Scar Management in Pediatric Patients
Abstract
1. Introduction
2. Methods
3. Managing the Scar Before It Forms
3.1. Incision Planning
3.2. Wound Closure
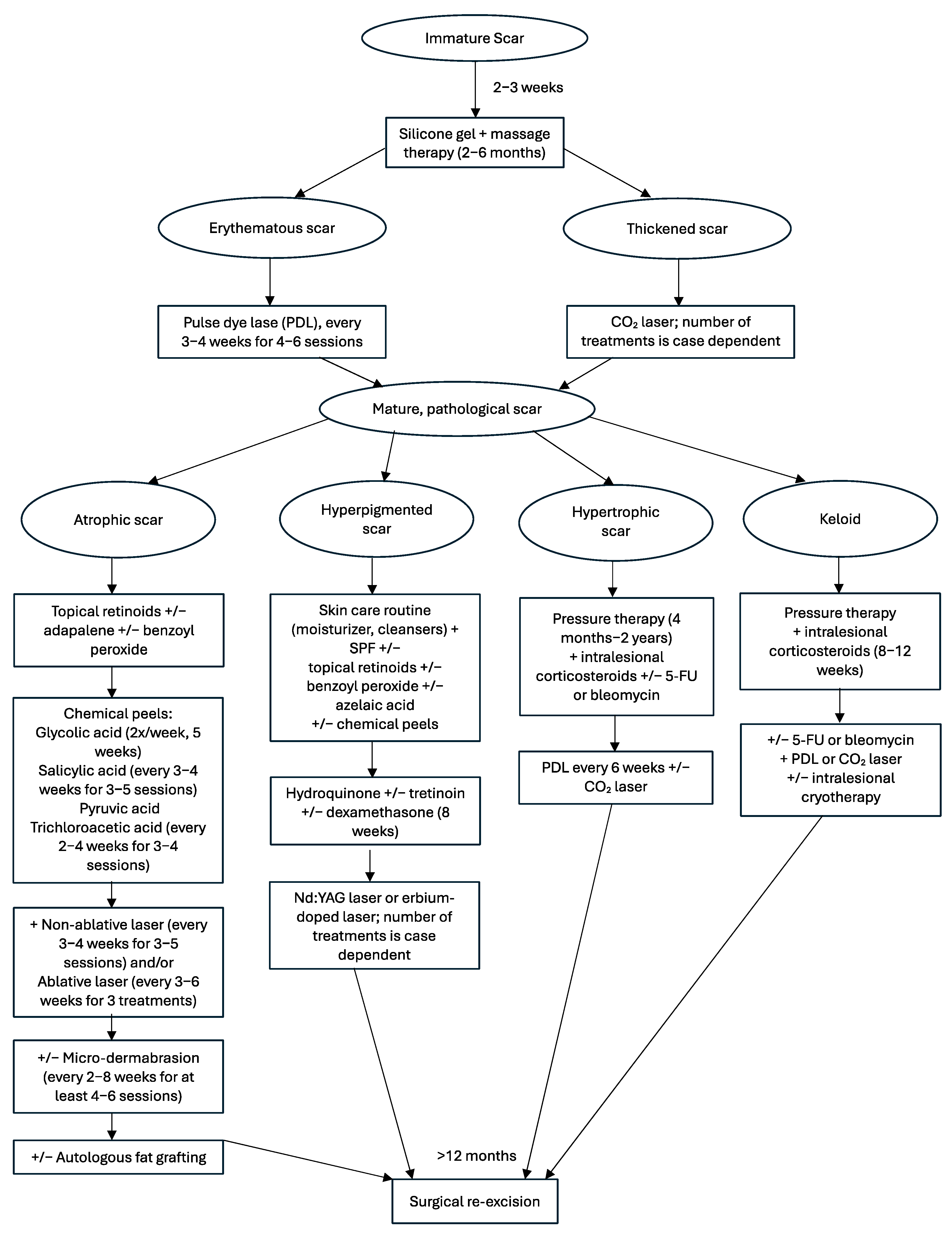
4. Immature Scars
5. Mature Scars
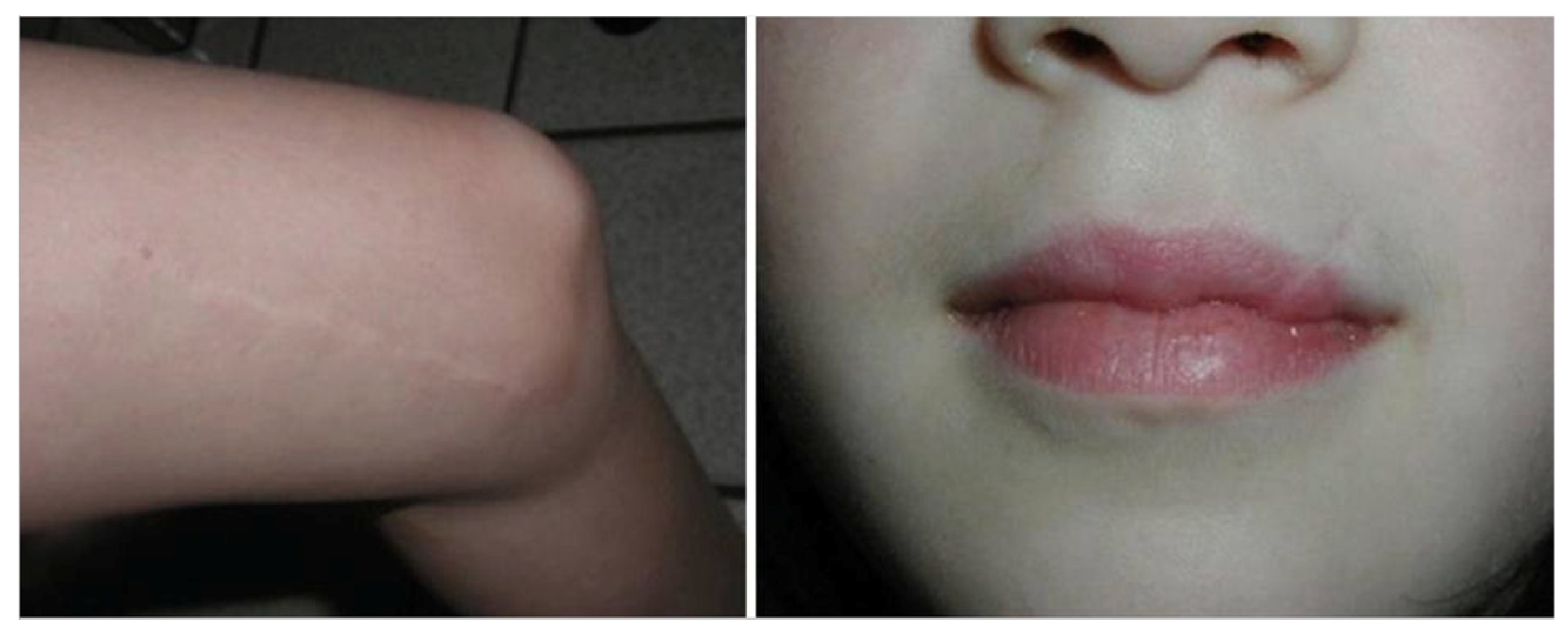
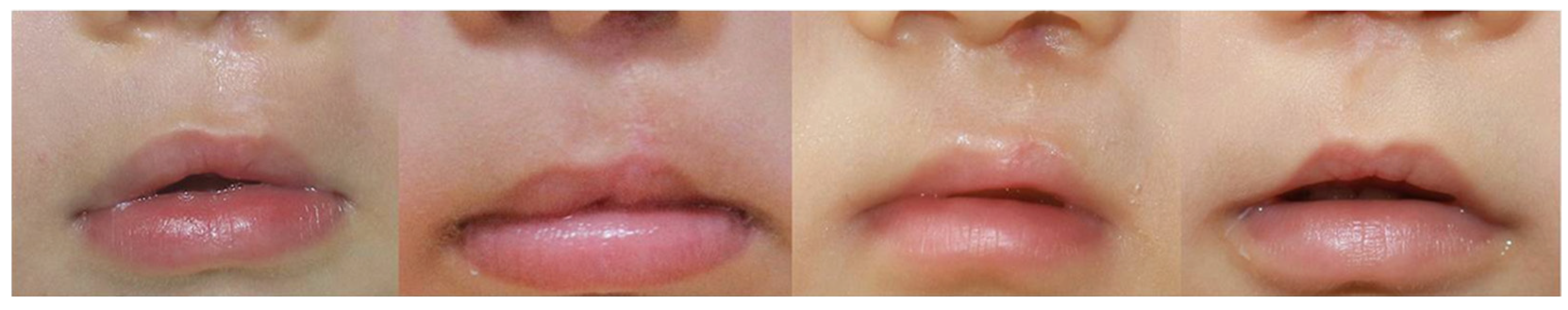
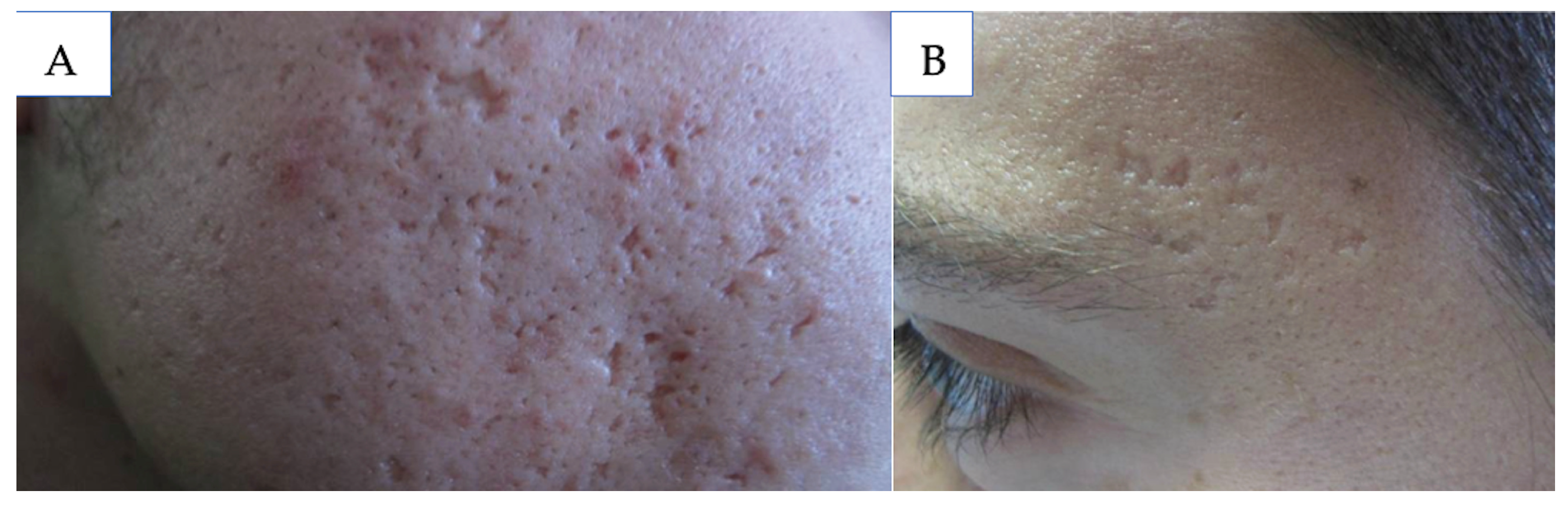
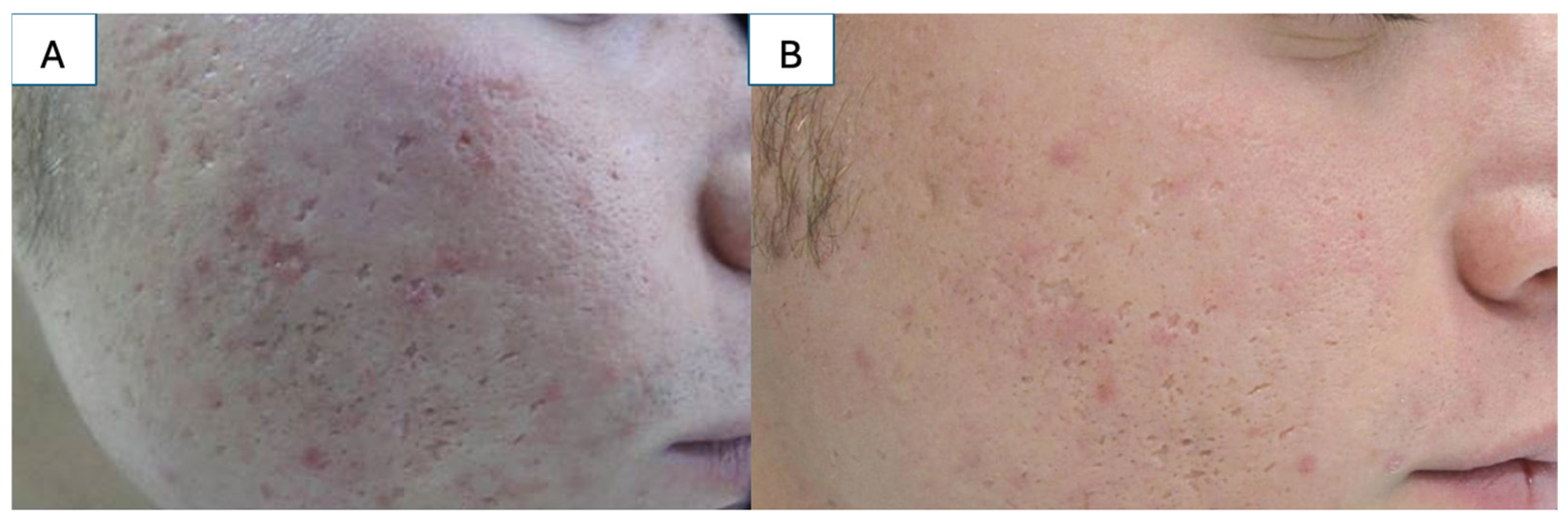
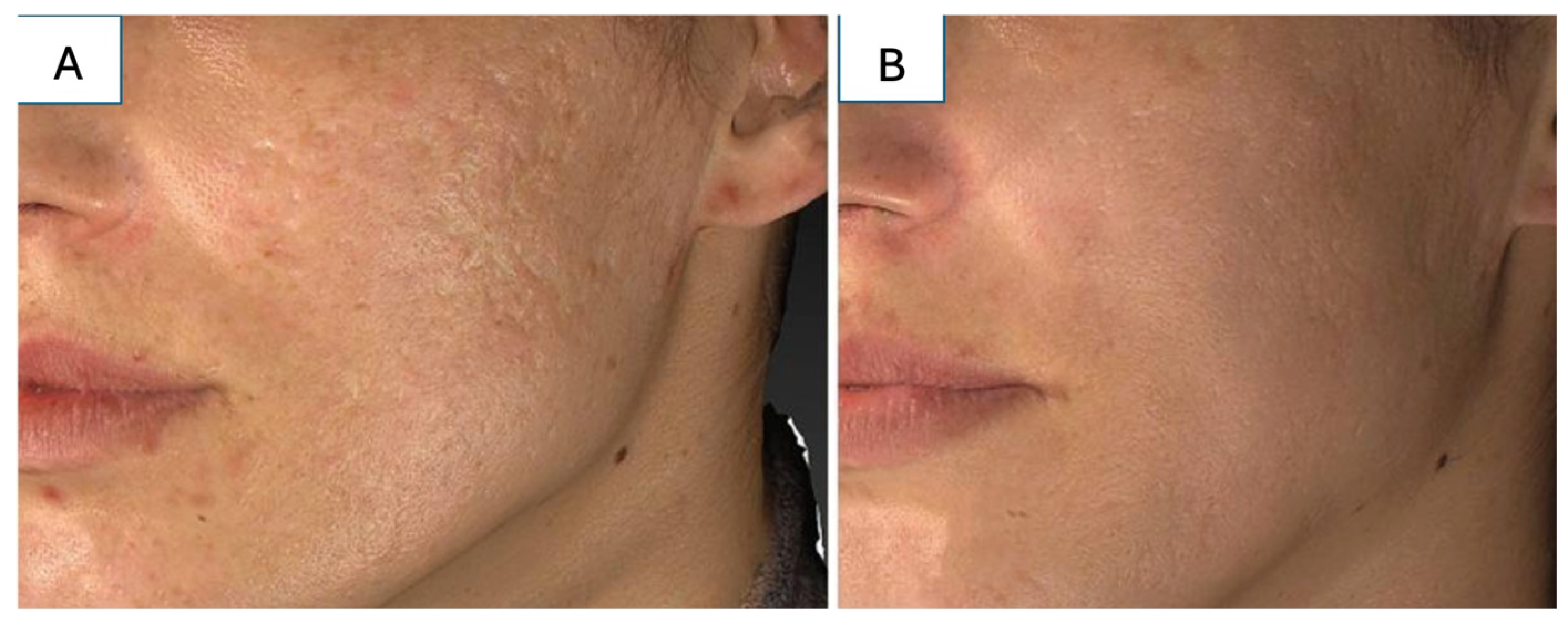
5.1. Atrophic
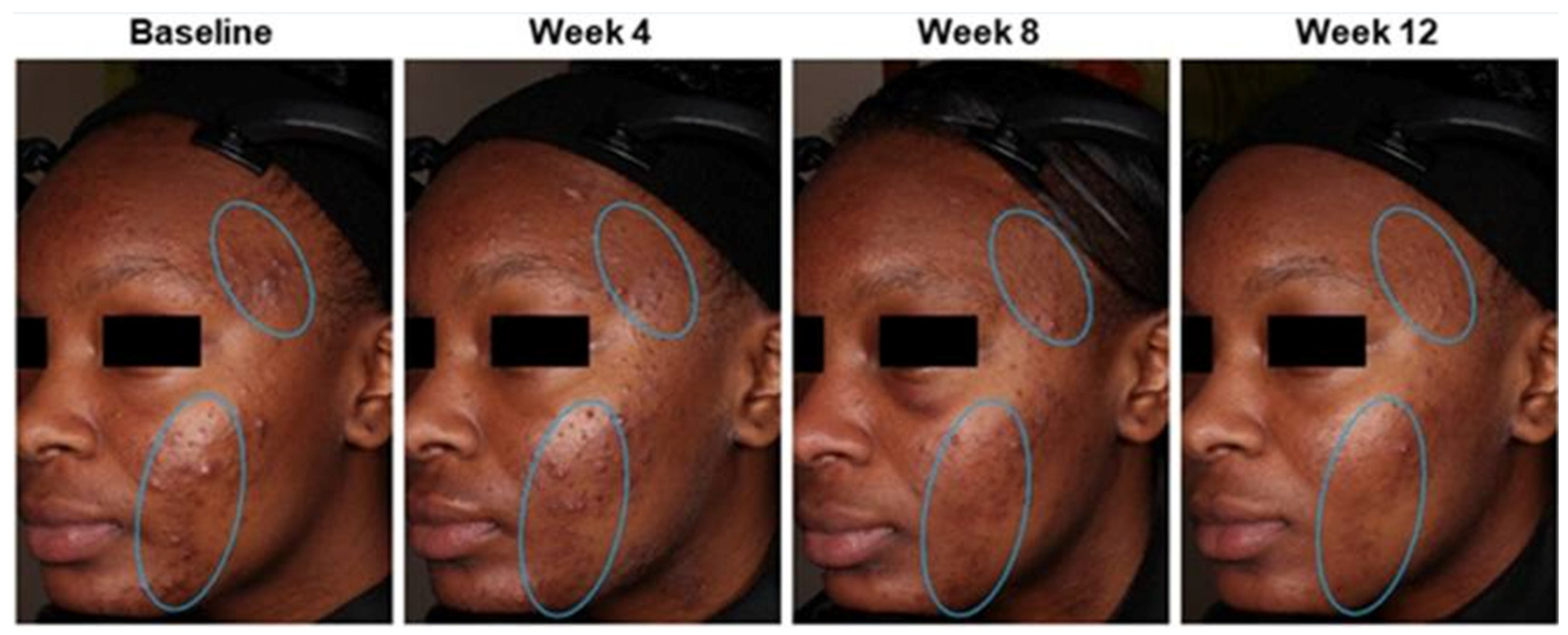
5.2. Hyperpigmented
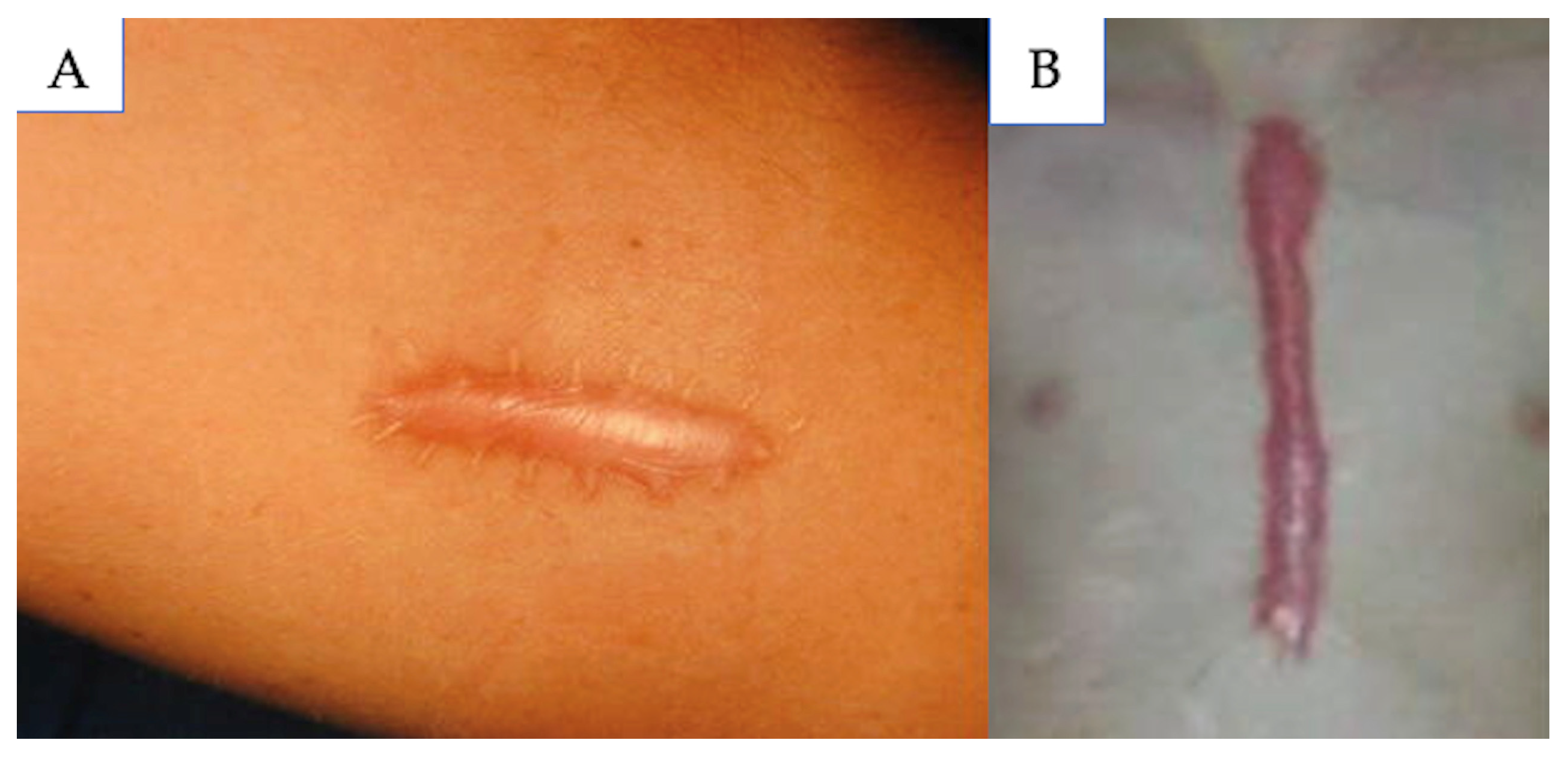
5.3. Hypertrophic
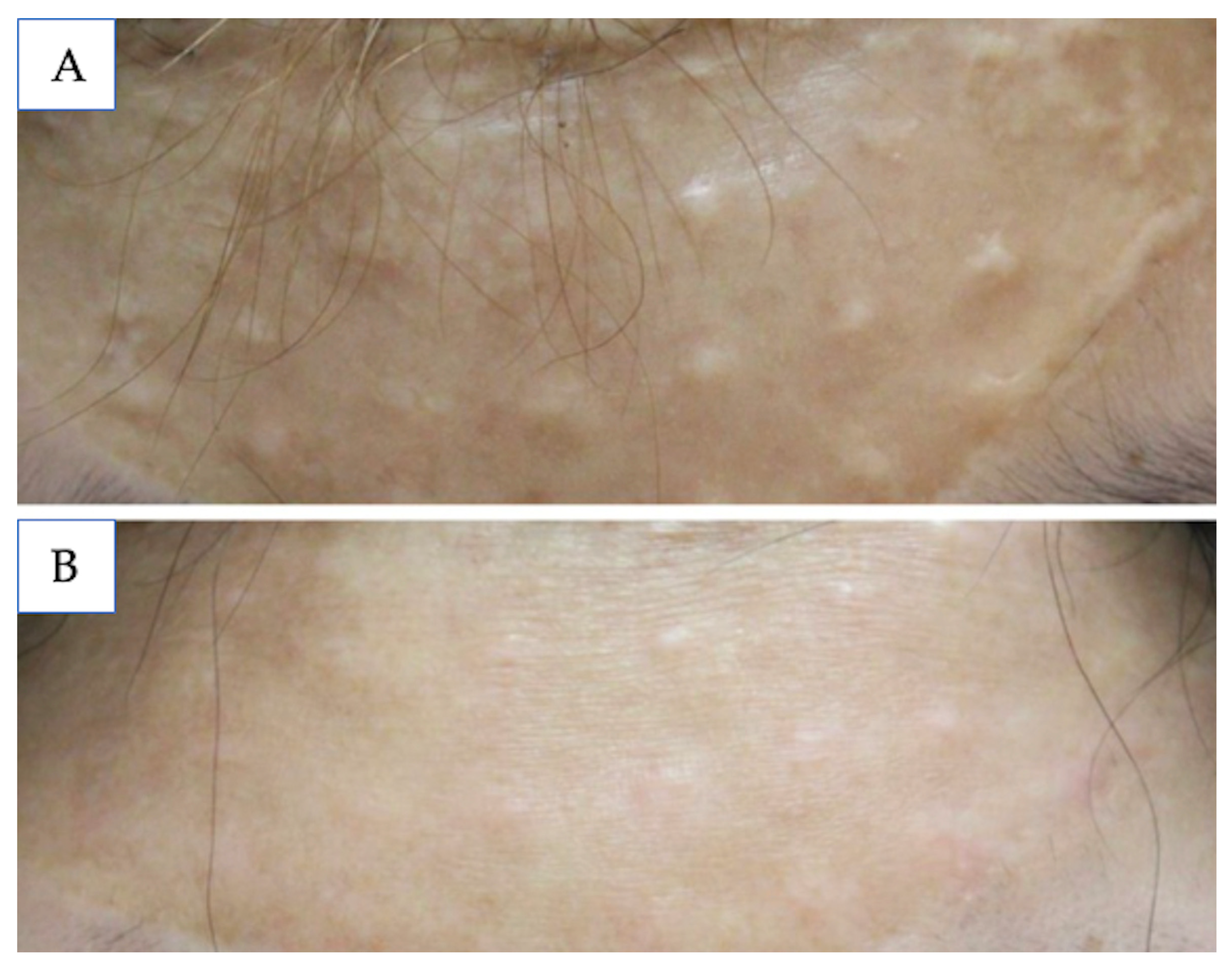
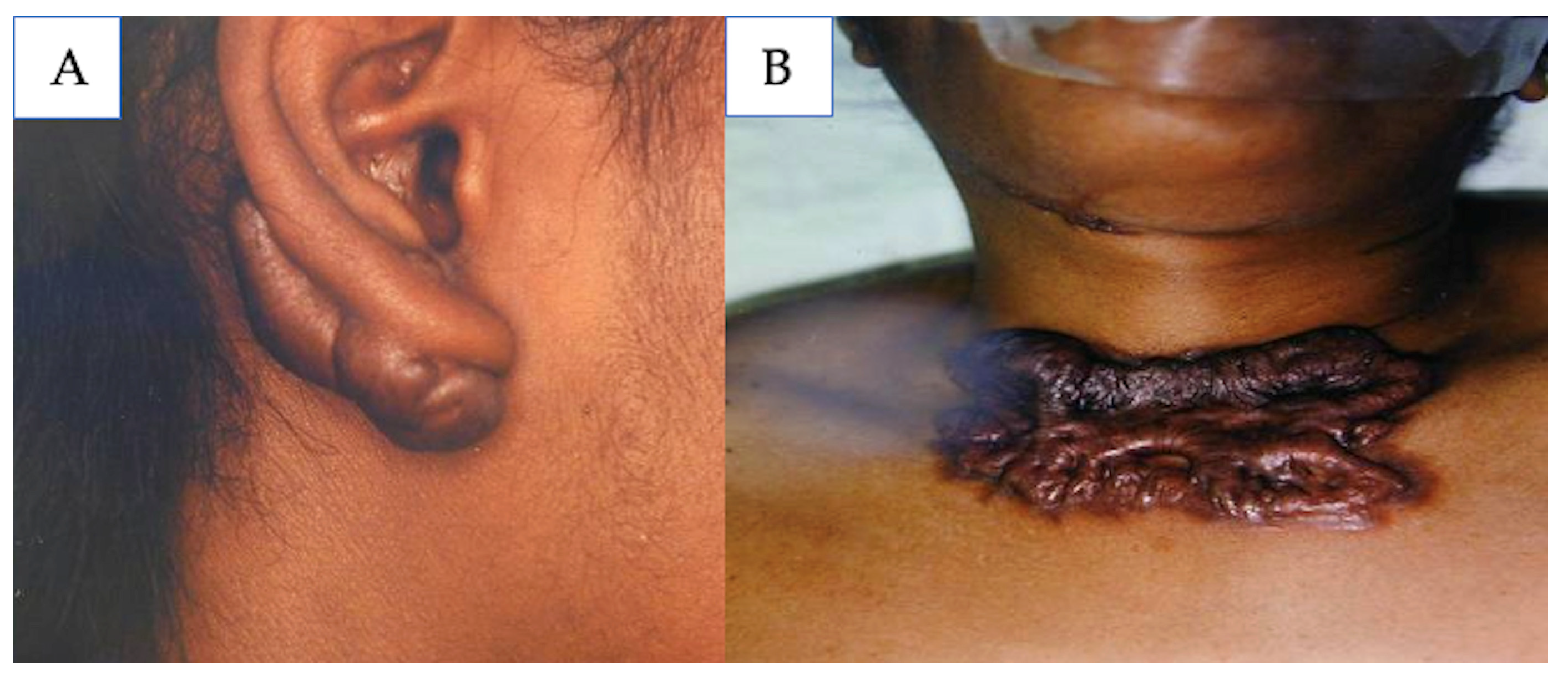
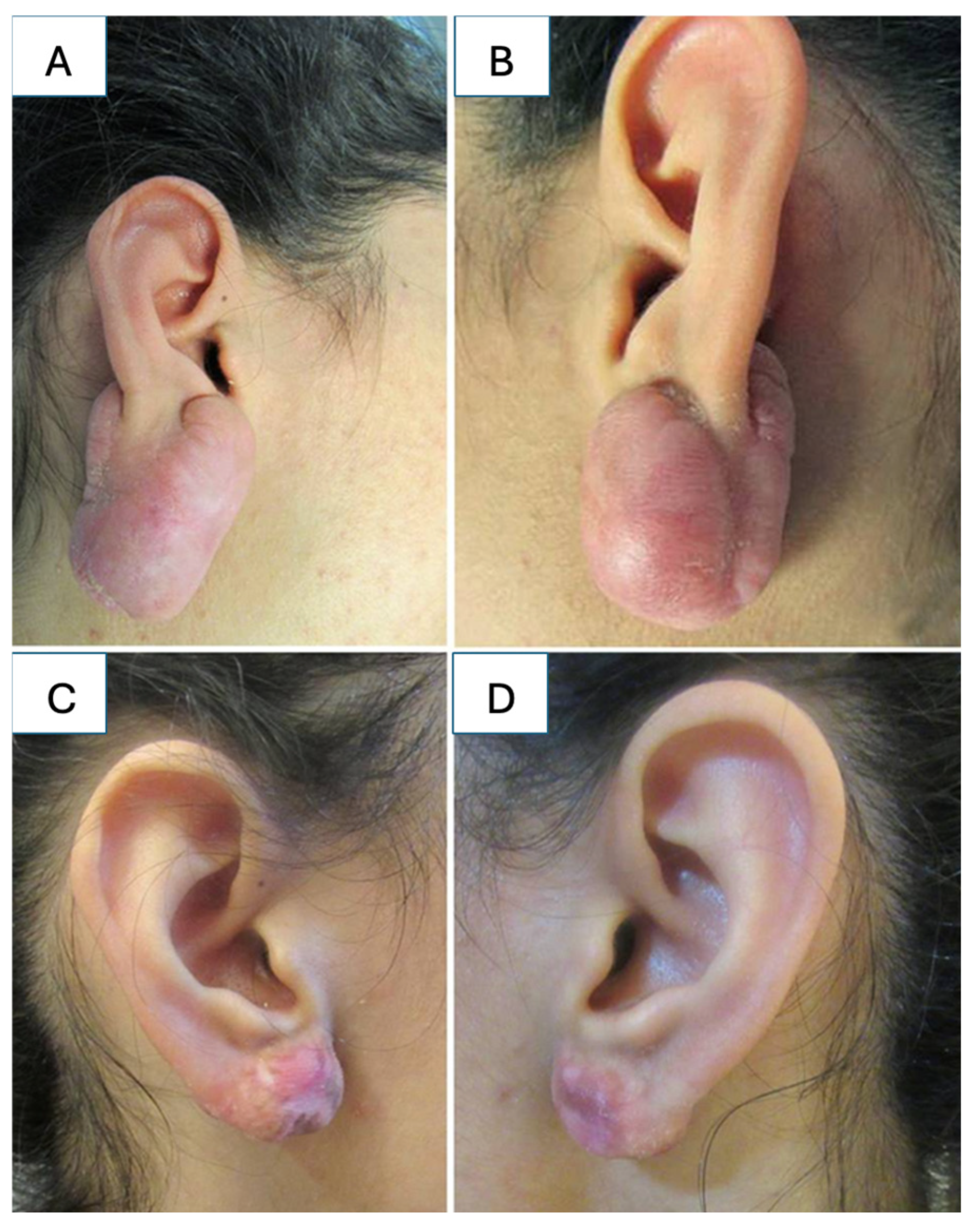
5.4. Keloids
6. Limitations and Future Directions
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garg, S.; Dahiya, N.; Gupta, S. Surgical scar revision: An overview. J. Cutan. Aesthetic Surg. 2014, 7, 3–13. [Google Scholar] [CrossRef]
- Le Touze, A. Scars in Pediatric Patients. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 46. [Google Scholar] [CrossRef]
- Ogawa, R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2022, 149, 79e–94e. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jang, Y.J. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Harijan, A. Overview of surgical scar prevention and management. J. Korean Med. Sci. 2014, 29, 751–757. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Dauskardt, R.H.; Wong, V.W.; Bhatt, K.A.; Wu, K.; Vial, I.N.; Padois, K.; Korman, J.M.; Longaker, M.T. Improving cutaneous scar formation by controlling the mechanical environment: Large animal and phase I studies. Ann. Surg. 2011, 254, 217–225. [Google Scholar] [CrossRef]
- Hom, D.B.; Bernstein, J.D. Reducing Risks of Facial Scarring. Facial Plast. Surg. Clin. N. Am. 2023, 31, 195–207. [Google Scholar] [CrossRef]
- Azmat, C.E.; Council, M. Wound Closure Techniques. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Wu, T. Plastic surgery made easy—Simple techniques for closing skin defects and improving cosmetic results. Aust. Fam. Physician 2006, 35, 492–496. [Google Scholar] [PubMed]
- Joo, J.S.; Zhuang, A.R.; Tchanque-Fossuo, C.; Tartar, D.; Armstrong, A.W.; King, T.H.; Sivamani, R.K.; Eisen, D.B. Dermal suture only versus layered closure: A randomized, split wound comparative effectiveness trial. J. Am. Acad. Dermatol. 2019, 81, 1346–1352. [Google Scholar] [CrossRef]
- Janis, J.E. Essentials of Plastic Surgery, 3rd ed.; Thieme: New York, NY, USA, 2023. [Google Scholar]
- Alinasab, B.; Haraldsson, P.O. Rapid Resorbable Sutures Are a Favourable Alternative to Non-resorbable Sutures in Closing Transcolumellar Incision in Rhinoplasty. Aesthet. Plast. Surg. 2016, 40, 449–452. [Google Scholar] [CrossRef]
- Bozan, A.; Dizdar, D. Absorbable Versus Non-Absorbable Sutures in Open Rhinoplasty: Analysis of Columellar Scarring. J. Craniofac. Surg. 2021, 32, 1075–1078. [Google Scholar] [CrossRef]
- Luo, W.; Tao, Y.; Wang, Y.; Ouyang, Z.; Huang, J.; Long, X. Comparing running vs interrupted sutures for skin closure: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Wallace, C.G.; Hsiao, Y.C.; Huang, J.J.; Chen, Z.C.; Chang, C.J.; Lo, L.J.; Chen, P.K.; Chen, J.P.; Chen, Y.R. Clinical evaluation of silicone gel in the treatment of cleft lip scars. Sci. Rep. 2018, 8, 7422. [Google Scholar] [CrossRef]
- Grigoryan, K.V.; Kampp, J.T. Summary and evidence grading of over-the-counter scar treatments. Int. J. Dermatol. 2020, 59, 1136–1143. [Google Scholar] [PubMed]
