Efficacy of Radiotherapy in Patients with Relapsing Primary Rosai–Dorfman Disease of the Nasal Cavity
Abstract
1. Introduction
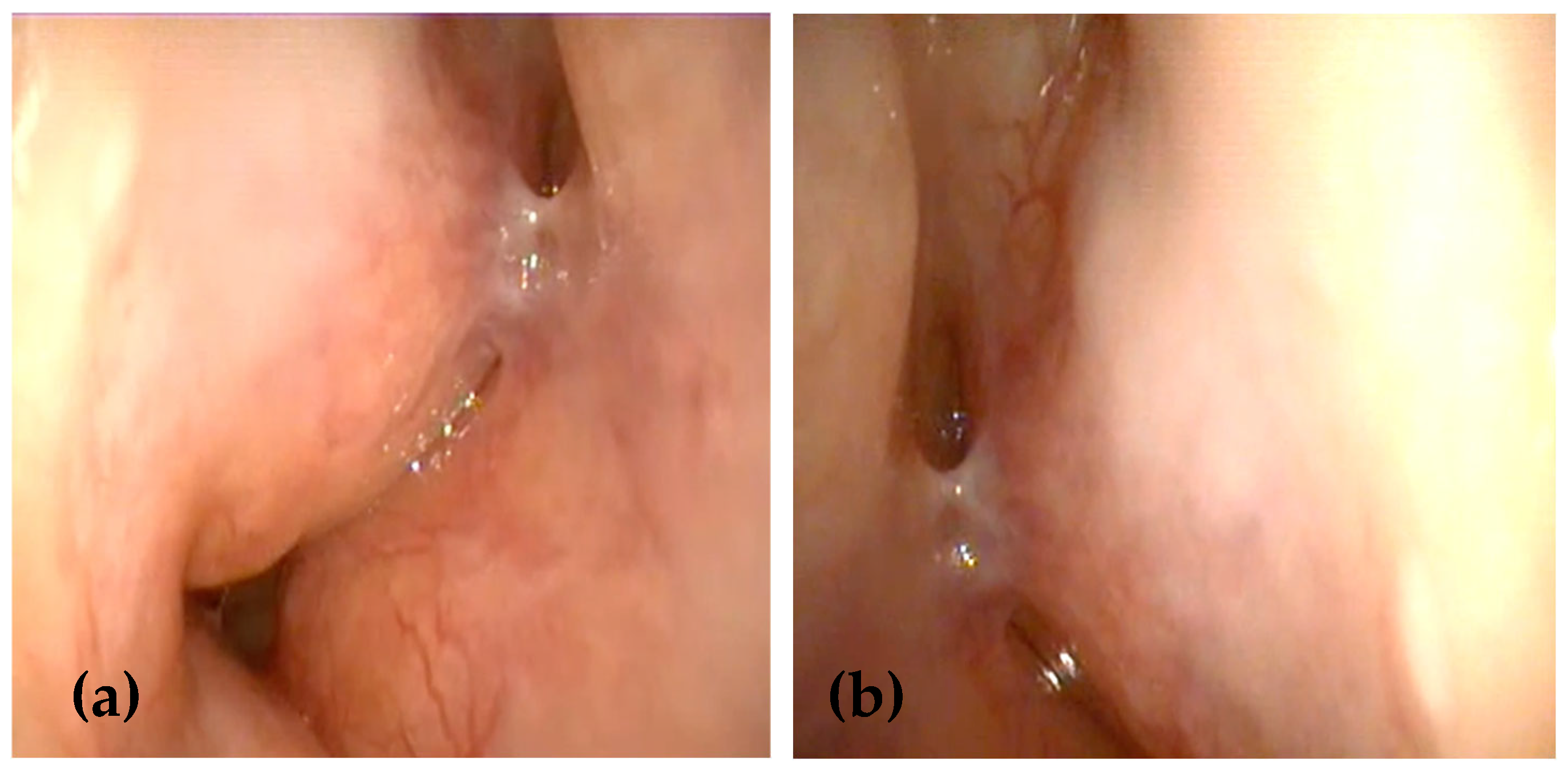
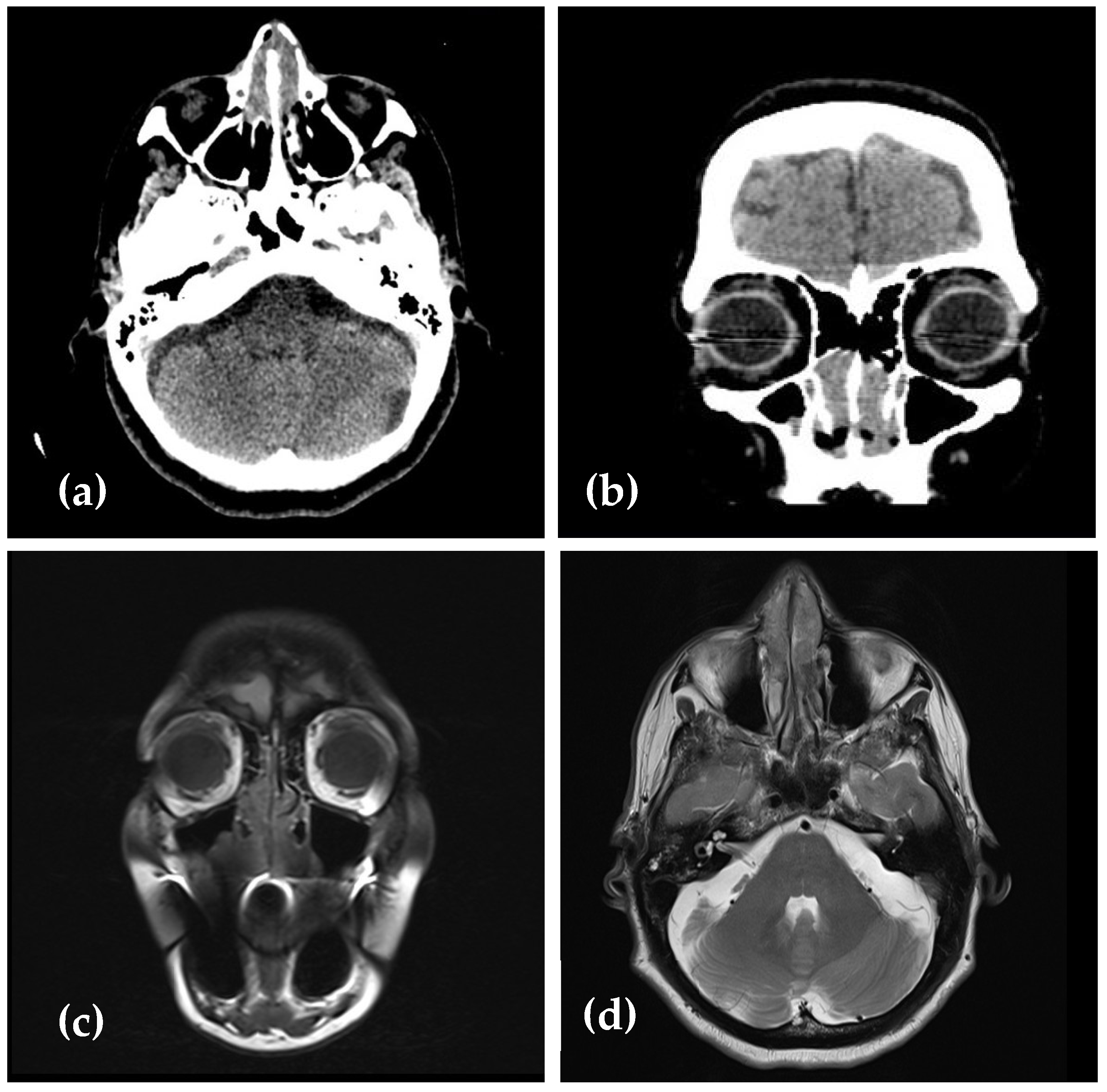
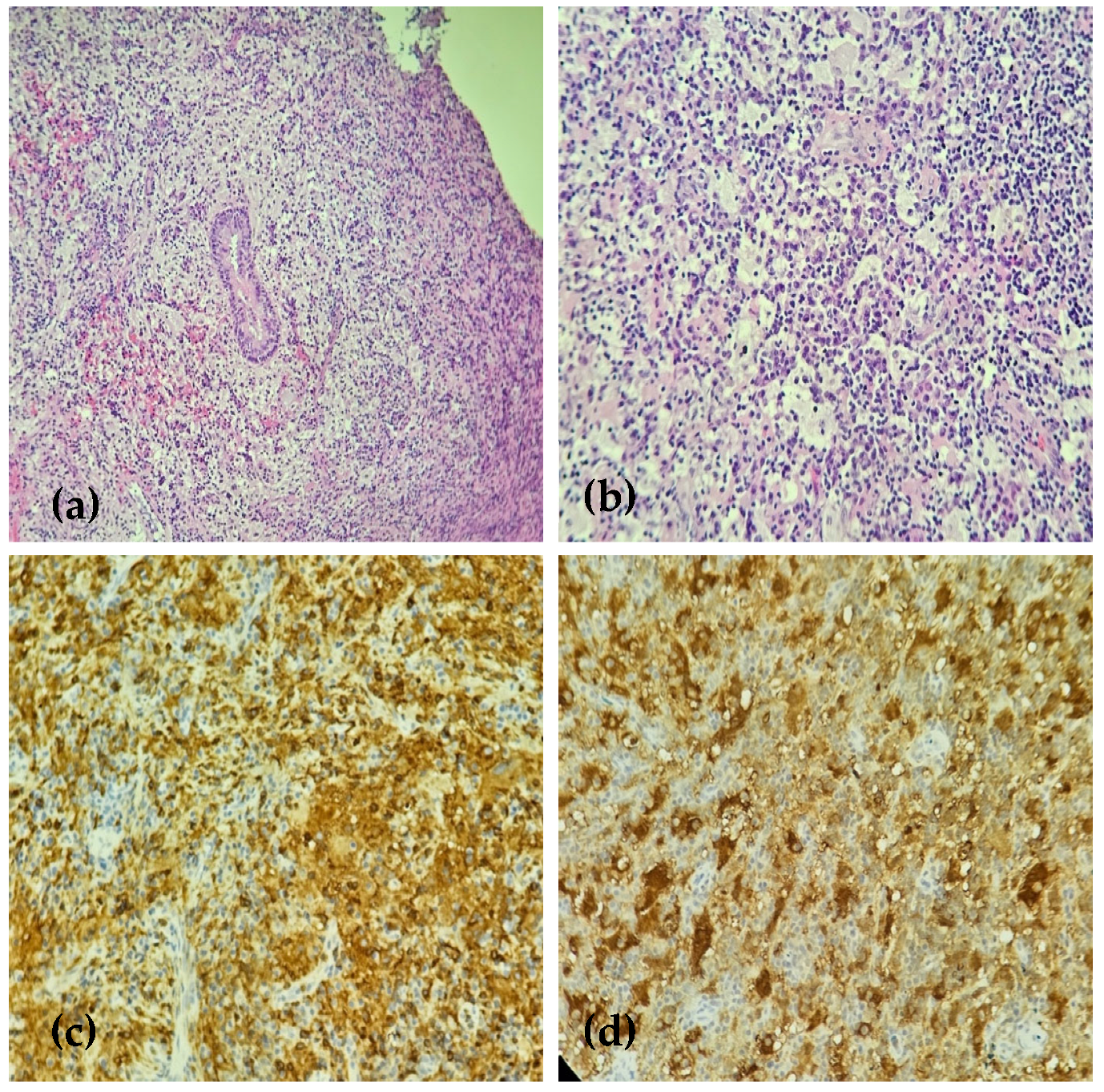
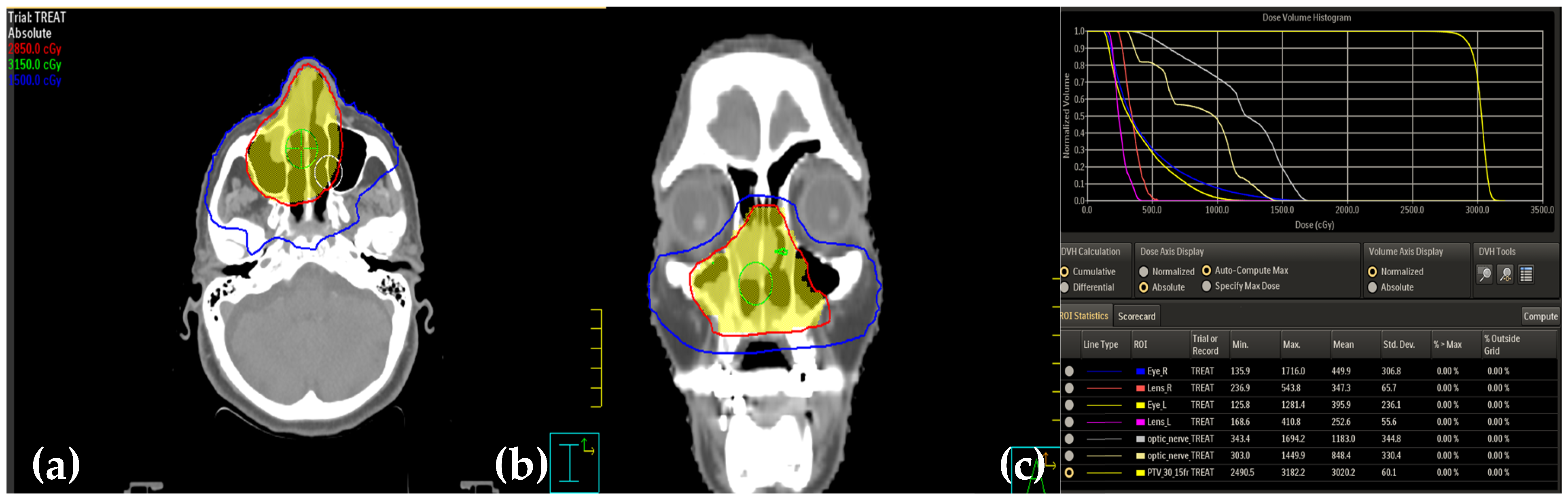
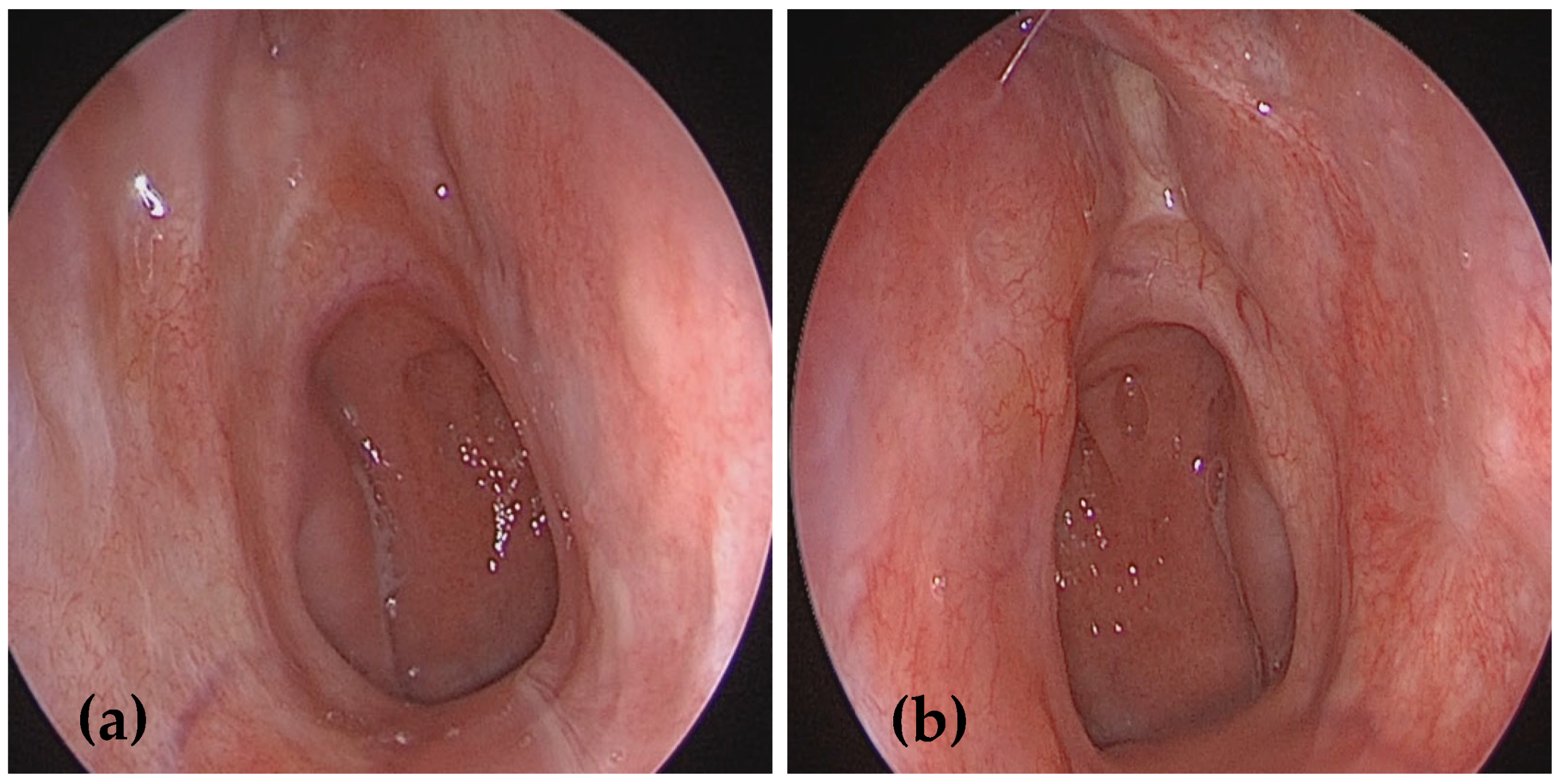
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| RDD | Rosai–Dorfman disease |
| SLC29A3 | Solute carrier family 29 member 3 |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| BRAF | V-Raf murine sarcoma viral oncogene homolog B |
| KRAS | Kirsten rat sarcoma 2 viral oncogene homolog |
| FDG-PET scan | Fluorodeoxyglucose-positron emission tomography |
| NRAS | Neuroblastoma ras viral oncogene homolog |
| MAP2K1 | Mitogen-activated protein kinase kinase 1 |
| IgG4-RD | Immunoglobulin G4-related disease |
References
- Destombes, P. Adenitis with lipid excess, in children or young adults, seen in the Antilles and mali (4 cases). Bull. Soc. Pathol. Exot. Fil. 1965, 58, 1169–1175. (In French) [Google Scholar] [PubMed]
- Rosai, J.; Dorfman, R. Sinus histiocytosis with massive lymphadenopathy: A pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer 1972, 30, 1174–1188. [Google Scholar] [CrossRef]
- Emile, J.-F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Foucar, E.; Rosai, J.; Dorfman, R.F.; Flanders, D. Sinus Histiocytosis with Massive Lymphadenopathy An Analysis of 14 Deaths Occurring in a Patient Registry. Cancer 1984, 54, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Abla, O.; Jacobsen, E.; Picarsic, J.; Krenova, Z.; Jaffe, R.; Emile, J.