Mean Platelet Volume-to-Albumin Ratio as a Predictor of Mortality in Patients with Febrile Neutropenia: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Sample Size Calculation
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
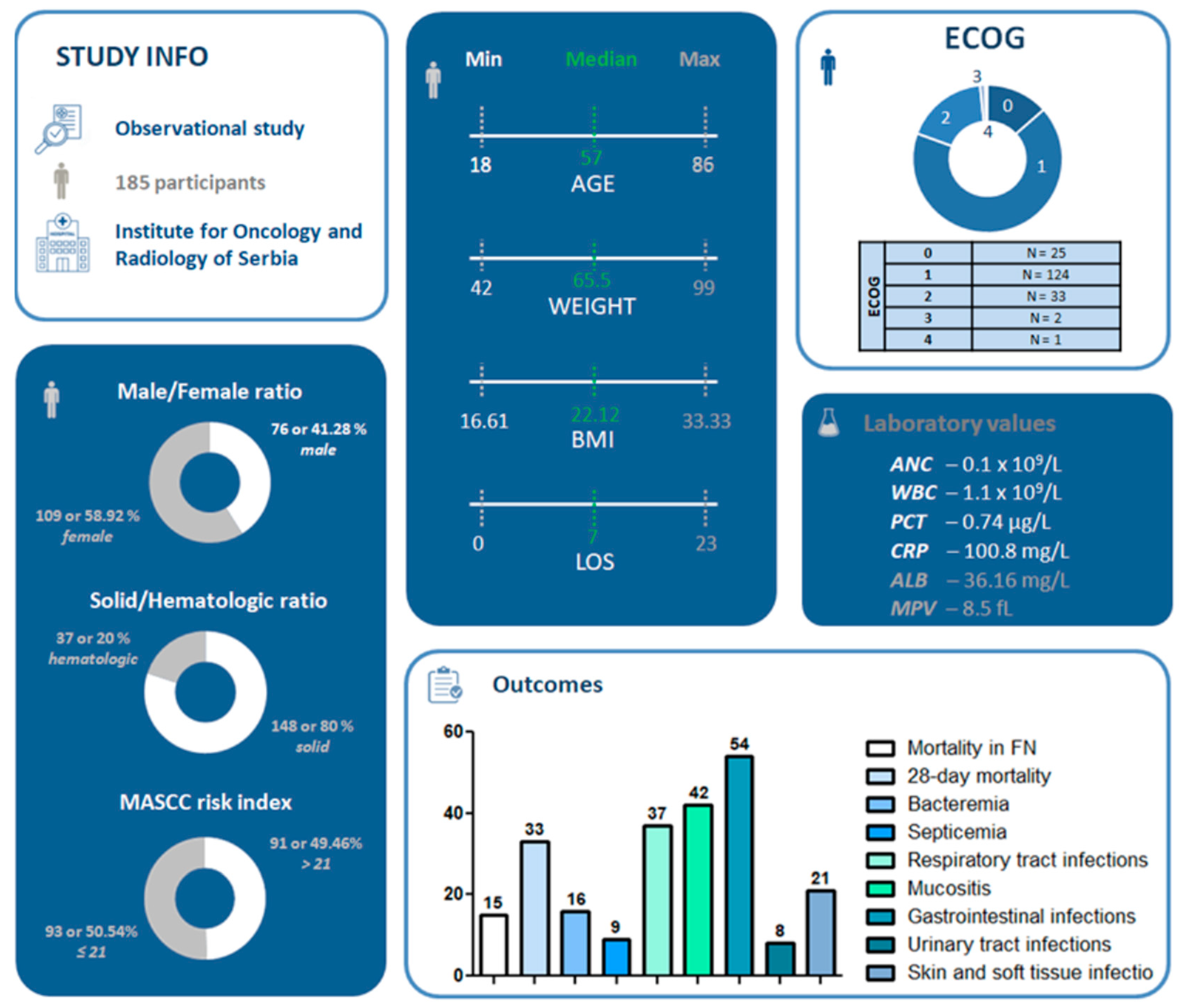
3.1. Study Population and Demographic Characteristics
3.2. Predictive Accuracy of MPV/ALB Ratio
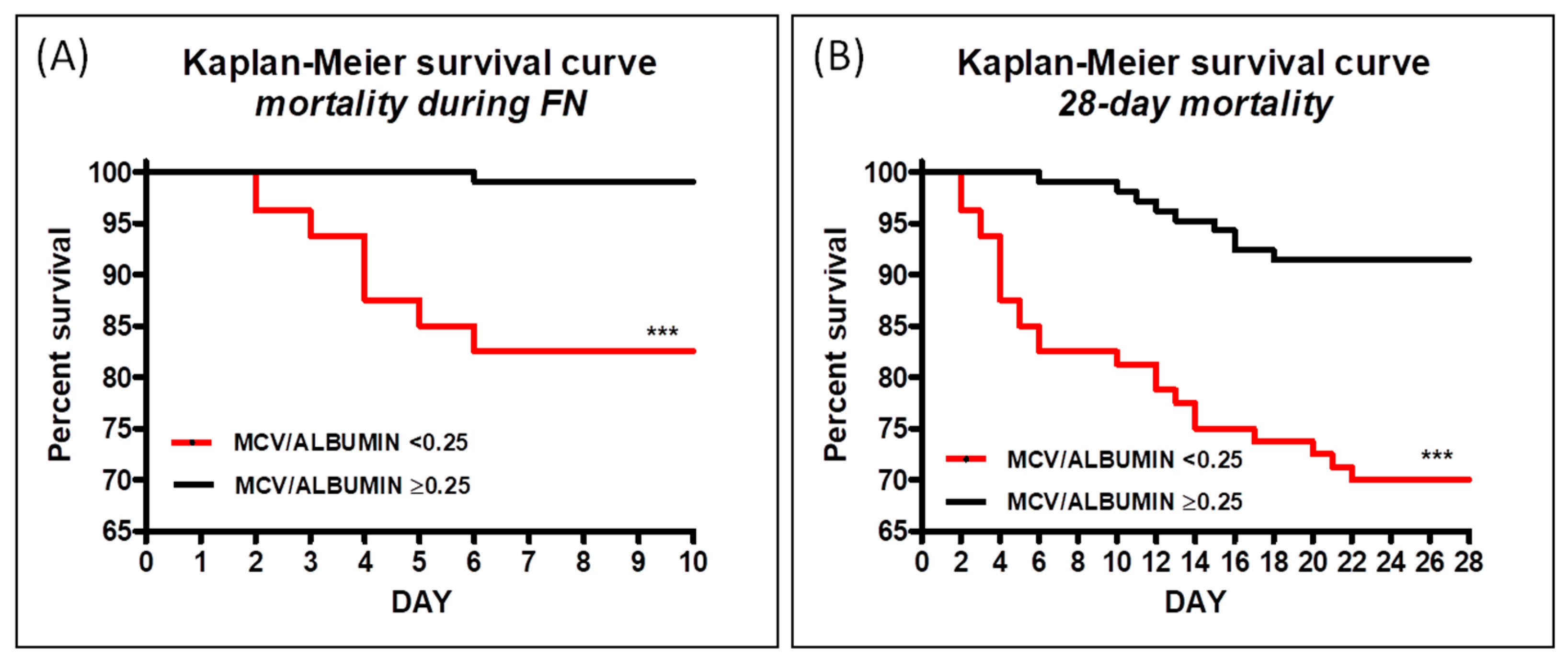
3.3. Kaplan–Meier Survival Analysis for MPV/ALB Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carpeño, J.D.C.; Gascón-Vilaplana, P.; Tejerina, A.M.; Antón-Torres, A.; López-López, R.; Barnadas-Molins, A.; Cruz-Hernández, J.J.; Massuti-Sureda, B.; Camps-Herrero, C.; Aranda-Aguilar, E.; et al. Epidemiology and characteristics of febrile neutropenia in oncology patients from Spanish tertiary care hospitals: PINNACLE study. Mol. Clin. Oncol. 2015, 3, 725–729. [Google Scholar] [CrossRef]
- Klastersky, J. Management of fever in neutropenic patients with different risks of complications. Clin. Infect. Dis. 2004, 39, S32–S37. [Google Scholar] [CrossRef]
- Aagaard, T.; Reekie, J.; Jørgensen, M.; Roen, A.; Daugaard, G.; Specht, L.; Sengeløv, H.; Mocroft, A.; Lundgren, J.; Helleberg, M. Mortality and admission to intensive care units after febrile neutropenia in patients with cancer. Cancer Med. 2020, 9, 3033–3042. [Google Scholar] [CrossRef]
- Li, Y.; Family, L.; Yang, S.; Klippel, Z.; Page, J.H.; Chao, C. Risk of Febrile Neutropenia Associated With Select Myelosuppressive Chemotherapy Regimens in a Large Community-Based Oncology Practice. J. Natl. Compr. Cancer Netw. 2017, 15, 1122–1130. [Google Scholar]
