Novel Insights into Addiction Management: A Meta-Analysis on Intervention for Relapse Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- Participants: studies that include patients diagnosed with alcohol use disorder (AUD) and high-risk drug addiction who were enrolled in relapse prevention programs. Participants were selected based on predefined eligibility criteria, including the severity of addiction, willingness to participate, and engagement in structured relapse prevention interventions.
- Study Design: Studies were selected based on specific inclusion criteria, such as publication date (e.g., studies published within the last 10 years), peer-reviewed status, and language (English only). These criteria were established to ensure the inclusion of high-quality, recent, and accessible evidence. Randomized trials were prioritized to minimize bias and establish causal relationships, while the cross-sectional study provided additional insights into population characteristics and trends.
- Intervention: Participants received various interventions, including pharmacological (e.g., medications like naltrexone or acamprosate) and non-pharmacological approaches (e.g., cognitive-behavioral therapy, motivational interviewing, and contingency management). The selection of interventions was based on their evidence-based efficacy in relapse prevention and their applicability to the target population.
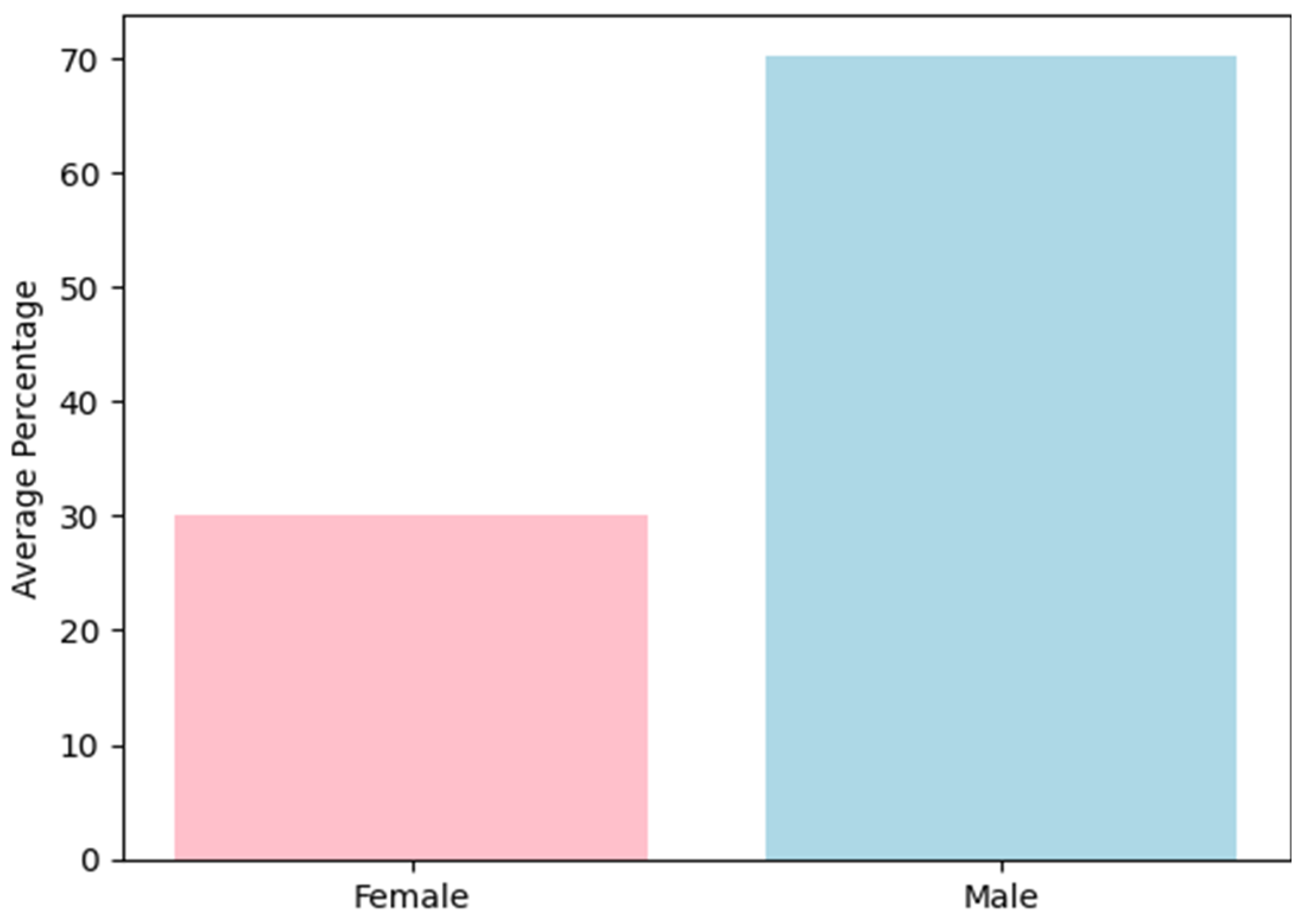
- Outcomes: The studies reported key outcomes such as gender distribution, type of addiction (alcohol vs. drug), and the effectiveness of interventions in reducing relapse rates. The primary outcome measure was the average relapse period, reported in months. Secondary outcomes included adherence to treatment, quality of life, and adverse effects of interventions.
