The Impact of Living Kidney Donor Glomerular Filtration Rate on Graft Survival
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Gupta, R.; Woo, K.; Yi, J.A. Epidemiology of end-stage kidney disease. Semin. Vasc. Surg. 2021, 34, 71–78. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Roy, R.; Piscoran, O.; Summers, A.; van Dellen, D.; Augustine, T. Living donor kidney transplantation: Let’s talk about it. Clin. Med. 2020, 20, 346–348. [Google Scholar] [CrossRef]
- Garg, N.; Lentine, K.L.; Inker, L.A.; Garg, A.X.; Rodrigue, J.R.; Segev, D.L.; Mandelbrot, D.A. The kidney evaluation of living kidney donor candidates: US practices in 2017. Am. J. Transplant. 2020, 20, 3379–3389. [Google Scholar] [CrossRef]
- Lentine, K.L.; Kasiske, B.L.; Levey, A.S.; Adams, P.L.; Alberú, J.; Bakr, M.A.; Gallon, L.; Garvey, C.A.; Guleria, S.; Li, P.K.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017, 101, S1–S109. [Google Scholar] [CrossRef]
- Andrews, P.A.; Burnapp, L. British Transplantation Society/Renal Association UK Guidelines for Living Donor Kidney Transplantation 2018: Summary of Updated Guidance. Transplantation 2018, 102, e307. [Google Scholar] [CrossRef]
- Orlandi, P.F.; Cristelli, M.P.; Aldworth, C.A.; Freitas, T.V.; Felipe, C.R.; Silva Junior, H.T.; Pestana, J.O. Long-term outcomes of elderly kidney transplant recipients. J. Bras. Nefrol. 2015, 37, 212–220. [Google Scholar] [CrossRef]
- Remport, A.; Keszei, A.; Vamos, E.P.; Novak, M.; Jaray, J.; Rosivall, L.; Mucsi, I.; Molnar, M.Z. Association of pre-transplant dialysis duration with outcome in kidney transplant recipients: A prevalent cohort study. Int. Urol. Nephrol. 2011, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.; Magnus, J.H.; Islam, T.M.; Jaffe, B.M.; Zhang, R.; Florman, S.S.; Hamm, L.L.; Mruthinti, N.; Sullivan, K.; Slakey, D.P. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J. Am. Coll. Surg. 2010, 210, 718–726. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Abramowicz, D.; Bolignano, D.; Gandolfini, I.; Hellemans, R.; Maggiore, U.; Nistor, I.; O’Neill, S.; Sever, M.S.; Koobasi, M.; et al. Management of obesity in kidney transplant candidates and recipients: A clinical practice guideline by the DESCARTES Working Group of ERA. Nephrol. Dial. Transplant. 2021, 37 (Suppl. 1), i1–i15. [Google Scholar] [CrossRef]