- Tran, B.; Wu, J.J.; Ratner, D.; Han, G. Topical Scar Treatment Products for Wounds: A Systematic Review. Dermatol. Surg. 2020, 46, 1564–1571. [Google Scholar]
- O’Brien, L.; Jones, D.J. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2013, 2013, CD003826. [Google Scholar]
- Kim, J.S.; Hong, J.P.; Choi, J.W.; Seo, D.K.; Lee, E.S.; Lee, H.S. The Efficacy of a Silicone Sheet in Postoperative Scar Management. Adv. Skin Wound Care 2016, 29, 414–420. [Google Scholar]
- Hsu, K.C.; Luan, C.W.; Tsai, Y.W. Review of Silicone Gel Sheeting and Silicone Gel for the Prevention of Hypertrophic Scars and Keloids. Wounds 2017, 29, 154–158. [Google Scholar]
- de Giorgi, V.; Sestini, S.; Mannone, F.; Papi, F.; Alfaioli, B.; Gori, A.; Lotti, T. The use of silicone gel in the treatment of fresh surgical scars: A randomized study. Clin. Exp. Dermatol. 2009, 34, 688–693. [Google Scholar]
- Rabello, F.B.; Souza, C.D.; Farina Júnior, J.A. Update on hypertrophic scar treatment. Clinics 2014, 69, 565–573. [Google Scholar]
- Mustoe, T.A. Silicone Gel for Scar Prevention. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 203–208. [Google Scholar]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burn. J. Int. Soc. Burn. Inj. 2014, 40, 1255–1266. [Google Scholar] [CrossRef]
- Commander, S.J.; Chamata, E.; Cox, J.; Dickey, R.M.; Lee, E.I. Update on Postsurgical Scar Management. Semin. Plast. Surg. 2016, 30, 122–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [PubMed]
- Berman, B.; Viera, M.H.; Amini, S.; Huo, R.; Jones, I.S. Prevention and management of hypertrophic scars and keloids after burns in children. J. Craniofac. Surg. 2008, 19, 989–1006. [Google Scholar] [PubMed]
- Shin, T.M.; Bordeaux, J.S. The role of massage in scar management: A literature review. Dermatol. Surg. 2012, 38, 414–423. [Google Scholar]
- Ward, R.E.; Sklar, L.R.; Eisen, D.B. Surgical and Noninvasive Modalities for Scar Revision. Dermatol. Clin. 2019, 37, 375–386. [Google Scholar]
- Bianchi, F.A.; Roccia, F.; Fiorini, P.; Berrone, S. Use of Patient and Observer Scar Assessment Scale for evaluation of facial scars treated with self-drying silicone gel. J. Craniofac. Surg. 2010, 21, 719–723. [Google Scholar]
- Xiao, A.; Ettefagh, L. Laser Revision of Scars. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Leszczynski, R.; da Silva, C.A.; Pinto, A.C.P.N.; Kuczynski, U.; da Silva, E.M. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2022, 9, CD011642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balaraman, B.; Geddes, E.R.; Friedman, P.M. Best Reconstructive Techniques: Improving the Final Scar. Dermatol. Surg. 2015, 41 (Suppl. S10), S265–S275. [Google Scholar]
- Kim, D.H.; Ryu, H.J.; Choi, J.E.; Ahn, H.H.; Kye, Y.C.; Seo, S.H. A comparison of the scar prevention effect between carbon dioxide fractional laser and pulsed dye laser in surgical scars. Dermatol. Surg. 2014, 40, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff TC, H.; Beekman, V.K.; van der List, J.P.; Niessen, F.B. Laser treatment of specific scar characteristics in hypertrophic scars and keloid: A systematic review. J. Plast. Reconstr. Aesthet. Surg. JPRAS 2021, 74, 48–64. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.S.; Kim, H.K.; Kim, S.Y.; Lee, J.; Kim, Y.C.; Park, Y.J. Very Early Pulsed Dye Laser Intervention for Optimal Cosmetic Outcome in Post-Thyroidectomy Scars. Clin. Cosmet. Investig. Dermatol. 2024, 17, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M.R.; Dover, J.S.; Arndt, K.A. The spectrum of laser skin resurfacing: Nonablative, fractional, and ablative laser resurfacing. J. Am. Acad. Dermatol. 2008, 58, 719–737; quiz 738–740. [Google Scholar] [CrossRef] [PubMed]