- Lynn, J.J.; Chen, K.F.; Weng, Y.M.; Chiu, T.F. Risk factors associated with complications in patients with chemotherapy-induced febrile neutropenia in emergency department. Hematol. Oncol. 2013, 31, 189–196. [Google Scholar] [CrossRef]
- Zecha, J.A.E.M.; Raber-Durlacher, J.E.; Laheij, A.M.G.A.; Westermann, A.M.; Epstein, J.B.; de Lange, J.; Smeele, L.E. The impact of the oral cavity in febrile neutropenia and infectious complications in patients treated with myelosuppressive chemotherapy. Support. Care Cancer 2019, 27, 3667–3679. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, Y.S.; Lim, K.S.; Lee, J.L. Adding procalcitonin to the MASCC risk-index score could improve risk stratification of patients with febrile neutropenia. Support. Care Cancer 2013, 21, 2303–2308. [Google Scholar] [CrossRef]
- Boccia, R.; Glaspy, J.; Crawford, J.; Aapro, M. Chemotherapy-Induced Neutropenia and Febrile Neutropenia in the US: A Beast of Burden That Needs to Be Tamed? Oncologist 2022, 27, 625–636. [Google Scholar] [CrossRef]
- Oberoi, S.; Das, A.; Trehan, A.; Ray, P.; Bansal, D. Can complications in febrile neutropenia be predicted? Report from a developing country. Support. Care Cancer 2017, 25, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.J.; Castillo, E.M.; Shatsky, R.A.; Chan, T.C. Procalcitonin as a Predictive Tool for Death and ICU Admission among Febrile Neutropenic Patients Visiting the Emergency Department. Medicina 2022, 58, 985. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, İ.C.; Çakıl Güzin, A.; Özdem Alataş, Ş.; Karaoğlu Asrak, H.; Akans, İ.; Akyol, Ş.; Özlü, C.; Tüfekçi, Ö.; Yılmaz, Ş.; Ören, H.; et al. Etiology and Factors Affecting Severe Complications and Mortality of Febrile Neutropenia in Children with Acute Leukemia. Turk. J. Haematol. 2023, 40, 143–153. [Google Scholar] [CrossRef]