- Lujan, P.R.; Chiurchiu, C.; Douthat, W.; de Arteaga, J.; de la Fuente, J.; Capra, R.H.; Massari, P.U. CKD-EPI instead of MDRD for candidates to kidney donation. Transplantation 2012, 94, 637–641. [Google Scholar] [CrossRef]
- Rizzari, M.D.; Suszynski, T.M.; Gillingham, K.J.; Matas, A.J. Consideration of donor age and human leukocyte antigen matching in the setting of multiple potential living kidney donors. Transplantation 2011, 92, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Noppakun, K.; Cosio, F.G.; Dean, P.G.; Taler, S.J.; Wauters, R.; Grande, J.P. Living donor age and kidney transplant outcomes. Am. J. Transplant. 2011, 11, 1279–1286. [Google Scholar] [CrossRef]
- Ferrari, P.; Lim, W.; Dent, H.; McDonald, S.P. Effect of donor-recipient age difference on graft function and survival in live-donor kidney transplantation. Nephrol. Dial. Transplant. 2011, 26, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.C.; Sasser, J.M.; Pollock, D.M.; Pollock, J.S. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 2005, 45, 406–411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lattimer, J.K. The Action of Testosterone Propionate Upon the Kidneys of Rats, Dogs and Men. J. Urol. 1942, 48, 778–794. [Google Scholar] [CrossRef]
- Quinkler, M.; Bujalska, I.J.; Kaur, K.; Onyimba, C.U.; Buhner, S.; Allolio, B.; Hughes, S.V.; Hewison, M.; Stewart, P.M. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension 2005, 46, 787–798. [Google Scholar] [CrossRef]
- Jacobs, S.C.; Nogueira, J.M.; Phelan, M.W.; Bartlett, S.T.; Cooper, M. Transplant recipient renal function is donor renal mass- and recipient gender-dependent. Transpl. Int. 2008, 21, 340–345. [Google Scholar] [CrossRef]
- Naderi, G.; Azadfar, A.; Yahyazadeh, S.R.; Khatami, F.; Aghamir, S.M.K. Impact of the donor-recipient gender matching on the graft survival from live donors. BMC Nephrol. 2020, 21, 5. [Google Scholar] [CrossRef]
- Cobo, G.; Hecking, M.; Port, F.K.; Exner, I.; Lindholm, B.; Stenvinkel, P.; Carrero, J.J. Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin. Sci. 2016, 130, 1147–1163. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Giral, M.; Foucher, Y.; Karam, G.; Labrune, Y.; Kessler, M.; Hurault de Ligny, B.; Büchler, M.; Bayle, F.; Meyer, C.; Trehet, N.; et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J. Am. Soc. Nephrol. 2010, 21, 1022–1029. [Google Scholar] [CrossRef]
