Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Material and Methods
2.1. Immunohistochemical Study
2.2. Statistical Methods
3. Results
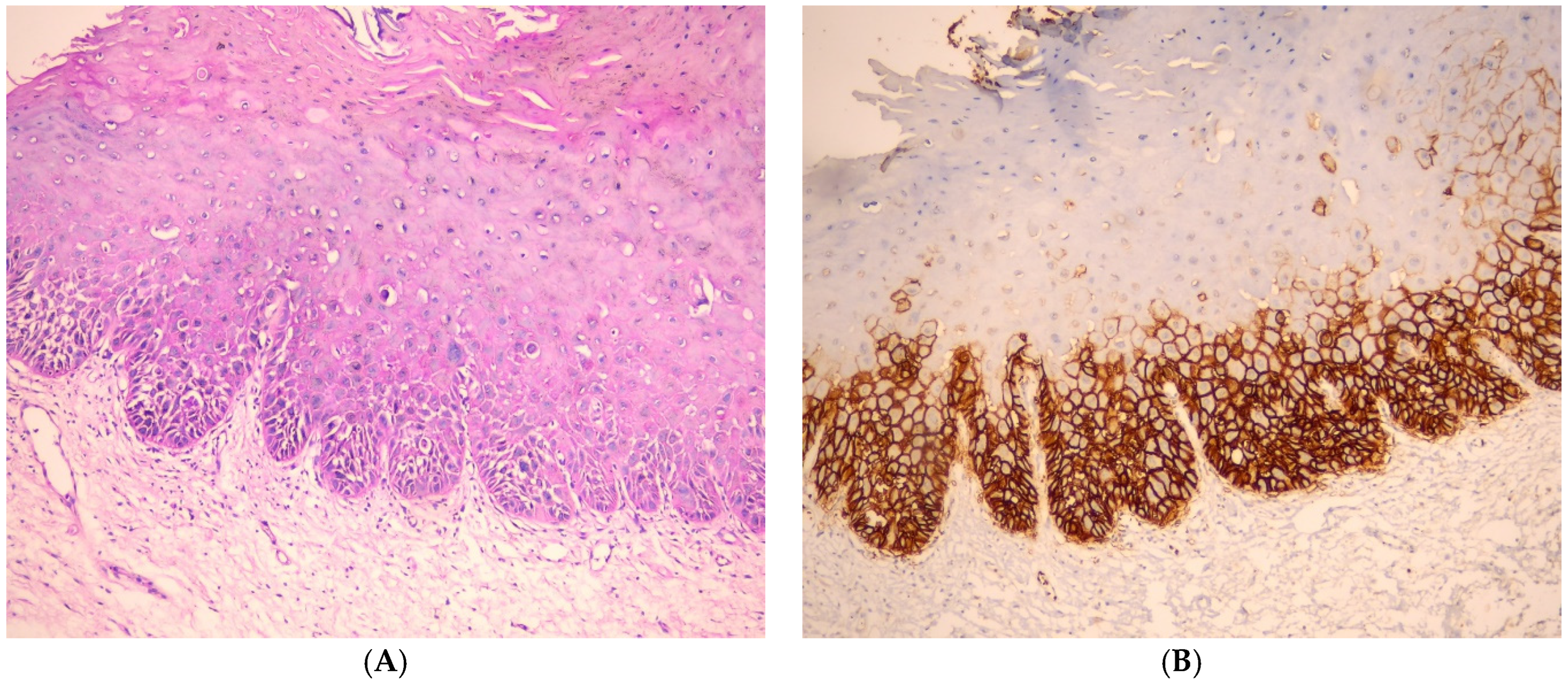
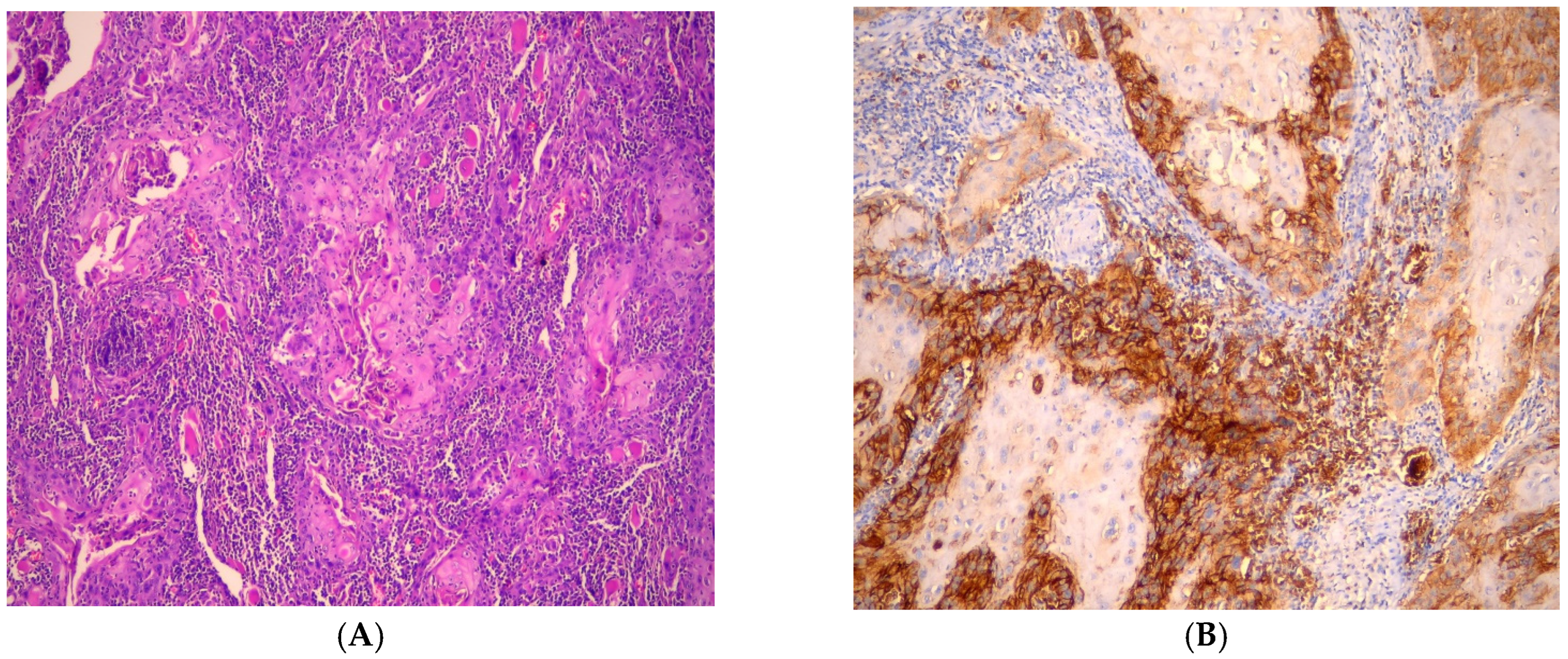
3.1. Immunohistochemical Findings of GLUT-1
3.2. Evaluation of GLUT-1 Immunostaining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zdrojewski, J.; Nowak, M.; Nijakowski, K.; Jankowski, J.; Scribante, A.; Gallo, S.; Pascadopoli, M.; Surdacka, A. Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review. Biomedicines 2024, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in oral cancer—A global scenario. Nepal J. Epidemiol. 2016, 6, 613–619. [Google Scholar] [CrossRef]
- Sriram, S.; Hasan, S.; Alqarni, A.; Alam, T.; Kaleem, S.M.; Aziz, S.; Durrani, H.K.; Ajmal, M.; Dawasaz, A.A.; Saeed, S. Efficacy of Platelet-Rich Plasma Therapy in Oral Lichen Planus: A Systematic Review. Medicina 2023, 59, 746. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef]
- Salama, D.E.; Hasan, A.; Balbola, G.A.; Taha, A.M.; Abdel-Samia, H.M.; Taha, A.K.; Radwan, G.S.; Rifat, R.; Abdel-Maqsoud, M.F.; Samman, A.; et al. Immunohistochemical comparison of CD44 and hypoxia inducible Factor-1 alpha (HIF-1α) in Oral squamous cell carcinoma and Oral epithelial dysplasia: A comparative study. J. Med. Chem. Sci. 2024, 7, 9–23. [Google Scholar]
- Fayed TM, M.E.; Abd Algaleel, M.A.; El Azony, H.A. Pattern of Recurrence of Oral Cavity Squamous Cell Carcinoma; A clinicopathological study. Al-Azhar Int. Med. J. 2024, 5, 33. [Google Scholar] [CrossRef]
- Ehtisham, M.; Gupta, S.; Khan, S. Expression of Glut-1 in Oral Epithelial Dysplasia and OSCC: An Immunohistochemical Study. Int. J. Contemp. Med. Res. 2020, 7, 15–19. [Google Scholar]
- Augustin, R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life 2010, 62, 315–333. [Google Scholar] [CrossRef]
- Poon, E.; Harris, A.L.; Ashcroft, M. Targeting the hypoxia-inducible factor (HIF) pathway in cancer. Expert Rev. Mol. Med. 2009, 11, e26. [Google Scholar] [CrossRef]