- Lederhandler, M.H.; Bloom, B.S.; Pomerantz, H.; Geronemus, R.G. Case Series of Fractional Ablative Laser Resurfacing of Pediatric Facial Traumatic and Surgical Scars. Lasers Surg. Med. 2021, 53, 50–54. [Google Scholar] [PubMed]
- Krakowski, A.C.; Totri, C.R.; Donelan, M.B.; Shumaker, P.R. Scar Management in the Pediatric and Adolescent Populations. Pediatrics 2016, 137, e20142065. [Google Scholar] [CrossRef]
- Bonnardeaux, E.; McCuaig, C. Surgical excision combined with fully ablative carbon dioxide laser therapy and triamcinolone injections as a potential treatment for keloids in children. Pediatr. Dermatol. 2020, 37, 137–141. [Google Scholar] [CrossRef]
- Mustoe, T.A. International Scar Classification in 2019. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Swizerland, 2020; Chapter 9. [Google Scholar] [CrossRef]
- Cohen, B.E.; Geronemus, R.G.; McDaniel, D.H.; Brauer, J.A. The Role of Elastic Fibers in Scar Formation and Treatment. Dermatol. Surg. 2017, 43 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, W.J.; Jung, J.M.; Won, C.H.; Chang, S.E.; Lee, M.W.; Moon, I.J. Morphological characteristics of facial scars: A retrospective analysis according to scar location, onset, age, and cause. Int. Wound J. 2024, 21, e14453. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Annunziata, M.C.; D’Arco, V.; De Vita, V.; Lodi, G.; Mauriello, M.C.; Pastore, F.; Monfrecola, G. Acne scars: Pathogenesis, classification and treatment. Dermatol. Res. Pract. 2010, 2010, 893080. [Google Scholar] [CrossRef]
- Loss, M.J.; Leung, S.; Chien, A.; Kerrouche, N.; Fischer, A.H.; Kang, S. Adapalene 0.3% Gel Shows Efficacy for the Treatment of Atrophic Acne Scars. Dermatol. Ther. 2018, 8, 245–257. [Google Scholar] [CrossRef]
- Schleicher, S.; Moore, A.; Rafal, E.; Gagne-Henley, A.; Johnson, S.M.; Dhawan, S.; Chavda, R.; York, J.P.; Sforzolini, B.; Holcomb, K.; et al. Trifarotene Reduces Risk for Atrophic Acne Scars: Results from A Phase 4 Controlled Study. Dermatol. Ther. 2023, 13, 3085–3096. [Google Scholar] [CrossRef]
- Dréno, B.; Bissonnette, R.; Gagné-Henley, A.; Barankin, B.; Lynde, C.; Kerrouche, N.; Tan, J. Prevention and Reduction of Atrophic Acne Scars with Adapalene 0.3%/Benzoyl Peroxide 2.5% Gel in Subjects with Moderate or Severe Facial Acne: Results of a 6-Month Randomized, Vehicle-Controlled Trial Using Intra-Individual Comparison. Am. J. Clin. Dermatol. 2018, 19, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ashton, R.; Weinstein, M. Acne Vulgaris in the Pediatric Patient. Pediatr. Rev. 2019, 40, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Gozali, M.V.; Zhou, B. Effective treatments of atrophic acne scars. J. Clin. Aesthet. Dermatol. 2015, 8, 33–40. [Google Scholar]
- Sharad, J. Glycolic acid peel therapy—A current review. Clin. Cosmet. Investig. Dermatol. 2013, 6, 281–288. [Google Scholar] [CrossRef]
- Chan, G.J. Use of superficial glycolic acid peels in clinical practice. Hong Kong J. Dermatol. Venereol. 2012, 20, 111–113. [Google Scholar]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Clark, E.; Scerri, L. Superficial and medium-depth chemical peels. Clin. Dermatol. 2008, 26, 209–218. [Google Scholar] [CrossRef]
- Kontochristopoulos, G.; Platsidaki, E. Chemical peels in active acne and acne scars. Clin. Dermatol. 2017, 35, 179–182. [Google Scholar] [CrossRef]