- Reese, P.P.; Feldman, H.I.; Asch, D.A.; Thomasson, A.; Shults, J.; Bloom, R.D. Short-term outcomes for obese live kidney donors and their recipients. Transplantation 2009, 88, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.; Nogueira, J.; Haririan, A.; Jacobs, S.; Weir, M.R.; Cooper, M. The Effect of Living Kidney Donor Obesity on Recipient Renal Outcomes. Am. J. Transplant. 2010, 10, 536. [Google Scholar]
- Tremblay, S.; Kaiser, T.E.; Alloway, R.R.; Woodle, E.S.; Diwan, T.S. Absence of the Effect of Pretransplant Body Mass Index on Post Kidney Transplant Outcomes. Prog. Transplant. 2016, 26, 183–190. [Google Scholar] [CrossRef]
- Espinoza, R.; Gracida, C.; Cancino, J.; Ibarra, A. Effect of obese living donors on the outcome and metabolic features in recipients of kidney transplantation. Transplant. Proc. 2006, 38, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Rotundo, S.; Chiodini, P.; Gagliardi, I.; Michael, A.; Angotti, E.; Borrelli, S.; Serra, R.; Foti, D.; De Sarro, G.; et al. Contribution of Predictive and Prognostic Biomarkers to Clinical Research on Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 5846. [Google Scholar] [CrossRef]
- Almeida, M.; Ribeiro, C.; Silvano, J.; Pedroso, S.; Tafulo, S.; Martins, S.; Ramos, M.; Malheiro, J. Living Donors’ Age Modifies the Impact of Pre-Donation Estimated Glomerular Filtration Rate on Graft Survival. J. Clin. Med. 2023, 12, 6777. [Google Scholar] [CrossRef]
- Nordén, G.; Lennerling, A.; Nyberg, G. Low absolute glomerular filtration rate in the living kidney donor: A risk factor for graft loss. Transplantation 2000, 70, 1360–1362. [Google Scholar] [CrossRef]
- Young, A.; Kim, S.J.; Garg, A.X.; Huang, A.; Knoll, G.; Prasad, G.V.R.; Treleaven, D.; Lok, C.E.; Donor Nephrectomy Outcomes Research (DONOR) Network; Arnold, J.; et al. Living kidney donor estimated glomerular filtration rate and recipient graft survival. Nephrol. Dial. Transplant. 2013, 29, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Savoye, E.; Santin, G.; Legeai, C.; Kerbaul, F.; Gaillard, F.; Pastural, M. Comparison of Kidney Graft Function and Survival in an Emulated Trial with Living Donors and Brain-Dead Donors. Transpl. Int. 2024, 37, 13208. [Google Scholar] [CrossRef] [PubMed]