-F.; Durham, B.H.; Braier, J.; Charlotte, F.; Donadieu, J.; et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 2018, 131, 2877–2890. [Google Scholar] [CrossRef]
- Mahzoni, P.; Hani, M.; Zavareh, T.; Bagheri, M.; Hani, N.; Moqtader, B. Intracranial Rosai-Dorfman disease. J. Res. Med. Sci. 2012, 17, 304–307. [Google Scholar]
- Li, C.; Chen, J.; He, P.; Cheng, L.; Wu, H. Clinical features, diagnosis, treatment and prognosis of otolaryngological extranodal sinus histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease, RDD). Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Li, S.H.; Liao, J.B.; Chiou, C.C.; Wu, C.S.; Chen, C.C. NRAS mutations may be involved in the pathogenesis of cutaneous rossi dorfman disease: A pilot study. Biology 2021, 10, 396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, T.E.; Wachsmann, M.; Oliver, D.; Abedin, Z.; Ye, D.; Burns, D.K.; Raisanen, J.M.; Greenberg, B.M.; Hatanpaa, K.J. BRAF mutation leading to central nervous system rosai-dorfman disease. Ann. Neurol. 2018, 84, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Garces, S.; Medeiros, L.J.; Patel, K.P.; Li, S.; Pina-Oviedo, S.; Li, J.; Garces, J.C.; Khoury, J.D.; Yin, C.C. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod. Pathol. 