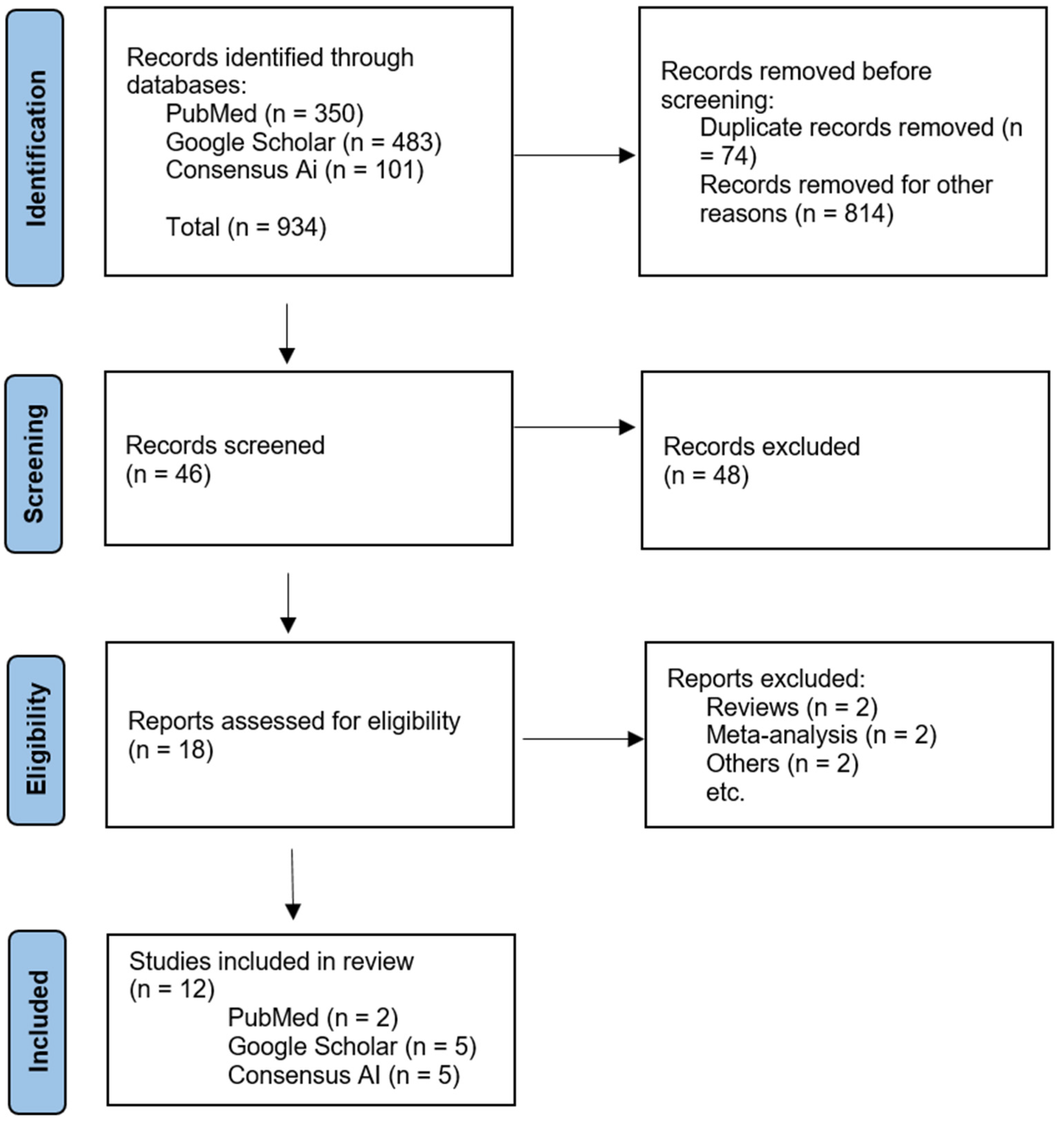
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Analysis
3. Results
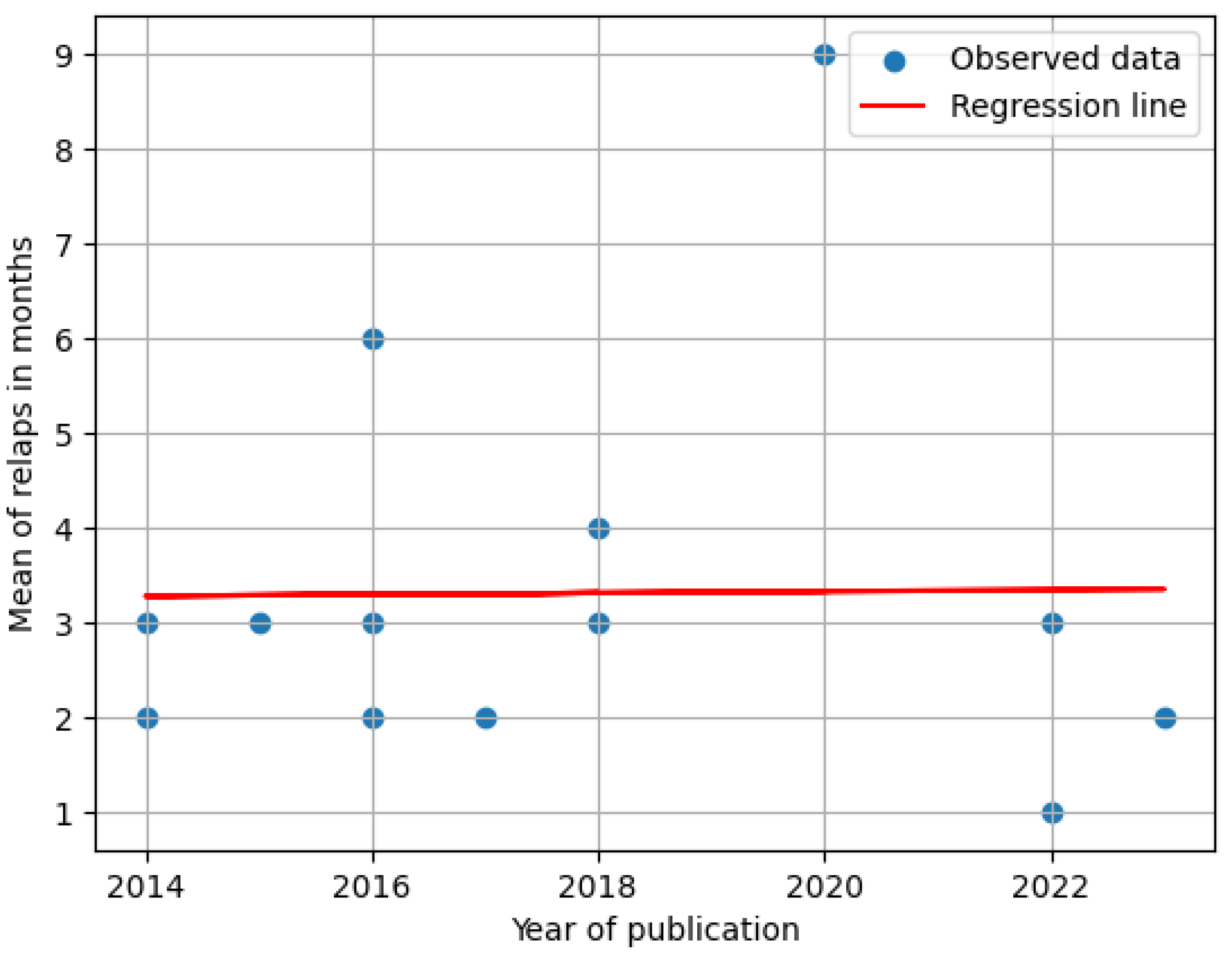
3.1. Correlation of the Mean Period of Relapse in Studies over Other Characteristics
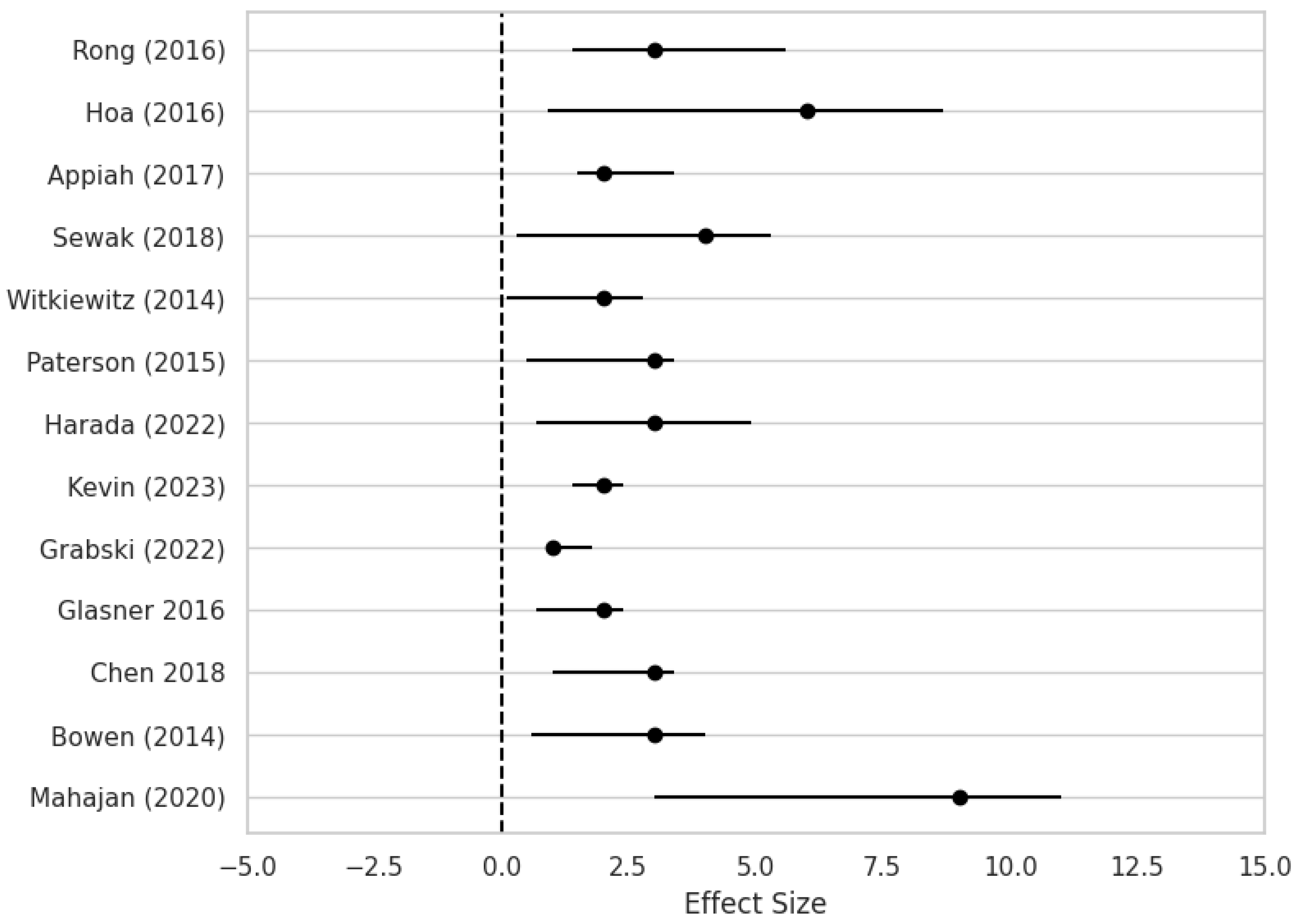
3.2. Effect of Interventions in Different Types of Addiction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, S.J.E.; Lee, H. Relapse to Substance Use: A Concept Analysis. Nurs. Forum 2020, 55, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, H.; Shen, J.; Wen, P.; Liu, X.; Hao, W. Estimating Prevalence of Illicit Drug Use in Yunnan, China, 2011–2015. Front. Psychiatry 2018, 9, 256. [Google Scholar] [CrossRef]
- Kuteesa, M.O.; Weiss, H.A.; Cook, S.; Seeley, J.; Ssentongo, J.N.; Kizindo, R.; Ngonzi, P.; Sewankambo, M.; Webb, E.L. Epidemiology of Alcohol Misuse and Illicit Drug Use Among Young People Aged 15–24 Years in Fishing Communities in Uganda. Int. J. Environ. Res. Public Health 2020, 17, 2401. [Google Scholar] [CrossRef]
- Barnett, B.S.; Parker, S.E.; Weleff, J. United States National Institutes of Health Grant Funding for Psychedelic-Assisted Therapy Clinical Trials from 2006–2020. Int. J. Drug Policy 2022, 99, 103473. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.; Wenzel, K.; Scodes, J.; Pavlicova, M.; Lee, J.D.; Rotrosen, J.; Nunes, E. Young Adults Have Worse Outcomes Than Older Adults: Secondary Analysis of a Medication Trial for Opioid Use Disorder. J. Adolesc. Health 2020, 67, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Shield, K.D.; Fischer, B.; Elton-Marshall, T.; Sornpaisarn, B.; Probst, C.; Rehm, J. Recent Changes in Trends of Opioid Overdose Deaths in North America. Subst. Abus. Treat Prev. Policy 2020, 15, 66. [Google Scholar] [CrossRef]
- Jalal, H.; Buchanich, J.M.; Roberts, M.S.; Balmert, L.C.; Zhang, K.; Burke, D.S. Changing Dynamics of the Drug Overdose Epidemic in the United States from 1979 through 2016. Science 2018, 361, eaau1184. [Google Scholar] [CrossRef]
- Menon, J.; Kandasamy, A. Relapse Prevention. Indian J. Psychiatry 2018, 60, 473. [Google Scholar] [CrossRef]
- Grant, S.; Colaiaco, B.; Motala, A.; Shanman, R.; Booth, M.; Sorbero, M.; Hempel, S. Mindfulness-Based Relapse Prevention for Substance Use Disorders: A Systematic Review and Meta-Analysis. J. Addict. Med. 2017, 11, 386–396. [Google Scholar] [CrossRef]
- Vo, H.T.; Robbins, E.; Westwood, M.; Lezama, D.; Fishman, M. Relapse Prevention Medications in Community Treatment for Young Adults with Opioid Addiction. Subst. Abus. 2016, 37, 392–397. [Google Scholar] [CrossRef]