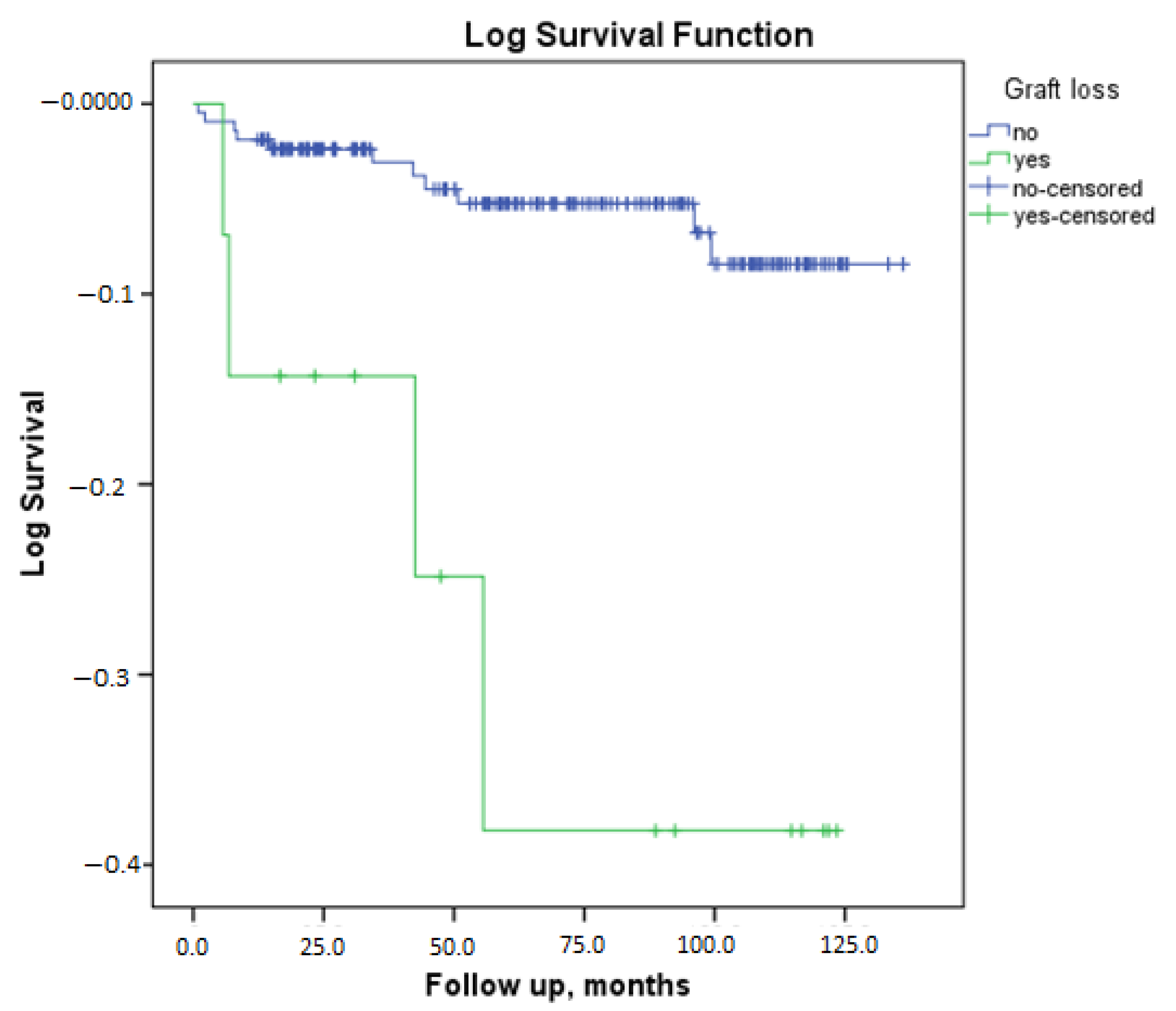
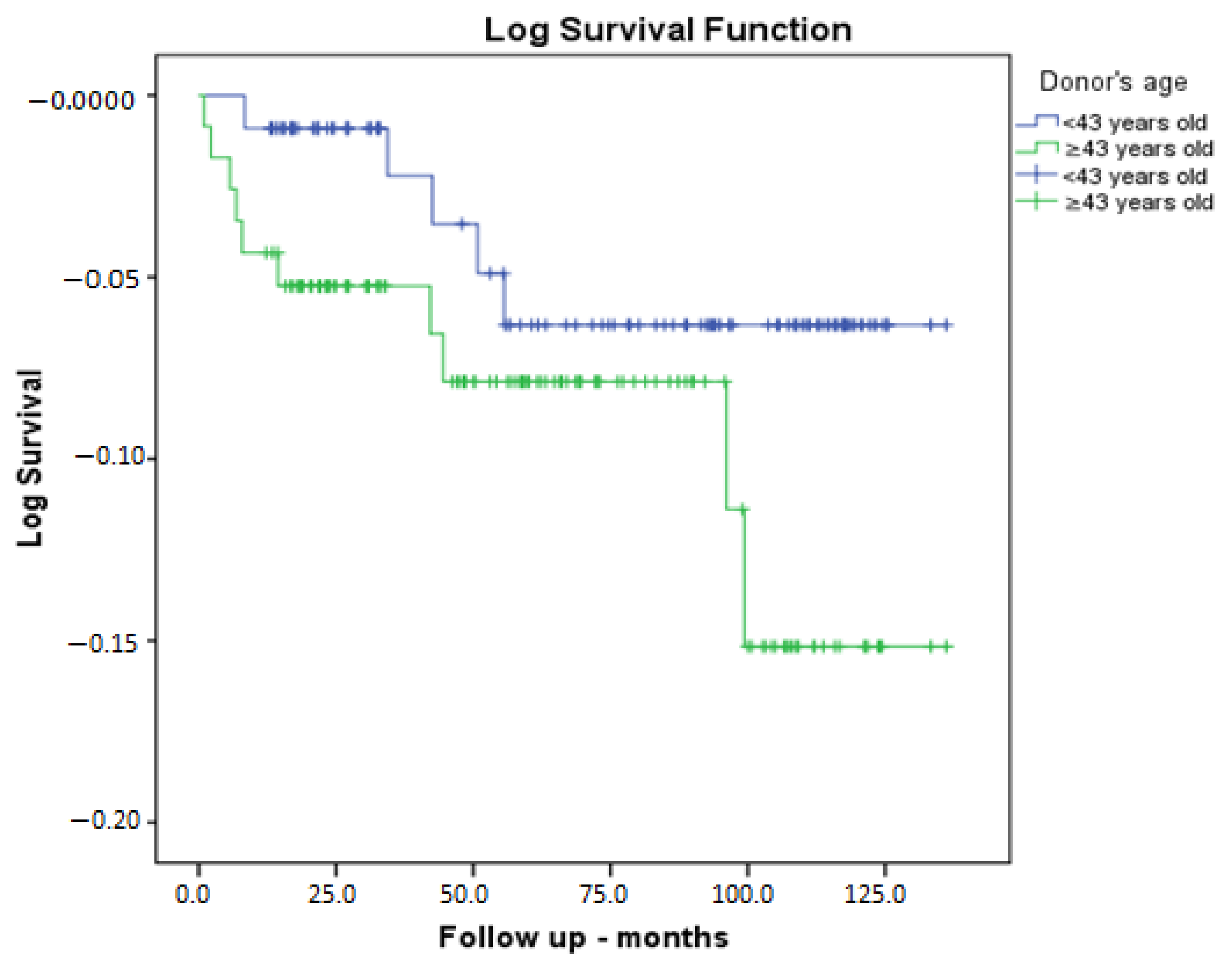
| Parameter | No Graft Loss (n = 214) | Graft Loss Is Present (n = 15) | p |
|---|---|---|---|
| Recipient gender, female/male (%) | 87/127 (40.7%) | 3/12 (20%) | 0.113 a |
| Recipient age | 35 (18–69) | 25 (18–63) | 0.084 b |
| Recipient BMI, kg/m2 | 22.6 (15.8–39.8) | 21.3 (16.8–26.1) | 0.076 b |
| Induction | |||
| No | 11 (5.1%) | 0 (0%) | 0.129 a |
| Simulect | 52 (24.3%) | 7 (46.7%) | |
| Anti-thymocyte globulin (ATG) | 151 (70.6%) | 8 (53.3%) | |
| Creatinine at discharge (mg/dL) | 1.09 (0.45–5.2) | 1.16 (0.65–6.58) | 0.438 b |
| GFR at discharge (mL/dk/1.73 mL2) | 83.7 ± 24.2 | 82.9 ± 39.9 | 0.941 c |
| Tacrolimus, yes/no (yes %) | 210/4 (98.1%) | 14/1 (93.3%) | 0.753 |
| Mycofenolat mofetil (MMF), yes/no (yes %) | 115/99 (53.7%) | 11/4 (73.3%) | 0.140 |
| Mycopheloic acid (MYF), yes/no (yes %) | 96/118 (44.9%) | 4/11 (26.7%) | 0.170 |
| Final creatinine | 1.14 (0.54–4.1) | 6.1 (1.76–12.2) | <0.001 b |
| Final GFR | 72 (14–135) | 11 (5–51) | <0.001 b |
| Greft biopsy, yes/no (%) | 42/172 (19.6%) | 11/4 (73.3%) | <0.001 a |
| Biopsy result | |||
| Normal | 11 (%26.8) | 0 (%0) | |
| Rejection | 16 (%39) | 7 (%63.6) | 0.288 a |
| BK virus-associated Nephropathy(BKVAN) | 2 (%4.9) | 1 (%9.1) | |
| GN | 7 (%17.1) | 1 (%9.1) | |
| Chronic allograft nephropathy(CAN) | 5 (%12.2) | 2 (%18.2) | |
| Recipient blood group | |||
| 0 | 61 (28.5%) | 6 (40%) | |
| A | 83 (38.8%) | 3 (20%) | 0.250 a |
| B | 52 (24.3%) | 3 (20%) | |
| AB | 18 (8.4%) | 3 (20%) | |
| Time spent on dialysis, months | 2 (0–240) | 8 (0–120) | 0.286 b |
| Preemptive, Yes/No (%) | 84/130 (539.3) | 5/10 (33.3%) | 0.649 a |
| Number of HLA mismatches | 3 (0–6) | 3 (0–6) | 0.696 b |
| Primary kidney disease | |||
| DN | 40 (18.7%) | 1 (6.7%) | |
| HT | 14 (6.5%) | 0 (0%) | |
| GN | 19 (8.9%) | 2 (13.3%) | |
| Obstructive pathologies | 19 (8.9%) | 1 (6.7%) | 0.477 a |
| PKD | 3 (1.4%) | 0 (0%) | |
| TIN | 5 (2.3%) | 0 (0%) | |
| Amyloidosis | 4 (1.9%) | 0 (0%) | |
| Hereditary | 2 (0.9%) | 1 (6.7%) | |
| Unknown etiologies | 108 (50.5%) | 10 (66.7%) | |
| Pre-tranplantation | |||
| recipient HT, Yes/No (%) | 56/158 (26.2%) | 9/6 (60%) | 0.005 a |
| DM | 12/2022 (5.6%) | 0/15 (0%) | 0.346 a |