- Agarwal, N.; Gupta, L.K.; Khare, A.K.; Kuldeep, C.M.; Mittal, A. Therapeutic response of 70% trichloroacetic acid CROSS in atrophic acne scars. Dermatol. Surg. 2015, 41, 597–604. [Google Scholar] [CrossRef]
- Castillo, D.E.; Keri, J.E. Chemical peels in the treatment of acne: Patient selection and perspectives. Clin. Cosmet. Investig. Dermatol. 2018, 11, 365–372. [Google Scholar] [CrossRef]
- Clementoni, M.T.; Azzopardi, E. Specific Attention Areas in Scar Management: Management of Atrophic Scars. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 41. [Google Scholar] [CrossRef]
- Alster, T.S.; Tanzi, E.L.; Lazarus, M. The use of fractional laser photothermolysis for the treatment of atrophic scars. Dermatol. Surg. 2007, 33, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hedelund, L.; Moreau, K.E.; Beyer, D.M.; Nymann, P.; Haedersdal, M. Fractional nonablative 1540-nm laser resurfacing of atrophic acne scars. A randomized controlled trial with blinded response evaluation. Lasers Med. Sci. 2010, 25, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Gokalp, H. Evaluation of nonablative fractional laser treatment in scar reduction. Lasers Med. Sci. 2017, 32, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Keen, A.; Sheikh, G.; Hassan, I.; Jabeen, Y.; Rather, S.; Mubashir, S.; Latif, I.; Zeerak, S.; Ahmad, M.; Hassan, A.; et al. Treatment of post-burn and post-traumatic atrophic scars with fractional CO2 laser: Experience at a tertiary care centre. Lasers Med. Sci. 2018, 33, 1039–1046. [Google Scholar] [CrossRef]
- Kim, S.; Cho, K.H. Clinical trial of dual treatment with an ablative fractional laser and a nonablative laser for the treatment of acne scars in Asian patients. Dermatol. Surg. 2009, 35, 1089–1098. [Google Scholar] [CrossRef]
- Rkein, A.; Ozog, D.; Waibel, J.S. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol. Surg. 2014, 40, 624–631. [Google Scholar]
- El-Hawary, E.E.; Nassar, S.; Hodeib, A.A.; Shareef, M.M.; Fawzy, M.M. Ablative Fractional Carbon Dioxide Laser and Autologous Platelet-Rich Plasma in the Treatment of Atrophic Acne Scars: A Comparative Clinico-Immuno-Histopathological Study. Lasers Surg. Med. 2021, 53, 458–467. [Google Scholar] [CrossRef]
- Taylor, M.B.; Zaleski-Larsen, L.; McGraw, T.A. Single Session Treatment of Rolling Acne Scars Using Tumescent Anesthesia, 20% Trichloracetic Acid Extensive Subcision, and Fractional CO2 Laser. Dermatol. Surg. 2017, 43 (Suppl. S1), S70–S74. [Google Scholar] [CrossRef]
- Faghihi, G.; Nouraei, S.; Asilian, A.; Keyvan, S.; Abtahi-Naeini, B.; Rakhshanpour, M.; Nilforoushzadeh, M.A.; Hosseini, S.M. Efficacy of Punch Elevation Combined with Fractional Carbon Dioxide Laser Resurfacing in Facial Atrophic Acne Scarring: A Randomized Split-face Clinical Study. Indian J. Dermatol. 2015, 60, 473–478. [Google Scholar] [CrossRef]
- Gupta, M.; Barman, K.D.; Sarkar, R. A Comparative Study of Microneedling Alone Versus Along with Platelet-Rich Plasma in Acne Scars. J. Cutan. Aesthet. Surg. 2021, 14, 64–71. [Google Scholar] [CrossRef]
- Sitohang IB, S.; Sirait SA, P.; Suryanegara, J. Microneedling in the treatment of atrophic scars: A systematic review of randomised controlled trials. Int. Wound J. 2021, 18, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, S. Microneedling: Advances and widening horizons. Indian Dermatol. Online J. 2016, 7, 244–254. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Barakat, M.; Awad, S.; Medhat, W.; El-Fakahany, H.; Farag, H. Microneedling Therapy for Atrophic Acne Scars: An Objective Evaluation. J. Clin. Aesthet. Dermatol. 2015, 8, 36–42. [Google Scholar]