- Sewak, R.; Spielholz, N.I. Relapse Prevention: Using Sound to Reduce the Probability of Recidivism and Suffering Following Detoxification. Med. Hypotheses 2018, 118, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Grabski, M.; McAndrew, A.; Lawn, W.; Marsh, B.; Raymen, L.; Stevens, T.; Hardy, L.; Warren, F.; Bloomfield, M.; Borissova, A.; et al. Adjunctive Ketamine with Relapse Prevention–Based Psychological Therapy in the Treatment of Alcohol Use Disorder. Am. J. Psychiatry 2022, 179, 152–162. [Google Scholar] [CrossRef]
- Lynch, K.G.; Plebani, J.; Spratt, K.; Morales, M.; Tamminga, M.; Feibush, P.; Kampman, K.M. Varenicline for the Treatment of Cocaine Dependence. J. Addict. Med. 2022, 16, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Mahajan, S.; Kaur, A.; Deepti, S.; Rally, S. Non-Pharmacological Approach for Prevention of Relapse during Recovery in Substance Abuse: A Study Done at Drug Deaddiction Center Attached to a Tertiary Hospital. Natl. J. Physiol. Pharm. Pharmacol. 2020, 11, 411–415. [Google Scholar] [CrossRef]
- Bowen, S.; Witkiewitz, K.; Clifasefi, S.L.; Grow, J.; Chawla, N.; Hsu, S.H.; Carroll, H.A.; Harrop, E.; Collins, S.E.; Lustyk, M.K.; et al. Relative Efficacy of Mindfulness-Based Relapse Prevention, Standard Relapse Prevention, and Treatment as Usual for Substance Use Disorders: A Randomized Clinical Trial. JAMA Psychiatry 2014, 71, 547. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, D.M.; Zhou, L.D.; Winkler, M.; Pauli, P.; Sui, N.; Li, Y.H. Mindfulness-Based Relapse Prevention Combined with Virtual Reality Cue Exposure for Methamphetamine Use Disorder: Study Protocol for a Randomized Controlled Trial. Contemp. Clin. Trials 2018, 70, 99–105. [Google Scholar] [CrossRef]
- Glasner, S.; Mooney, L.J.; Ang, A.; Garneau, H.C.; Hartwell, E.; Brecht, M.-L.; Rawson, R.A. Mindfulness-Based Relapse Prevention for Stimulant Dependent Adults: A Pilot Randomized Clinical Trial. Mindfulness 2017, 8, 126–135. [Google Scholar] [CrossRef]
- Harada, T.; Aikawa, Y.; Takahama, M.; Yumoto, Y.; Umeno, M.; Hasegawa, Y.; Ohsawa, S.; Asukai, N. A 12-session Relapse Prevention Program vs Psychoeducation in the Treatment of Japanese Alcoholic Patients: A Randomized Controlled Trial. Neuropsychopharm. Rep. 2022, 42, 205–212. [Google Scholar] [CrossRef]
- Paterson, L.M.; Flechais, R.S.; Murphy, A.; Reed, L.J.; Abbott, S.; Boyapati, V.; Elliott, R.; Erritzoe, D.; Ersche, K.D.; Faluyi, Y.; et al. The Imperial College Cambridge Manchester (ICCAM) Platform Study: An Experimental Medicine Platform for Evaluating New Drugs for Relapse Prevention in Addiction. Part A: Study Description. J. Psychopharmacol. 2015, 29, 943–960. [Google Scholar] [CrossRef]