2017, 30, 1367–1377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, N.V.; Morris, M.R.; Cangul, H.; Gleeson, D.; Straatman-Iwanowska, A.; Davies, N.; Keenan, S.; Pasha, S.; Rahman, F.; Gentle, D.; et al. Mutations in SLC29a3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Pittaluga, S.; Price, S.; Raffeld, M.; Hahn, J.; Jaffe, E.S.; Rao, V.K.; Maric, I. Bone marrow findings in autoimmune lymphoproliferative syndrome with germline FAS mutation. Haematologica 2017, 102, 364–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menon, M.P.; Evbuomwan, M.O.; Rosai, J.; Jaffe, E.S.; Pittaluga, S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: Another red herring or a true association with IgG4-related disease? Histopathology 2014, 64, 455–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragotte, R.J.; Dhanrajani, A.; Pleydell-Pearce, J.; Del Bel, K.L.; Tarailo-Graovac, M.; van Karnebeek, C.; Terry, J.; Senger, C.; McKinnon, M.L.; Seear, M.; et al. The importance of considering monogenic causes of autoimmunity: A somatic mutation in KRAS causing pediatric Rosai-Dorfman syndrome and systemic lupus erythematosus. Clin. Immunol. 2017, 175, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Estalilla, O.C.; Manning, J.T.; Medeiros, L.J. Sinus Histiocytosis with Massive Lymphadenopathy and Malignant Lymphoma Involving the Same Lymph Node: A Report of Four Cases and Review of the Literature. Mod. Pathol. 2000, 13, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Akyigit, A.; Akyol, H.; Sakallioglu, O.; Polat, C.; Keles, E.; Alatas, O. Rosai-Dorfman Disease Originating from Nasal Septal Mucosa. Case Rep. Otolaryngol. 2015, 2015, 232898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.