- Pawar, M.; Singh, M. Microneedling with autologous platelet-rich plasma versus microneedling with topical insulin in the treatment of postacne atrophic scars: A simultaneous split-face comparative study. J. Am. Acad. Dermatol. 2021, 84, 810–811. [Google Scholar] [CrossRef]
- Ali, B.; ElMahdy, N.; Elfar, N.N. Microneedling (Dermapen) and Jessner’s solution peeling in treatment of atrophic acne scars: A comparative randomized clinical study. J. Cosmet. Laser Ther. 2019, 21, 357–363. [Google Scholar] [CrossRef]
- Rana, S.; Mendiratta, V.; Chander, R. Efficacy of microneedling with 70% glycolic acid peel vs microneedling alone in treatment of atrophic acne scars-A randomized controlled trial. J. Cosmet. Dermatol. 2017, 16, 454–459. [Google Scholar] [CrossRef]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef]
- Vingan, N.R.; Wamsley, C.E.; Panton, J.A.; Mangalagiri, D.; Turer, D.; Akgul, Y.; Barillas, J.; Culver, A.; Kenkel, J.M. Investigating the Efficacy of Modified Lipoaspirate Grafting to Improve the Appearance of Atrophic Acne Scars: A Pilot Study. Aesthet. Surg. J. 2023, 43, NP613–NP630. [Google Scholar] [CrossRef]
- Roh, M.R.; Jung, J.Y.; Chung, K.Y. Autologous fat transplantation for depressed linear scleroderma-induced facial atrophic scars. Dermatol. Surg. 2008, 34, 1659–1665. [Google Scholar] [CrossRef]
- Gu, Z.; Li, Y.; Li, H. Use of Condensed Nanofat Combined With Fat Grafts to Treat Atrophic Scars. JAMA Facial Plast. Surg. 2018, 20, 128–135. [Google Scholar] [CrossRef]
- Heath, C.R. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis 2019, 103, 71–73. [Google Scholar] [PubMed]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar]
- American Academy of Dermatology Association. Sunscreen FAQs. 15 April 2024. Available online: https://www.aad.org/media/stats-sunscreen (accessed on 18 March 2025).
- Taylor, S.; Elbuluk, N.; Grimes, P.; Chien, A.; Hamzavi, I.; Alexis, A.; Gonzalez, N.; Weiss, J.; Kang, S.; Desai, S.R. Treatment recommendations for acne-associated hyperpigmentation: Results of the Delphi consensus process and a literature review. J. Am. Acad. Dermatol. 2023, 89, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; Baldwin, H.; Cook-Bolden, F.E.; Alexis, A.F.; Stein Gold, L.; Guenin, E. Effects of Topical Retinoids on Acne and Post-inflammatory Hyperpigmentation in Patients with Skin of Color: A Clinical Review and Implications for Practice. Am. J. Clin. Dermatol. 2022, 23, 69–81. [Google Scholar] [CrossRef]
- Saedi, N. Postinflammatory Hyperpigmentation. UpToDate. 2023. Available online: https://www.uptodate.com/contents/postinflammatory-hyperpigmentation (accessed on 8 March 2025).
- Chan, R.; Park, K.C.; Lee, M.H.; Lee, E.S.; Chang, S.E.; Leow, Y.H.; Tay, Y.K.; Legarda-Montinola, F.; Tsai, R.Y.; Tsai, T.H.; et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br. J. Dermatol. 2008, 159, 697–703. [Google Scholar] [CrossRef]
- Chaowattanapanit, S.; Silpa-Archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Sugarman, J.L.; Guenin, E.; Harris, S.; Bhatt, V. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris in a preadolescent population. Pediatr. Dermatol. 2019, 36, 193–199. [Google Scholar] [CrossRef]
- Siegfried, E.C.; Jaworski, J.C.; Kaiser, J.D.; Hebert, A.A. Systematic review of published trials: Long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 2016, 16, 75. [Google Scholar] [CrossRef]
