −0.064 |
| P | 0.713 | |
| N | 35 | |
| Ventral DC left | Pearson correlation coefficient (r) | −0.111 |
| P | 0.526 | |
| N | 35 | |
| Anterior cingulate gyrus total | Pearson correlation coefficient (r) | 0.003 |
| P | 0.985 | |
| N | 35 | |
| Anterior cingulate gyrus right | Pearson correlation coefficient (r) | −0.080 |
| P | 0.648 | |
| N | 35 | |
| Anterior cingulate gyrus left | Pearson correlation coefficient (r) | 0.106 |
| P | 0.548 | |
| N | 35 | |
| Middle cingulate gyrustotal | Pearson correlation coefficient (r) | −0.024 |
| P | 0.889 | |
| N | 35 | |
| Middle cingulate gyrus right | Pearson correlation coefficient (r) | −0.013 |
| P | 0.941 | |
| N | 35 | |
| Middle cingulate gyrus left | Pearson correlation coefficient (r) | −0.030 |
| P | 0.864 | |
| N | 35 | |
| Posterior cingulate gyrustotal | Pearson correlation coefficient (r) | 0.075 |
| P | 0.669 | |
| N | 35 | |
| Posterior cingulate gyrus right | Pearson correlation coefficient (r) | 0.087 |
| P | 0.620 | |
| N | 35 | |
| Posterior cingulate gyrus left | Pearson correlation coefficient (r) | 0.046 |
| P | 0.794 | |
| N | 35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perić, R.; Kozić, D.; Brkić, S.; Lendak, D.; Ostojić, J.; Bugarski Ignjatović, V.; Boban, J. Reduction in Brain Parenchymal Volume Correlates with Depression and Cognitive Decline in HIV-Positive Males. Medicina 2025, 61, 632. https://doi.org/10.3390/medicina61040632
Perić R, Kozić D, Brkić S, Lendak D, Ostojić J, Bugarski Ignjatović V, Boban J. Reduction in Brain Parenchymal Volume Correlates with Depression and Cognitive Decline in HIV-Positive Males. Medicina. 2025; 61(4):632. https://doi.org/10.3390/medicina61040632
Chicago/Turabian StylePerić, Radmila, Duško Kozić, Snežana Brkić, Dajana Lendak, Jelena Ostojić, Vojislava Bugarski Ignjatović, and Jasmina Boban. 2025. "Reduction in Brain Parenchymal Volume Correlates with Depression and Cognitive Decline in HIV-Positive Males" Medicina 61, no. 4: 632. https://doi.org/10.3390/medicina61040632
APA StylePerić, R., Kozić, D., Brkić, S., Lendak, D., Ostojić, J., Bugarski Ignjatović, V., & Boban, J. (2025). Reduction in Brain Parenchymal Volume Correlates with Depression and Cognitive Decline in HIV-Positive Males. Medicina, 61(4), 632. https://doi.org/10.3390/medicina61040632