-L.; Liang, Z.-P.; Xu, S.-E.; Yang, Z.-H.; Peng, Y.; Sun, X.-Q.; Qin, G. Case Report Rosai-Dorfman disease (RDD) in the paraglottic space: Report of a case and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 13532–13538. [Google Scholar]
- Wenig, B.M.; Abbondanzo, S.L.; Childers, E.L.; Kapadia, B.; Heffner, D.R. Extranodal Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease) of the Head and Neck. Hum. Pathol. 1993, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Toguri, D.; Louie, A.V.; Rizkalla, K.; Franklin, J.; Rodrigues, G.B.; Venkatesan, V. Radiotherapy for steroid-resistant laryngeal Rosai-Dorfman disease. Curr. Oncol. 2011, 18, e158–e162. [Google Scholar] [CrossRef]
- Naidu, R.K.; Urken, M.L.; Som, P.M.; Danon, A.; Biller, H.F.; York, N. Extranodal head and neck sinus histiocytosis with massive lymphadenopathy. Otolaryngol. Head. Neck Surg. 1990, 102, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Li, G.; Wang, C.; Yu, G.; Sun, Y. Rosai-Dorfman disease originating from nasal septal mucosa and presenting with nasal dorsum collapse: A case report with literature review. Ear Nose Throat J. 2024, 103, NP604–NP609. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; White, M.J.; Duffield, A.S.; Gagan, J.; London, N.R.; Montgomery, E.A.; Bishop, J.A. Limited sinonasal Rosai–Dorfman disease presenting as chronic sinusitis. Histopathology 2022, 81, 99–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paulli, M.; Rosso, R.; Kindl, S.; Boveri, E.; Marocolo, D.; Chioda, C.; Agostini, C.; Magrini, U.; Facchetti, F. lmmunophenotypic Characterization of the Cell Infiltrate in Five Cases of Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease). Hum. Pathol. 