- Wallo, W.; Nebus, J.; Leyden, J.J. Efficacy of a soy moisturizer in photoaging: A double-blind, vehicle-controlled, 12-week study. J. Drugs Dermatol. 2007, 6, 917–922. [Google Scholar]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: A randomized, double-blind, vehicle-controlled trial. Ski. Res. Technol. 2014, 20, 208–212. [Google Scholar] [CrossRef]
- Castanedo-Cazares, J.P.; Lárraga-Piñones, G.; Ehnis-Pérez, A.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Smoller, B.R.; Torres-Álvarez, B. Topical niacinamide 4% and desonide 0.05% for treatment of axillary hyperpigmentation: A randomized, double-blind, placebo-controlled study. Clin. Cosmet. Investig. Dermatol. 2013, 6, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Kaczvinsky, J.R.; Li, J.; Robinson, L.R.; Matts, P.J.; Berge, C.A.; Miyamoto, K.; Bissett, D.L. Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: Results of a randomized, double-blind, vehicle-controlled trial. Br. J. Dermatol. 2010, 162, 435–441. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Yatskayer, M.; Bhushan, P.; Pillai, S.; Oresajo, C. Evaluation of a kojic acid, emblica extract, and glycolic acid formulation compared with hydroquinone 4% for skin lightening. Cutis 2010, 86, 153–158. [Google Scholar] [PubMed]
- Kircik, L.H. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: A 16-week, baseline-controlled study. J. Drugs Dermatol. 2011, 10, 586–590. [Google Scholar]
- Abels, C.; Kaszuba, A.; Michalak, I.; Werdier, D.; Knie, U.; Kaszuba, A. A 10% glycolic acid containing oil-in-water emulsion improves mild acne: A randomized double-blind placebo-controlled trial. J. Cosmet. Dermatol. 2011, 10, 202–209. [Google Scholar] [CrossRef]
- Afarideh, M.; Rodriguez Baisi, K.E.; Davis DM, R.; Hand, J.L.; Tollefson, M.M. Trends in utilization of non-first-line topical acne medications among children, adolescents, and adults in the United States, 2012–2016. Pediatr. Dermatol. 2021, 38, 1066–1073. [Google Scholar] [CrossRef]
- Agbai, O.; Hamzavi, I.; Jagdeo, J. Laser Treatments for Postinflammatory Hyperpigmentation: A Systematic Review. JAMA Dermatol. 2017, 153, 199–206. [Google Scholar] [CrossRef]
- Cho, S.B.; Park, S.J.; Kim, J.S.; Kim, M.J.; Bu, T.S. Treatment of post-inflammatory hyperpigmentation using 1064-nm Q-switched Nd:YAG laser with low fluence: Report of three cases. J. Eur. Acad. Dermatol. Venereol. JEADV 2009, 23, 1206–1207. [Google Scholar] [CrossRef]
- Rokhsar, C.K.; Ciocon, D.H. Fractional photothermolysis for the treatment of postinflammatory hyperpigmentation after carbon dioxide laser resurfacing. Dermatol. Surg. 2009, 35, 535–537. [Google Scholar] [CrossRef]
- Katz, T.M.; Goldberg, L.H.; Firoz, B.F.; Friedman, P.M. Fractional photothermolysis for the treatment of postinflammatory hyperpigmentation. Dermatol. Surg. 2009, 35, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Frech, F.S.; Hernandez, L.; Urbonas, R.; Zaken, G.A.; Dreyfuss, I.; Nouri, K. Hypertrophic Scars and Keloids: Advances in Treatment and Review of Established Therapies. Am. J. Clin. Dermatol. 2023, 24, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Ghazawi, F.M.; Zargham, R.; Gilardino, M.S.; Sasseville, D.; Jafarian, F. Insights into the Pathophysiology of Hypertrophic Scars and Keloids: How Do They Differ? Adv. Skin. Wound Care. 2018, 31, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Block, L.; Gosain, A.; King, T.W. Emerging Therapies for Scar Prevention. Adv. Wound Care 2015, 4, 607–614. [Google Scholar] [CrossRef]