- Witkiewitz, K.; Warner, K.; Sully, B.; Barricks, A.; Stauffer, C.; Thompson, B.L.; Luoma, J.B. Randomized Trial Comparing Mindfulness-Based Relapse Prevention with Relapse Prevention for Women Offenders at a Residential Addiction Treatment Center. Subst. Use Misuse 2014, 49, 536–546. [Google Scholar] [CrossRef]
- Rong, C.; Jiang, H.-F.; Zhang, R.-W.; Zhang, L.-J.; Zhang, J.-C.; Zhang, J.; Feng, X.-S. Factors Associated with Relapse among Heroin Addicts: Evidence from a Two-Year Community-Based Follow-Up Study in China. Int. J. Environ. Res. Public Health 2016, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Appiah, R.; Boakye, K.E.; Ndaa, P.; Aziato, L. “Tougher than Ever”: An Exploration of Relapse Prevention Strategies among Patients Recovering from Poly-Substance Use Disorders in Ghana. Drugs Educ. Prev. Policy 2018, 25, 467–474. [Google Scholar] [CrossRef]
- Gonzales, R.; Anglin, M.D.; Beattie, R.; Ong, C.A.; Glik, D.C. Understanding Recovery Barriers: Youth Perceptions About Substance Use Relapse. Am. J. Health Behav. 2012, 36, 602–614. [Google Scholar] [CrossRef]
- Satre, D.D.; Chi, F.W.; Mertens, J.R.; Weisner, C.M. Effects of Age and Life Transitions on Alcohol and Drug Treatment Outcome Over Nine Years. J. Stud. Alcohol. Drugs 2012, 73, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Salehi, L.; Alizadeh, L. Efficacy of a Cognitive-Behavioral Relapse Prevention Model in the Treatment of Opioid Dependence in Iran: A Randomized Clinical Trial. Shiraz E-Med. J. 2018, in press. [Google Scholar] [CrossRef]
- Hsu, S.H.; Marlatt, G.A. Addiction Syndrome: Relapse and Relapse Prevention. In APA Addiction Syndrome Handbook, Vol. 2: Recovery, Prevention, and Other Issues; Shaffer, H., LaPlante, D.A., Nelson, S.E., Eds.; American Psychological Association: Washington, DC, USA, 2012; pp. 105–132. ISBN 978-1-4338-1105-0. [Google Scholar]
- Parekh, N.; Ali, K.; Page, A.; Roper, T.; Rajkumar, C. Incidence of Medication-Related Harm in Older Adults After Hospital Discharge: A Systematic Review. J. Am. Geriatr. Soc. 2018, 66, 1812–1822. [Google Scholar] [CrossRef]
- Walsh, A.E.L.; Naughton, G.; Sharpe, T.; Zajkowska, Z.; Malys, M.; Van Heerden, A.; Mondelli, V. Remote Measurement Technologies for Depression in Young People: A Realist Review with Meaningful Lived Experience Involvement and Recommendations for Future Research and Practice. medRxiv 2022. [Google Scholar] [CrossRef]
- Patalano, R.; De Luca, V.; Vogt, J.; Birov, S.; Giovannelli, L.; Carruba, G.; Pivonello, C.; Stroetmann, V.; Triassi, M.; Colao, A.; et al. An Innovative Approach to Designing Digital Health Solutions Addressing the Unmet Needs of Obese Patients in Europe. Int. J. Environ. Res. Public Health 2021, 18, 579. [Google Scholar] [CrossRef]