1992, 23, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Katwal, S.; Ghimire, A.; Bhusal, A.; Yogi, T.N. Uncommon presentation of Rosai-Dorfman disease: Nasal and nasopharyngeal involvement: A case report and discussion. Radiol. Case Rep. 2024, 19, 956–960. [Google Scholar] [CrossRef]
- Ottaviano, G.; Doro, D.; Marioni, G.; Mirabelli, P.; Marchese-Ragona, R.; Tognon, S.; Marino, F.; Staffieri, A. Extranodal Rosai-Dorfman disease: Involvement of eye, nose and trachea. Acta Otolaryngol. 2006, 126, 657–660. [Google Scholar] [CrossRef] [PubMed]
- McClain, K.L.; Bigenwald, C.; Collin, M.; Haroche, J.; Marsh, R.A.; Merad, M.; Picarsic, J.; Ribeiro, K.B.; Allen, C.E. Histiocytic disorders. Nat. Rev. Dis. Primers 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ku, P.K.M.; Tong, M.C.F.; Leung, C.Y.; Pak, M.W.; van Hasselt, C.A. Nasal manifestation of extranodal Rosai-Dorfman disease-diagnosis and management. J. Laryngol. Otol. 1999, 113, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Foucar, E.; Rosai, J.; Dorfman, R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Review of the entity. Semin. Diagn. Pathol. 1990, 7, 19–73. [Google Scholar] [PubMed]
- Komp, D. The treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Semin. Diagn. Pathol. 1990, 7, 6–83. [Google Scholar] [PubMed]