- Ai, J.W.; Liu, J.T.; Pei, S.D.; Liu, Y.; Li, D.S.; Lin, H.M.; Pei, B. The effectiveness of pressure therapy (15–25 mmHg) for hypertrophic burn scars: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 40185. [Google Scholar] [CrossRef]
- Gold, M.H.; McGuire, M.; Mustoe, T.A.; Pusic, A.; Sachdev, M.; Waibel, J.; Murcia, C.; International Advisory Panel on Scar Management. Updated international clinical recommendations on scar management: Part 2--algorithms for scar prevention and treatment. Dermatol. Surg. 2014, 40, 825–831. [Google Scholar]
- Morelli Coppola, M.; Salzillo, R.; Segreto, F.; Persichetti, P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: Patient selection and perspectives. Clin. Cosmet. Investig. Dermatol. 2018, 11, 387–396. [Google Scholar] [CrossRef]
- Hamrick, M.; Boswell, W.; Carney, D. Successful treatment of earlobe keloids in the pediatric population. J. Pediatr. Surg. 2009, 44, 286–288. [Google Scholar] [CrossRef]
- Reish, R.G.; Eriksson, E. Scars: A review of emerging and currently available therapies. Plast. Reconstr. Surg. 2008, 122, 1068–1078. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; Wei, Z.; Lin, W.; Fan, B.; Feng, S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 480–487. [Google Scholar] [CrossRef]
- Mavilakandy, A.K.; Vayalapra, S.; Minty, I.; Parekh, J.N.; Charles, W.N.; Khajuria, A. Comparing Combination Triamcinolone Acetonide and 5-Fluorouracil with Monotherapy Triamcinolone Acetonide or 5-Fluorouracil in the Treatment of Hypertrophic Scars: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2024, 153, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.I.; Kim, S.; Cho, S.W.; Cho, M.K. The efficacy of bleomycin for treating keloid and hypertrophic scar: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Sabry, H.H.; Sorour, N.E.; Moharm, F.M. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J. Dermatol. Dermatol. Surg. 2016, 20, 32–39. [Google Scholar] [CrossRef]
- Artzi, O.; Koren, A.; Niv, R.; Mehrabi, J.N.; Friedman, O. The Scar Bane, Without the Pain: A New Approach in the Treatment of Elevated Scars: Thermomechanical Delivery of Topical Triamcinolone Acetonide and 5-Fluorouracil. Dermatol. Ther. 2019, 9, 321–326. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. Emerging Technologies in Scar Management: Laser-Assisted Delivery of Therapeutic Agents. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 50. [Google Scholar] [CrossRef]
- Ogawa, R. Keloids and Hypertrophic Scars. UpToDate. 2024. Available online: https://www.uptodate.com/contents/keloids-and-hypertrophic-scars (accessed on 7 March 2025).
- de las Alas, J.M.; Siripunvarapon, A.H.; Dofitas, B.L. Pulsed dye laser for the treatment of keloid and hypertrophic scars: A systematic review. Expert Rev. Med. Devices 2012, 9, 641–650. [Google Scholar] [CrossRef]
- Hultman, C.S.; Yoshida, S. Laser Therapy for Hypertrophic Scars and Keloids. UpToDate. 2023. Available online: https://www.uptodate.com/contents/laser-therapy-for-hypertrophic-scars-and-keloids (accessed on 27 January 2024).
- Hultman, C.S.; Edkins, R.E.; Wu, C.; Calvert, C.T.; Cairns, B.A. Prospective, before-after cohort study to assess the efficacy of laser therapy on hypertrophic burn scars. Ann. Plast. Surg. 2013, 70, 521–526. [Google Scholar] [CrossRef]
- Elrod, J.; Schiestl, C.; Neuhaus, D.; Mohr, C.; Neuhaus, K. Patient- and Physician-Reported Outcome of Combined Fractional CO2 and Pulse Dye Laser Treatment for Hypertrophic Scars in Children. Ann. Plast. Surg. 2020, 85, 237–244. [Google Scholar] [CrossRef]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Téot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef]