| Coronary artery disease(CAD) | 13/201 (6.1%) | 2/13 (13.3%) | 0.256 a |
| Post-transplantation | |||
| HT, Yes/No (%) | 150/64 (70.1%) | 13/2 (86.7%) | 0.171 a |
| DM | 47/167 (22%) | 2/13 (13.3%) | 0.431 a |
| Coronary artery disease(CAD) | 13/201 (6.1%) | 2/13 (13.3%) | 0.256 a |
| Median follow up duration, months | 65.9 (1–136) | 55.6 (5.7–123.5 | 0.981 |
| Mortality rate | 11/203 (5.1%) | 4/11 (26.7%) | 0.001 a |
| Parameter | No Graft Loss (n = 214) | Graft Loss Is Present (n = 15) | p |
|---|---|---|---|
| Donor gender, female/male (%) | 135/79 (63.1%) | 11/4 (73.3%) | 0.425 a |
| Donor age | 43 (22–74) | 47 (21–62) | 0.348 b |
| Donor BMI, kg/m2 | 26.3 (16.6–39.5) | 26,8 (19.9–38) | 0.609 b |
| Pre-transplantation donor HT Yes/No (%) | 13/204 (6%) | 2/13 (13.3%) | 0.251 |
| Pre-transplantation donor CAD Yes/No (%) | 5/212 (2.3%) | 1/14 (6.7%) | 0.850 |
| Donor operation type | |||
| Open nephrectomy | 90 (42.1%) | 60 (40%) | 0.876 a |
| Laparoscopic nephrectomy | 124 (57.9%) | 9 (60%) | |
| Kidney removed left/right (%left) | 142/72 (%66.4) | 13/2 (86.7%) | 0.104 a |
| Number of arteries | 1 (1–3) | 1 (1–2) | 0.760 b |
| Donor GFR, mL/min/1.73 m2 | 111.3 ± 11.5 | 106.3 ± 10.7 | 0.108 c |
| Donor blood group | |||
| O | 112 (52.3%) | 7 (46.7%) | |
| A | 58 (27.1%) | 4 (26.7%) | 0.196 a |
| B | 38 (17.8%) | 2 (13.3%) | |
| AB | 6 (2.8%) | 2 (13.3%) |
| Recipients’ Post-Transplant Discharge Creatinine | Recipients’ Post-Transplant Discharge GFR | Recipients’ Final Creatinin | Recipients’ Final GFR | ||
|---|---|---|---|---|---|
| Donor eGFR | Correlation Coefficient (r) | −0.219 * | 0.255 * | −0.240 * | 0.302 * |
| p | 0.001 | <0.001 | <0.001 | <0.001 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| CI (%95) | OR | p | RR CI (%95) | OR | p | |
| Recipient characteristic | ||||||
| Gender | Referance male | |||||
| 0.100–1.331 | 0.365 | 0.127 | ||||
| Age | 0.921–1.013 | 0.966 | 0.149 | |||
| BMI | 0.747–1.007 | 0.867 | 0.062 | |||
| Induction | ||||||
| No | Referance | Referance | ||||
| Simulect | 0.000 | 0.000 | 0.999 | 0.000 | 949 | 0.999 |
| ATG | 0.878–7.350 | 2.541 | 0.085 | 0.000 | 2087 | 0.999 |
| Pre-transplatation HT | 1.441–12.425 | 4.232 | 0.009 | 1.937–28.882 | 7.479 | 0.004 |
| Post-transplantation HT | 0.608–12.645 | 2.773 | 0.188 | |||
| CAD | 0.485–11.675 | 2.379 | 0.286 | |||