- Horneff, G.; Jurgens, H.; Hort, W.; Karitzky, D.; Cobel, U. Sinus histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease): Response to Methotrexate and Mercaptopurine. Med. Pediatr. Oncol. 1996, 27, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Goodnight, J.W.; Wang, M.B.; Sercarz, J.A.; Fu, Y.S. Extranodal Rosai-Dorfman Disease of the Head and Neck. Laryngoscope 1996, 106 Pt 1, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Assal, A.; Scherba, M.; Elman, J.; White, R.; Verma, A.; Strakhan, M.; Mohammadi, F.; Janakiram, M. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am. J. Hematol. 2016, 91, E1. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.; Shanmugam, V.; Jagannathan, J. Rosai–Dorfman Disease with Activating KRAS Mutation—Response to Cobimetinib. N. Engl. J. Med. 2017, 377, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.L.; Mollah, M.A.H. Rosai-Dorfman Disease: A Case Report. J. Bangladesh Coll. Phys. Surg. 2022, 40, 299–301. [Google Scholar] [CrossRef]
- Hu, P.-P.; Wei, F.; Liu, X.-G.; Liu, Z.-J. Diagnosis and treatment of Rosai-Dorfman disease of the spine: A systematic literature review. Syst. Rev. 2021, 10, 31. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhou, S.H.; Wang, S.Q.; Teng, X.D.; Fan, J. Factors associated with recurrence and therapeutic strategies for sinonasal Rosai-Dorfman disease. Head Neck 2012, 34, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Ojha, J.; Rawal, Y.B.; Hornick, J.L.; Magliocca, K.; Montgomey, D.R.; Foss, R.D.; Accurso, B. Extranodal Rosai-Dorfman Disease Originating in the Nasal and paranasal Complex and Gnathic Bones: A Systematic Analysis of Seven Cases and Review of Literature. Head Neck Pathol. 2019, 14, 442–453. [Google Scholar] [CrossRef]