- Dimitrijević, J.; Čalamać, M.; Đurmez, O.; Krstić, D.; Stojanović, M. Serum Albumin as a Prognostic Biomarker for Febrile Neutropenia Outcome and Complications: A Prospective Observational Trial. Clin. Med. Insights Oncol. 2024, 18, 11795549241281330. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur. J. Surg. Oncol. 2024, 50, 107074. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2019, 2019, 9213074. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Enkobahry, A.; Sime, T.; Kene, K.; Mateos, T.; Dilnesa, S.; Zawdie, B. Blood biomarkers as potential malnutrition screening alternatives among adult patients with cancer on treatment in oncology unit of jimma tertiary hospital: A cross-sectional analysis. BMC Nutr. 2023, 9, 38. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Jia, J.; Yang, J.; Song, Y.; Duan, H. Total Parenteral Nutrition Treatment Improves the Nutrition Status of Gynecological Cancer Patients by Improving Serum Albumin Level. Front. Med. 2022, 8, 759387. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Shmuely, H.; Monely, L.; Shvidel, L. All-Cause Mortality and Its Predictors in Haemato-Oncology Patients with Febrile Neutropenia. J. Clin. Med. 2023, 12, 5635. [Google Scholar] [CrossRef]
- Sereeaphinan, C.; Kanchanasuwan, S.; Julamanee, J. Mortality-associated clinical risk factors in patients with febrile neutropenia: A retrospective study. IJID Reg. 2021, 1, 5–11. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Hinedi, K.; Khairallah, H.; Saadeh, B.; Abbasi, S.; Noureen, M.; Raza, S.; Alkhatti, A. Epidemiology and source of infection in patients with febrile neutropenia: A ten-year longitudinal study. J. Infect. Public. Health 2019, 12, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Parodi, R.L.; Lagrutta, M.; Tortolo, M.; Navall, E.; Rodríguez, M.S.; Sasia, G.F.; De Candia, L.F.; Gruvman, M.A.; Bottasso, O.; Greca, A.A. A multicenter prospective study of 515 febrile neutropenia episodes in Argentina during a 5-year period. PLoS ONE 2019, 14, e0224299. [Google Scholar] [CrossRef]
- Weerasubpong, B.; Makruasi, N.; Linasmita, P.; Rattanamongkolgul, S. Factors Associated with Survival Outcomes of Febrile Neutropenia in Hematologic Malignancy Patients. J. Med. Assoc. Thai 2016, 99, S53–S62. [Google Scholar] [PubMed]
- Tajarernmuang, P.; Phrommintikul, A.; Limsukon, A.; Pothirat, C.; Chittawatanarat, K. The Role of Mean Platelet Volume as a Predictor of Mortality in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care Res. Pract. 2016, 2016, 4370834. [Google Scholar] [CrossRef]
- Jellinge, M.E.; Henriksen, D.P.; Hallas, P.; Brabrand, M. Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS ONE 2014, 9, e105983. [Google Scholar] [CrossRef]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am. J. Med. 2017, 130, e11–e1465. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Chandra Sekhar, G.; Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian. J. Ophthalmol. 2008, 56, 45–50. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Diagnostic tests 1: Sensitivity and specificity. BMJ 1994, 308, 1552. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Diagnostic tests 2: Predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef]