| Graft biopsy | 3.416–37.132 | 11.262 | <0.001 | 5.387–113.609 | 24.74 | <0.001 |
| Donor characteristics | ||||||
| Kidney side | ||||||
| left | Referance | |||||
| right | 0.067–1.381 | 0.303 | 0.123 | |||
| Blood group | ||||||
| O | Referance | Referance | ||||
| A | 0.310–3.924 | 1.103 | 0.879 | 0.269–5.342 | 1.199 | 0.812 |
| B | 0.168–4.230 | 0.842 | 0.835 | 0.060–4.638 | 0.527 | 0.564 |
| AB | 0.906–31.410 | 5.333 | 0.064 | 0.987–83.106 | 9.055 | 0.051 |
| GFR | 0.918–1.009 | 0.962 | 0.089 | 0.870–0.983 | 0.925 | 0.013 |
| Male Recipient | p | Female Recipient | p | p * | |||
|---|---|---|---|---|---|---|---|
| Recipient Characteristic | Female Donor | Male Donor | Male Donor | Female Donor | |||
| Age | 37 (18–66) | 31 (18–58) | 0.152 | 37.5 (18–69) | 31 (18–59) | 0.008 | 0.023 |
| Recipient BMI, kg/m2 | 23.3 (15.8–34.2) | 21.9 (17.7–34.6) | 0.942 | 23 (16.4–39.8) | 21 (16.2–36.7) | 0.068 | 0.107 |
| Induction | |||||||
| No | 4 (3.8%) | 2 (8.6%) | 2 (4.2%) | 2 (4.8%) | |||
| Simulect | 23 (22.1%) | 12 (34.3%) | 0.15 | 12 (25%) | 12 (28.6%) | 0.91 | 0.666 |
| ATG | 77 (74%) | 20 (57.1%) | 2 | 34 (70.8%) | 28 (67.2%) | 3 | |
| Creatinine atdischarge (mg/dL) | 1.19 (0.47–6.58) | 1.17 (0.68–1.62) | 0.297 | 0.86 (0.45–2.1) | 0.88 (0.46–2.2) | 0.607 | <0.001 |
| GFR at discharge (mL/dk/1.73 mL2) | 78.8 ± 28.4 | 86.7 ± 23.5 | 0.099 | 88.3 ± 26.7 | 87.9 ± 25.5 | 0.951 | 0.059 |
| Final creatinine | 1.29 (0.7–12.2) | 1.2 (0.8–6.1) | 0.232 | 0.9 (0.54–8.23) | 0.95 (0.56–8.7) | 0.786 | <0.001 |
| Final GFR | 66 (5–118) | 78 (11–128) | 0.173 | 72.9 ± 29.9 | 75.9 ± 29.6 | 0.641 | 0.118 |
| Hospitalization days | 9 (6–42) | 8 (6–27) | 0.197 | 9 (6–39) | 9 (6–30) | 0.616 | 0.609 |
| Greft biopsy, yes/no (%) | 30/74 (28.8%) | 7/28 (20%) | 0.306 | 10/38 (20.8%) | 6/36 (14.3%) | 0.418 | 0.252 |
| Biopsy result | |||||||
| Normal | 7 (23.3%) | 2 (28.6%) | |||||
| Rejection | 12 (40%) | 2 (28.6%) | 1 (11.1%) | 1 (16.7%) | |||
| BKVAN | 3 (10%) | 0 (0%) | 0.777 | 6 (66.7%) | 3 (50%) | 0.937 | 0.935 |
| GN | 4 (13.3%) | 2 (28.6%) | 1 (11.1%) | 1 (16.7%) | |||
| CAN | 4 (13.3%) | 1 (14.3%) | 1 (11.1%) | 1 (16.7%) | |||
| Biopsy result | |||||||
| Normal | 1 (3.3%) | 0 (%0) | 1.000 | 0 (%0) | 0 (0%) | 1.000 | 0.862 |
| Anormal | 29 (96.7%) | 7 (%100) | 9 (%100) | 6 (100%) | |||