- Jassal, D.S.; Kasper, K.; Morales, C.; Rubinger, M. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Report of a case and literature review. Am. J. Hematol. 2002, 69, 67–71. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafoleanu, C.-C.; Badea, F.-C.; Georgescu, A.-M.; Musat, G.-C.; Andrei, A.; Tanase, I. Efficacy of Radiotherapy in Patients with Relapsing Primary Rosai–Dorfman Disease of the Nasal Cavity. Medicina 2025, 61, 585. https://doi.org/10.3390/medicina61040585
Sarafoleanu C-C, Badea F-C, Georgescu A-M, Musat G-C, Andrei A, Tanase I. Efficacy of Radiotherapy in Patients with Relapsing Primary Rosai–Dorfman Disease of the Nasal Cavity. Medicina. 2025; 61(4):585. https://doi.org/10.3390/medicina61040585
Chicago/Turabian StyleSarafoleanu, Caius-Codrut, Florentina-Carmen Badea, Alina-Maria Georgescu, Gabriela-Cornelia Musat, Anica Andrei, and Ionut Tanase. 2025. "Efficacy of Radiotherapy in Patients with Relapsing Primary Rosai–Dorfman Disease of the Nasal Cavity" Medicina 61, no. 4: 585. https://doi.org/10.3390/medicina61040585
APA StyleSarafoleanu, C.-C., Badea, F.-C., Georgescu, A.-M., Musat, G.-C., Andrei, A., & Tanase, I. (2025). Efficacy of Radiotherapy in Patients with Relapsing Primary Rosai–Dorfman Disease of the Nasal Cavity. Medicina, 61(4), 585. https://doi.org/10.3390/medicina61040585