- Reddon, H.; Milloy, M.-J.; Wood, E.; Nosova, E.; Kerr, T.; DeBeck, K. High-Intensity Cannabis Use and Hospitalization: A Prospective Cohort Study of Street-Involved Youth in Vancouver, Canada. Harm Reduct. J. 2021, 18, 53. [Google Scholar] [CrossRef]
- Ti, L.; Fast, D.; Small, W.; Kerr, T. Perceptions of a Drug Prevention Public Service Announcement Campaign among Street-Involved Youth in Vancouver, Canada: A Qualitative Study. Harm Reduct. J. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Wibawa, A.P.; Nabila, K.; Utama, A.B.P.; Purnomo, P.; Dwiyanto, F.A. Social Informatics and CDIO: Revolutionizing Technological Education. Int. J. Educ. Learn. 2023, 5, 89–99. [Google Scholar] [CrossRef]
- ALHussaini, M.H.; Shahbaz, M.; Ahsan, M. The Impact of Parental Socioeconomic Status on Social Behavior of Students. Knowledge 2022, 1, 34–41. [Google Scholar] [CrossRef]
- Sokoliuk, M. Neuropsychological and Psychosomatic Factors Influencing Rehabilitation Potential in Oncology Patients: An Integrative Review. Bull. Natl. Def. Univ. Ukr. 2024, 3, 134–140. [Google Scholar] [CrossRef]
- Andersson, H.W.; Lauvsnes, A.D.F.; Nordfjærn, T. Emerging Adults in Inpatient Substance Use Treatment: A Prospective Cohort Study of Patient Characteristics and Treatment Outcomes. Eur. Addict. Res. 2021, 27, 206–215. [Google Scholar] [CrossRef]
- Hui, C.L.M.; Chiu, C.P.Y.; Li, Y.-K.; Law, C.-W.; Chang, W.-C.; Chan, S.K.W.; Lee, E.H.M.; Sham, P.; Chen, E.Y.H. The Effect of Paternal Age on Relapse in First-Episode Schizophrenia. Can. J. Psychiatry 2015, 60, 346–353. [Google Scholar] [CrossRef]
- Ma, L.; Mor, S.; Anderson, P.L.; Baños, R.M.; Botella, C.; Bouchard, S.; Cárdenas-López, G.; Donker, T.; Fernández-Álvarez, J.; Lindner, P.; et al. Integrating Virtual Realities and Psychotherapy: SWOT Analysis on VR and MR Based Treatments of Anxiety and Stress-Related Disorders. Cogn. Behav. Ther. 2021, 50, 509–526. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R.; Wan, C.; Zhang, Z.; Zhang, S.; Yang, W. Unveiling the Evolution of Virtual Reality in Medicine: A Bibliometric Analysis of Research Hotspots and Trends over the Past 12 Years. Healthcare 2024, 12, 1266. [Google Scholar] [CrossRef]
- Schoenberg, P.L.A. Welcoming the “Metaverse” in Integrative and Complementary Medicine: Introductory Overview. OBM Integr. Complement. Med. 2023, 8, 46. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, J.; Han, R.; Peng, C.; Huang, A. Using Virtual Reality Exposure Therapy in Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Value Health 2022, 25, 288–301. [Google Scholar] [CrossRef]