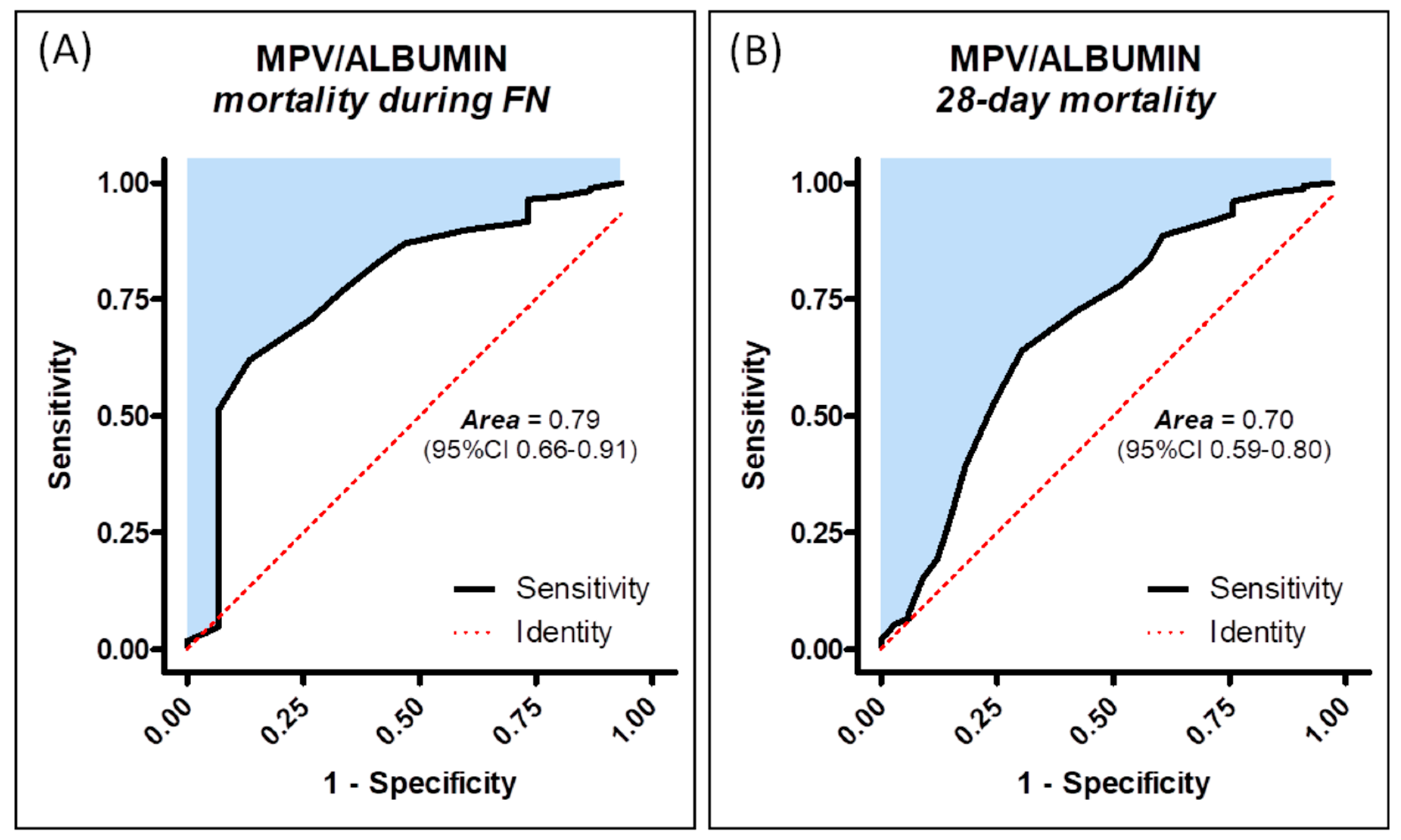
| MPV/ALB | Sensitivity | Specificity | PPV | NPV | Likelihood Ratio |
|---|---|---|---|---|---|
| Mortality in FN | 0.62 (0.54–0.69) | 0.87 (0.60–0.98) | 0.29 (0.1–0.75) | 0.96 (0.94–0.97) | 4.77 |
| 28-day mortality | 0.64 (0.56–0.72) | 0.69 (0.51–0.84) | 0.31 (0.20–0.50) | 0.90 (0.84–0.93) | 2.06 |
| MPV/ALBUMIN | Chi Square | Hazard Ratio (95%CI) | p Value |
|---|---|---|---|
| Mortality during FN | 16.94 | 8.79 (3.12–24.73) | <0.0001 |
| 28-day mortality | 15.22 | 4.04 (2.00–8.14) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrijević, J.; Čalamać, M.; Đurmez, O.; Stojanović, M. Mean Platelet Volume-to-Albumin Ratio as a Predictor of Mortality in Patients with Febrile Neutropenia: An Observational Study. Medicina 2025, 61, 601. https://doi.org/10.3390/medicina61040601
Dimitrijević J, Čalamać M, Đurmez O, Stojanović M. Mean Platelet Volume-to-Albumin Ratio as a Predictor of Mortality in Patients with Febrile Neutropenia: An Observational Study. Medicina. 2025; 61(4):601. https://doi.org/10.3390/medicina61040601
Chicago/Turabian StyleDimitrijević, Jelena, Marina Čalamać, Ognjen Đurmez, and Marko Stojanović. 2025. "Mean Platelet Volume-to-Albumin Ratio as a Predictor of Mortality in Patients with Febrile Neutropenia: An Observational Study" Medicina 61, no. 4: 601. https://doi.org/10.3390/medicina61040601
APA StyleDimitrijević, J., Čalamać, M., Đurmez, O., & Stojanović, M. (2025). Mean Platelet Volume-to-Albumin Ratio as a Predictor of Mortality in Patients with Febrile Neutropenia: An Observational Study. Medicina, 61(4), 601. https://doi.org/10.3390/medicina61040601