- Kumar, D. Regulation of glycolysis in head and neck squamous cell carcinoma. Postdoc J. J. Postdr. Res. Postdr. Aff. 2017, 5, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhong, J.-T.; Zhou, S.-H.; Han, H.-M. Roles of GLUT-1 and HK-II expression in the biological behavior of head and neck cancer. Oncotarget 2019, 10, 3066–3083. [Google Scholar] [CrossRef]
- Nakazato, K.; Mogushi, K.; Kayamori, K.; Tsuchiya, M.; Takahashi, K.-I.; Sumino, J.; Michi, Y.; Yoda, T.; Uzawa, N. Glucose metabolism changes during the development and progression of oral tongue squamous cell carcinomas. Oncol. Lett. 2019, 18, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, P.; Shyam, N.D.V.N.; Kumar, G.K.; Narayen, V.; Konda, P.; Mudududla, P. Evaluation of glucose transporter-1 expression in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211. [Google Scholar] [CrossRef]
- Pereira, K.M.A.; Chaves, F.N.; Viana, T.S.A.; Carvalho, F.S.R.; Costa, F.W.G.; Alves, A.P.N.N.; Sousa, F.B. Oxygen metabolism in oral cancer: HIF and GLUTs (Review). Oncol. Lett. 2013, 6, 311–316. [Google Scholar] [CrossRef]
- Singh, D.; Arora, R.; Kaur, P.; Singh, B.; Mannan, R.; Arora, S. Overexpression of hypoxia-inducible factor and metabolic pathways: Possible targets of cancer. Cell Biosci. 2017, 7, 62. [Google Scholar] [CrossRef]
- Pereira, K.M.A.; Feitosa, S.G.; Lima, A.T.T.; Luna, E.C.M.; Cavalcante, R.B.; Lima, K.C.D.; Chaves, F.N.; Costa, F.W.G. Immunohistochemical Evaluation of Glucose Transporter Type 1 in Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2016, 17, 147–151. [Google Scholar] [CrossRef]
- Reisser, C.; Eichhorn, K.; Herold-Mende, C.; Born, A.I.; Bannasch, P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int. J. Cancer 1999, 80, 194–198. [Google Scholar] [CrossRef]
- Airley, R.; Loncaster, J.; Davidson, S.; Bromley, M.; Roberts, S.; Patterson, A.; Hunter, R.; Stratford, I.; West, C. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 928–934. [Google Scholar]
- Haneef, A.N.M.; Gopalakrishnan, S.; Jayaseelan, V.P.; Ramamurthi, R.; Muniapillai, S. GLUT1 Expression in Oral Squamous Cell Carcinoma and Its Significance. Middle East J. Cancer 2024, 15, 189–198. [Google Scholar]