| Recipient blood group | |||||||
| 0 | 24 (23.1%) d | 15 (%42.9) e | 14 (%39.2) | 14 (33.3%) | |||
| A | 39 (37.5%) d | 13 (%37.1) d | 0.042 | 18 (%37.5) | 16 (38.1%) | 0.495 | 0.257 |
| B | 30 (28.8%) d | 3 (%8.6) e | 11 (%22.9) | 11 (26.2%) | |||
| AB | 11 (%10.6) d | 4 (%11.4) d | 5 (%10.4) | 1 (2.4%) | |||
| Time spent on dialysis, months | 2 (0–240) | 3 (0–197) | 0.622 | 2.5 (0–132) | 0 (0–120) | 0.243 | 0.649 |
| Preemptive, Yes/No (%) | 34/70 (%32.7) | 14/21 (%40) | 0.432 | 20/28 (%41.7) | 21/21 (%50) | 0.428 | 0.257 |
| Number of mismatches | 3.5 (0–6) | 3 (0–6) | 0.025 | 3.5 (0–6) | 3 (0–5) | 0.008 | 0.004 |
| Primary Kidney Disease | |||||||
| DN | 11 (%10.6) | 5 (%14.3) | 3 (%6.3) | 0 (%0) | |||
| HT | 16 (%15.4) | 0 (%0) | 12 (%25) | 8 (%19) | |||
| GN | 10 (%9.6) | 5 (%14.3) | 3 (%6.3) | 3 (%7.1) | |||
| Obstructive pathologies | 8 (%7.7) | 3 (%8.6) | 5 (%10.4) | 4 (%9.5) | |||
| PKD | 1 (%1) | 1 (%2.9) | 0.091 | 0 (%0) | 1 (%2.4) | 0.639 | 0.250 |
| TIN | 1 (%1) | 3 (%8.6) | 1 (%2.1) | 0 (%0) | |||
| Amyloidosis | 1 (%1) | 0 (%0) | 2 (%4.2) | 1 (%2.4) | |||
| Hereditary | 0 (%0) | 1 (%2.9) | 1 (%2.1) | 1 (%2.4) | |||
| Unknown etiologies | 56 (%53.8) | 17 (%48.6) | 21 (%43.8) | 24 (%57.1) | |||
| Pre-transplantation recipient | |||||||
| HT | 34/70 (%32.7) | 9/26 (%74.3) | 0.440 | 13/35 (%27.1) | 9/33 (%21.4) | 0.533 | 0.550 |
| DM | 7/97 (%6.7) | 0/35 (%0) | 0.115 | 4/44 (%8.3) | 1/41 (%2.4) | 0.367 | 0.260 |
| CAD | 9/95 (%8.7) | 3/32 (%8.6) | 1.000 | 2/46 (%4.2) | 1/41 (%2.4) | 1.000 | 0.453 |
| Post-transplantation | |||||||
| HT | 79/25 (%76) | 28/7 (%80) | 0.623 | 33/15 (%68.8) | 23/19 (%54.8) | 0.172 | 0.050 |
| DM, | 29/75 (%27.9) d | 4/31 (%11.4) d,e | 0.048 | 13/35 (%27.1) d,e | 3/39 (%7.1) e | 0.014 | 0.014 |
| CAD | 9/95 (%8.7) | 3/32 (%8.6) | 1.000 | 2/46 (%4.2) | 1/41 (%2.4) | 1.000 | 0.453 |
| Graft loss | 10/94 (%9.6) | 2/33 (%5.7) | 0.477 | 2/46 (%4.2) | 1/41 (%2.4) | 1.000 | 0.351 |
| Mortality rate | 11/93 (%10.6) | 1/34 (%2.9) | 0.295 | 3/45 (%6.3) | 0/42 (%0) | 0.245 | 0.090 |
| Donor age | 42 ± 11.4 | 43.4 ± 11.7 | 0.550 | 44 (21–61) | 46.8 (25–74) | 0.128 | 0.180 |
| Donor BMI, kg/m2 | 26.7 (19.6–39.5) | 26.4 (19.1–34) | 0.325 | 25.6 ± 3.6 | 27.6 ± 4.7 | 0.035 | 0.093 |
| Donor operation type | 0.293 | 0.172 | 0.377 | ||||