- Dessy, E.; Van Puyvelde, M.; Mairesse, O.; Neyt, X.; Pattyn, N. Cognitive Performance Enhancement: Do Biofeedback and Neurofeedback Work? J. Cogn. Enhanc. 2018, 2, 12–42. [Google Scholar] [CrossRef]
- Gkora, V.; Driga, A.M. Virtual Reality, Digital Technologies and Brain Rewiring Techniques for Intervention in Attention-Deficit/Hyperactivity Disorder (ADHD). Revista Saúde e Tecnologia (JHT) 2023, 2, e2237. [Google Scholar] [CrossRef]
- Becker, J.B.; McClellan, M.; Reed, B.G. Sociocultural Context for Sex Differences in Addiction. Addict. Biol. 2016, 21, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Du, J.; Jiang, H.; Zhao, M. Application of Digital Medicine in Addiction. J. Shanghai Jiaotong Univ. (Sci.) 2022, 27, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.; Zlebnik, N.E.; Farokhnia, M.; Leggio, L.; Ikemoto, S.; Shaham, Y. Sex Differences in Opioid and Psychostimulant Craving and Relapse: A Critical Review. Pharmacol. Rev. 2021, 74, 119–140. [Google Scholar] [CrossRef]
- Angres, D.; Bologeorges, S.; Chou, J. A Two Year Longitudinal Outcome Study of Addicted Health Care Professionals: An Investigation of the Role of Personality Variables. Subst. Abus. 2013, 7, SART.S10556. [Google Scholar] [CrossRef]
- Grella, C.E.; Scott, C.K.; Foss, M.A.; Dennis, M.L. Gender Similarities and Differences in the Treatment, Relapse, and Recovery Cycle. Eval. Rev. 2008, 32, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.F.; Trucco, E.M.; McHugh, R.K.; Lincoln, M.; Gallop, R.J. The Women’s Recovery Group Study: A Stage I Trial of Women-Focused Group Therapy for Substance Use Disorders versus Mixed-Gender Group Drug Counseling. Drug Alcohol Depend. 2007, 90, 39–47. [Google Scholar] [CrossRef]
- Chie, Q.T.; Tam, C.L.; Bonn, G.; Wong, C.P.; Dang, H.M.; Khairuddin, R. Drug Abuse, Relapse, and Prevention Education in Malaysia: Perspective of University Students Through a Mixed Methods Approach. Front. Psychiatry 2015, 6, 65. [Google Scholar] [CrossRef]
- Walton, M.A.; Blow, F.C.; Booth, B.M. Diversity in Relapse Prevention Needs: Gender and Race Comparisons Among Substance Abuse Treatment Patients. Am. J. Drug Alcohol Abus. 2001, 27, 225–240. [Google Scholar] [CrossRef]
- Sonbol, H.M.; Amr, M.A.; Simon, M.A. Family-Based Contributors in Relapse and Relapse Prevention Among Patients with Substance Use Disorder: An Exploration of Risk and Prognostic Factors. Addict. Health 2024, 16, 17–22. [Google Scholar] [CrossRef]
- Eropean Drug Report 2023: Trends and Developments. 2023. Available online: https://www.scribd.com/document/749427901/edr-23-english-single-pdf-27-feb-2024-0 (accessed on 9 March 2025).