- Ogawa, R.; Dohi, T.; Tosa, M.; Aoki, M.; Akaishi, S. The Latest Strategy for Keloid and Hypertrophic Scar Prevention and Treatment: The Nippon Medical School (NMS) Protocol. J. Nippon. Med. Sch. 2021, 88, 2–9. [Google Scholar] [CrossRef]
- Acosta, S.; Ureta, E.; Yañez, R.; Oliva, N.; Searle, S.; Guerra, C. Effectiveness of Intralesional Triamcinolone in the Treatment of Keloids in Children. Pediatr. Dermatol. 2016, 33, 75–79. [Google Scholar] [CrossRef]
- Laspro, M.; Onuh, O.C.; Cohen, R.F.; Cooper, B.T.; Chiu, E.S. The Role of Radiation Therapy in Adult and Pediatric Keloid Management: A National Survey of Radiation Oncologists. Ann. Plast. Surg. 2023, 91, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A.D.; Style, C.C.; Olutoye, O.O. A Review of Hypertrophic Scar and Keloid Treatment and Prevention in the Pediatric Population: Where Are We Now? Adv. Wound Care 2022, 11, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, X.; Gao, Z.; Xia, L. Minimally Invasive Technologies for Treatment of HTS and Keloids: Low-Dose 5-Fluorouracil. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 30. [Google Scholar] [CrossRef]
- Vanhooteghem, O. Remarkable efficiency of surgical shave excision of keloids followed by intralesional injection of Bleomycin. A retrospective study of 314 cases. Dermatol. Ther. 2022, 35, e15425. [Google Scholar] [CrossRef] [PubMed]
- Nischwitz, S.P.; Lumenta, D.B.; Spendel, S.; Kamolz, L. Minimally Invasive Technologies for Treatment of HTS and Keloids: Pulsed-Dye Laser. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 31. [Google Scholar] [CrossRef]
- Ekstein, S.F.; Wyles, S.P.; Moran, S.L.; Meves, A. Keloids: A review of therapeutic management. Int. J. Dermatol. 2021, 60, 661–671. [Google Scholar] [CrossRef]
- Har-Shai, Y.; Har-Shai, L. Minimally Invasive Technologies for the Treatment of Hypertrophic Scars and Keloids: Intralesional Cryosurgery. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; Chapter 28. [Google Scholar] [CrossRef]
- van Leeuwen, M.C.; Bulstra, A.E.; Ket, J.C.; Ritt, M.J.; van Leeuwen, P.A.; Niessen, F.B. Intralesional Cryotherapy for the Treatment of Keloid Scars: Evaluating Effectiveness. Plast. Reconstr. Surg. Glob. Open. 2015, 3, e437. [Google Scholar] [CrossRef]
- Goldenberg, G.; Luber, A.J. Use of intralesional cryosurgery as an innovative therapy for keloid scars and a review of current treatments. J. Clin. Aesthet. Dermatol. 2013, 6, 23–26. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, S.; Bao, E.; Rothberg, S.; Palacios, J.F.; Smith, I.T.; Tanna, N.; Bastidas, N. Scar Management in Pediatric Patients. Medicina 2025, 61, 553. https://doi.org/10.3390/medicina61040553
Barone S, Bao E, Rothberg S, Palacios JF, Smith IT, Tanna N, Bastidas N. Scar Management in Pediatric Patients. Medicina. 2025; 61(4):553. https://doi.org/10.3390/medicina61040553
Chicago/Turabian StyleBarone, Sydney, Eric Bao, Stephanie Rothberg, Jose F. Palacios, Isabelle T. Smith, Neil Tanna, and Nicholas Bastidas. 2025. "Scar Management in Pediatric Patients" Medicina 61, no. 4: 553. https://doi.org/10.3390/medicina61040553
APA StyleBarone, S., Bao, E., Rothberg, S., Palacios, J. F., Smith, I. T., Tanna, N., & Bastidas, N. (2025). Scar Management in Pediatric Patients. Medicina, 61(4), 553. https://doi.org/10.3390/medicina61040553