| Open nephrectomy | 40 (%38.5) | 17 (%48.6) | 24 (%50) | 15 (%35.7) | |||
| Laparoscopic nephrectomy | 64 (%61.5) | 18 (%51.4) | 24 (%50) | 27 (%67.3) | |||
| Kidney removed left/right (%left) | 74/30 (%71.2) | 24/11 (%68.6) | 0.772 | 32/16 (%66.7) | 25/17 (%59.5) | 0.483 | 0.596 |
| Number of arteries | 1 (1–3) | 1 (1–3) | 0.864 | 1 (1–2) | 1 (1–3) | 0.783 | 0.590 |
| Donor GFR, mL/min/1.73 m2 | 111.5 ± 11.8 | 111.7 ± 11.8 | 0.913 | 110.8 ± 11.9 | 109 ± 9.9 | 0.432 | 0.712 |
| Donor GFR/Recipient BMI | 4.85 ± 0.92 | 4.83 ± 1 | 0.905 | 4.7 (2.5–7.4) | 5.5 (2.4–6.7) | 0.143 | 0.296 |
| Donor blood group | |||||||
| 0 | 57 (%54.8) | 18 (%51.4) | 24 (%50) | 20 (%47.6) | |||
| A | 27 (%26) | 11 (%31.4) | 0.495 | 12 (%25) | 12 (%28.6) | 0.797 | 0.461 |
| B | 16 (%15.4) | 3 (%8.6) | 11 (%22.9) | 10 (%23.8) | |||
| AB | 4 (%3.8) | 3 (%8.6) | 1 (%2.1) | 0 (%0) | |||
| Donor Age | p | ||
|---|---|---|---|
| <43 (n = 111) | ≥43 (n = 118) | ||
| Donor GFR | 118 (83–143) | 107 (73–131) | <0.001 a |
| Donor BMI | 25.9 (16.6–36.8) | 27.1 (18.9–39.5) | 0.001 a |
| Recipient GFR at discharge (mL/dk/1.73 mL2) | 88.3 ± 23.4 | 79.4 ± 26.6 | 0.009 b |
| Donor final creatinin | 1.09 (0.56–9.8) | 1.24 (0.54–12.2) | 0.026 a |
| Donor final GFR | 75 (5–135) | 65.5 (5–126) | 0.008 a |
| Median follow up time, month | 78.6 (8.4–136) | 59 (1–136.5) | 0.029 a |
| Graft loss, yes/no (yes %) | 6/105 (%) | 9/109 (%) | 0.497 c |
| Mortality rate, yes/no (yes %) | 5/106 (4.5%) | 10/108 (8.5%) | 0.225 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakmak, U.; Merhametsiz, O.; Ay, N. The Impact of Living Kidney Donor Glomerular Filtration Rate on Graft Survival. Medicina 2025, 61, 580. https://doi.org/10.3390/medicina61040580
Cakmak U, Merhametsiz O, Ay N. The Impact of Living Kidney Donor Glomerular Filtration Rate on Graft Survival. Medicina. 2025; 61(4):580. https://doi.org/10.3390/medicina61040580
Chicago/Turabian StyleCakmak, Umit, Ozgur Merhametsiz, and Nurettin Ay. 2025. "The Impact of Living Kidney Donor Glomerular Filtration Rate on Graft Survival" Medicina 61, no. 4: 580. https://doi.org/10.3390/medicina61040580
APA StyleCakmak, U., Merhametsiz, O., & Ay, N. (2025). The Impact of Living Kidney Donor Glomerular Filtration Rate on Graft Survival. Medicina, 61(4), 580. https://doi.org/10.3390/medicina61040580