- Falk, D. An Epidemiologic Analysis of Co-Occurring Alcohol and Drug Use and Disorders. Alcohol Res. Health 2008, 31, 100. [Google Scholar] [PubMed]
- Torres-Ruiz, M.; Robinson-Ector, K.; Attinson, D.; Trotter, J.; Anise, A.; Clauser, S. A Portfolio Analysis of Culturally Tailored Trials to Address Health and Healthcare Disparities. Int. J. Environ. Res. Public Health 2018, 15, 1859. [Google Scholar] [CrossRef] [PubMed]
- Asumbrado, R.R.; Canoy, N.A. A Critical Narrative Inquiry to Understand Relapse among Filipino Methamphetamine Polydrug Users in Low-Income Communities. Drugs Educ. Prev. Policy 2021, 28, 286–295. [Google Scholar] [CrossRef]
- Stitzer, M.L.; Cox, W.M. Introduction to Special Section: Relapse to Substance Abuse: Recent Findings from Basic and Clinical Research. Exp. Clin. Psychopharmacol. 1996, 4, 3–4. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Kim, J.; Jung, Y. Non-Pharmacological Nursing Interventions for Prevention and Treatment of Delirium in Hospitalized Adult Patients: Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 8853. [Google Scholar] [CrossRef]
- Agabio, R.; Camposeragna, A.; Saulle, R.; Krupchanka, D.; Leggio, L.; Minozzi, S. Combined Pharmacological and Psychosocial Interventions for Alcohol Use Disorder. Cochrane Database Syst. Rev. 2023, 2023, CD015673. [Google Scholar] [CrossRef]
- Motter, A.F.; Magalhães, R.G.C.D.; Kelner, M.; Silva, S.J.B.D.; Zamprogno, S.B.; Machado, L.P.; Bolzan, L.G.D.M.; Rolo, K.G.T.; Ferreira, C.C.; Lopes, T.C.; et al. Evidence-based interventions for tourette syndrome: An updated review of pharmacological, behavioral and non-pharmacological therapies. JHS 2023, 3, 2–10. [Google Scholar] [CrossRef]
- Skeva, R.; Gregg, L.; Jay, C.; Pettifer, S. Views of Practitioners and Researchers on the Use of Virtual Reality in Treatments for Substance Use Disorders. Front. Psychol. 2021, 12, 606761. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.-J.; Wen, Y.-T.; Winkler, M.H.; Paul, P.; He, Y.-L.; Wang, L.; Chen, H.-X.; Li, Y.-H. Memory Retrieval-Extinction Combined With Virtual Reality Reducing Drug Craving for Methamphetamine: Study Protocol for a Randomized Controlled Trial. Front. Psychiatry 2020, 11, 322. [Google Scholar] [CrossRef]
- Hung, M.-W.; Hou, C.-T.; Ho, C.-J.; Yuan, C.W.; Bi, N.; Chen, S.-H.; Huang, M.-C.; You, C.-W. Exploring the Opportunities and Challenges of Enabling Clinical-Friendly Drug Psychotherapy with Virtual Reality and Biofeedback Technologies. In Proceedings of the Extended Abstracts of the 2021 CHI Conference on Human Factors in Computing Systems, Yokohama, Japan, 8 May 2021; pp. 1–7. [Google Scholar]
- Caponnetto, P.; Casu, M. Update on Cyber Health Psychology: Virtual Reality and Mobile Health Tools in Psychotherapy, Clinical Rehabilitation, and Addiction Treatment. Int. J. Environ. Res. Public Health 2022, 19, 3516. [Google Scholar] [CrossRef] [PubMed]
- Wetterling, T.; Veltrup, C.; Junghanns, K.; Krömer-Olbrisch, T.; Schneider, U. Acceptance of Pharmacotherapy for Relapse Prevention by Chronic Alcoholics. Pharmacopsychiatry 2001, 34, 142–146. [Google Scholar] [CrossRef]
- Donovan, D.M. Relapse Prevention in Substance Abuse Treatment. In Drug Abuse Treatment Through Collaboration: Practice and Research Partnerships that Work; Sorensen, J.L., Rawson, R.A., Guydish, J., Zweben, J.E., Eds.; American Psychological Association: Washington, DC, USA, 2003; pp. 121–137. ISBN 978-1-55798-985-7. [Google Scholar]
- Kim, B.; Schwartz, W.; Catacora, D.; Vaughn-Cooke, M. Virtual Reality Behavioral Therapy. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2016, 60, 356–360. [Google Scholar] [CrossRef]
- Jerdan, S.W.; Grindle, M.; Van Woerden, H.C.; Kamel Boulos, M.N. Head-Mounted Virtual Reality and Mental Health: Critical Review of Current Research. JMIR Serious Games 2018, 6, e14. [Google Scholar] [CrossRef]
- Just, S.A.; Lütt, A.; Siegle, P.; Döring-Brandl, E.J. Feasibility of Using Virtual Reality in Geriatric Psychiatry. Int. J. Geriatr. Psychiatry 2024, 39, e6060. [Google Scholar] [CrossRef] [PubMed]
- Tsamitros, N.; Sebold, M.; Gutwinski, S.; Beck, A. Virtual Reality-Based Treatment Approaches in the Field of Substance Use Disorders. Curr. Addict. Rep. 2021, 8, 399–407. [Google Scholar] [CrossRef]
- Hernández-Serrano, O.; Ghiţă, A.; Figueras-Puigderrajols, N.; Fernández-Ruiz, J.; Monras, M.; Ortega, L.; Mondon, S.; Teixidor, L.; Gual, A.; Ugas-Ballester, L.; et al. Predictors of Changes in Alcohol Craving Levels during a Virtual Reality Cue Exposure Treatment among Patients with Alcohol Use Disorder. J. Clin. Med. 2020, 9, 3018. [Google Scholar] [CrossRef]
- Ghiţă, A.; Teixidor, L.; Monras, M.; Ortega, L.; Mondon, S.; Gual, A.; Paredes, S.M.; Villares Urgell, L.; Porras-García, B.; Ferrer-García, M.; et al. Identifying Triggers of Alcohol Craving to Develop Effective Virtual Environments for Cue Exposure Therapy. Front. Psychol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Simon, J.; Etienne, A.-M.; Bouchard, S.; Quertemont, E. Alcohol Craving in Heavy and Occasional Alcohol Drinkers After Cue Exposure in a Virtual Environment: The Role of the Sense of Presence. Front. Hum. Neurosci. 2020, 14, 124. [Google Scholar] [CrossRef]
- Ghiţă, A.; Gutiérrez-Maldonado, J. Applications of Virtual Reality in Individuals with Alcohol Misuse: A Systematic Review. Addict. Behav. 2018, 81, 1–11. [Google Scholar] [CrossRef]