- Attur, S.K.; Patel, A.; Attur, K.M. Study of expression of GLUT-1 in oral potentially malignant disorders and oral squamous cell carcinoma: An immuno-histochemical analysis. J. Oral Maxillofac. Pathol. 2024, 28, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The Prognostic value of GLUT-1 in cancers:A systematic review and meta-analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]



| Groups | Mean GLUT1 Area% | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | F-Value | p-Value | |
| OED | 17.13 | 3.02 | 14.209 | 20.239 | 348.026 | <0.0001 * |
| WDSCC | 13.42 | 3.309 | 10 | 17.208 | ||
| MDSCC | 38.78 | 3.692 | 35.887 | 42.939 | ||
| PDSCC | 47.37 | 2.392 | 43.913 | 50.552 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, R.G.; Hashim, M.I.; Bawahab, A.A.; Baloush, R.A.A.; Abdelwahed, M.S.; Hasan, A.; Ismail, K.A.; Abd-Elhameed, N.R.; Embaby, A.; Sharfeldeen, A.E.R.M. Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma. Medicina 2025, 61, 557. https://doi.org/10.3390/medicina61040557
Mostafa RG, Hashim MI, Bawahab AA, Baloush RAA, Abdelwahed MS, Hasan A, Ismail KA, Abd-Elhameed NR, Embaby A, Sharfeldeen AERM. Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma. Medicina. 2025; 61(4):557. https://doi.org/10.3390/medicina61040557
Chicago/Turabian StyleMostafa, Rahma Gamal, Mohammad Ibrahim Hashim, Ahmed Abdulwahab Bawahab, Razan Abed A. Baloush, Mohammed S. Abdelwahed, Abdulkarim Hasan, Khadiga A. Ismail, Nageh Rady Abd-Elhameed, Ahmed Embaby, and Abd El Rahman M. Sharfeldeen. 2025. "Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma" Medicina 61, no. 4: 557. https://doi.org/10.3390/medicina61040557
APA StyleMostafa, R. G., Hashim, M. I., Bawahab, A. A., Baloush, R. A. A., Abdelwahed, M. S., Hasan, A., Ismail, K. A., Abd-Elhameed, N. R., Embaby, A., & Sharfeldeen, A. E. R. M. (2025). Immunohistochemical Expression of Glucose Transporter-1 in Oral Epithelial Dysplasia and Different Grades of Oral Squamous Cell Carcinoma. Medicina, 61(4), 557. https://doi.org/10.3390/medicina61040557