- Mazza, M.; Squillacioti, M.R.; Pecora, R.D.; Janiri, L.; Bria, P. Effect of Aripiprazole on Self-Reported Anhedonia in Bipolar Depressed Patients. Psychiatry Res. 2009, 165, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Lebiecka, Z.; Skoneczny, T.; Tyburski, E.; Samochowiec, J.; Kucharska-Mazur, J. Is Virtual Reality Cue Exposure a Promising Adjunctive Treatment for Alcohol Use Disorder? J. Clin. Med. 2021, 10, 2972. [Google Scholar] [CrossRef] [PubMed]
- Emmelkamp, P.M.G.; Meyerbröker, K. Virtual Reality Therapy in Mental Health. Annu. Rev. Clin. Psychol. 2021, 17, 495–519. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Year | Study Type | No. Participants | Mean Age | Percent Male | Percent Female | Substance Use Issue | Mean Relapse Period (Months) | Intervention Type | Effect Size/Key Findings | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowen S. [16] | USA | 2014 | RCT | 286 | 54 | 71.50% | 42.10% | drug use, heavy drinking | 3 | Mindfulness-Based Relapse Prevention (MBRP), Relapse Prevention (RP), Treatment As Usual (TAU) | MBRP led to significantly fewer days of substance use and heavy drinking at 12-month follow-up vs RP and TAU; effect sizes not explicitly provided | 12 months |
| Chen X. [17] | China | 2018 | RCT | 180 | 36.5 | 83% | 17% | methamphetamine | 3 | MBRP + Virtual Reality Cue Exposure (VRCE), MBRP alone, Treatment As Usual (TAU) | Study protocol only; no outcome data or effect sizes available yet | 3 and 6 months planned |
| Glasner S. [18] | USA | 2016 | RCT | 63 | 45.3 | 71.40% | 28.60% | stimulants | 2 | MBRP + Contingency Management (CM) vs Health Education + CM | Medium effect sizes for reduced depression (d=0.58) and psychiatric severity (d=0.61); lower odds of stimulant use in MBRP group (OR=0.78 for depression, OR=0.68 for anxiety) | 1 month post-treatment |
| Grabski M. [12] | UK | 2022 | double blind clinical trial | 96 | 44.07 | 53.54% | 36.46% | alcohol use | 1 | Ketamine infusions (with or without MBRP) vs placebo infusions (with or without alcohol education) | Ketamine + therapy group had 15.9% more abstinent days vs control (95% CI: 3.8%, 28.1%) at 6 months; well tolerated | 6 months |
| Lynch K.G. [13] | USA | 2023 | double blind clinical trial | 156 | 51 | 78% | 22% | cocaine use | 2 | Varenicline + Cognitive Behavioral Therapy (CBT) vs Placebo + CBT | No significant differences in cocaine abstinence, craving, or withdrawal symptoms between groups | 12 weeks |
| Harada T. [19] | Japan | 2022 | RCT | 48 | 53.3 | 75% | 25% | alcohol use | 3 | CBT-based Relapse Prevention (RP) vs Psychoeducation (PE) | No significant differences between RP and PE groups in relapse rate or psychological measures | 3 and 6 months |
| Paterson L. [20] | UK | 2015 | RCT | 87 | 42.5 | 81% | 19% | alcohol, opiate, cocaine | 3 | Pharmacological (naltrexone, GSK598809, aprepitant) in experimental medicine study with fMRI | Study focused on feasibility and brain response; no clinical relapse outcome or effect size reported | Not applicable |
| Witkiewitz K. [21] | USA | 2014 | RCT | 105 | 35.8 | 0% | 100% | methamphetamine, heroin, cocaine, alcohol, marijuana, nicotine | 2 | Mindfulness-Based Relapse Prevention (MBRP) vs Relapse Prevention (RP) | MBRP group had fewer drug use days and fewer legal/medical issues at 15-week follow-up | 15 weeks |
| Sewak R. [11] | USA | 2018 | RCT | 116 | 40 | 62.93% | 37.06% | drugs use | 4 | Sound-based auditory stimulation (binaural beats, music, subliminal messages) | Preliminary hypothesis and early RCT suggest sound may reduce relapse risk; no standardized effect size provided | Not specified |
| Appiah R. [23] | Ghana | 2017 | clinical trial | 15 | 43.5 | 86.60% | 13.30% | drugs use | 2 | Multilevel relapse prevention strategies: clinical, spiritual, social, individual | Qualitative findings suggest contextual and spiritual strategies enhance recovery in Ghana | 1 year (post-treatment interviews) |
| Vo H.T. [10] | USA | 2016 | clinical trial | 56 | 23.1 | 70% | 30% | opioid use | 6 | Buprenorphine or Extended-Release Naltrexone (XR-NTX) | Retention ~65% at 12 weeks, 40% at 24 weeks; no significant differences between medications in opioid abstinence | 24 weeks |
| Rong C. [22] | China | 2016 | RCT | 554 | 41.6 | 80% | 20% | heroin use | 3 | Methadone or Jitai tablets with psychological counseling and social support | Psychological counseling significantly reduced relapse (OR = 3.56); longer drug history increased relapse risk | 2 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabugan, D.C.; Bredicean, A.C.; Anghel, T.; Dumache, R.; Muresan, C.; Corsaro, L.; Hogea, L. Novel Insights into Addiction Management: A Meta-Analysis on Intervention for Relapse Prevention. Medicina 2025, 61, 619. https://doi.org/10.3390/medicina61040619
Tabugan DC, Bredicean AC, Anghel T, Dumache R, Muresan C, Corsaro L, Hogea L. Novel Insights into Addiction Management: A Meta-Analysis on Intervention for Relapse Prevention. Medicina. 2025; 61(4):619. https://doi.org/10.3390/medicina61040619
Chicago/Turabian StyleTabugan, Dana Cătălina, Ana Cristina Bredicean, Teodora Anghel, Raluca Dumache, Camelia Muresan, Leonardo Corsaro, and Lavinia Hogea. 2025. "Novel Insights into Addiction Management: A Meta-Analysis on Intervention for Relapse Prevention" Medicina 61, no. 4: 619. https://doi.org/10.3390/medicina61040619
APA StyleTabugan, D. C., Bredicean, A. C., Anghel, T., Dumache, R., Muresan, C., Corsaro, L., & Hogea, L. (2025). Novel Insights into Addiction Management: A Meta-Analysis on Intervention for Relapse Prevention. Medicina, 61(4), 619. https://doi.org/10.3390/medicina61040619







