Attitudes and Barriers Toward Antiretroviral Therapeutic Drug Monitoring Among Infectious Disease Providers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Demographic Characteristics
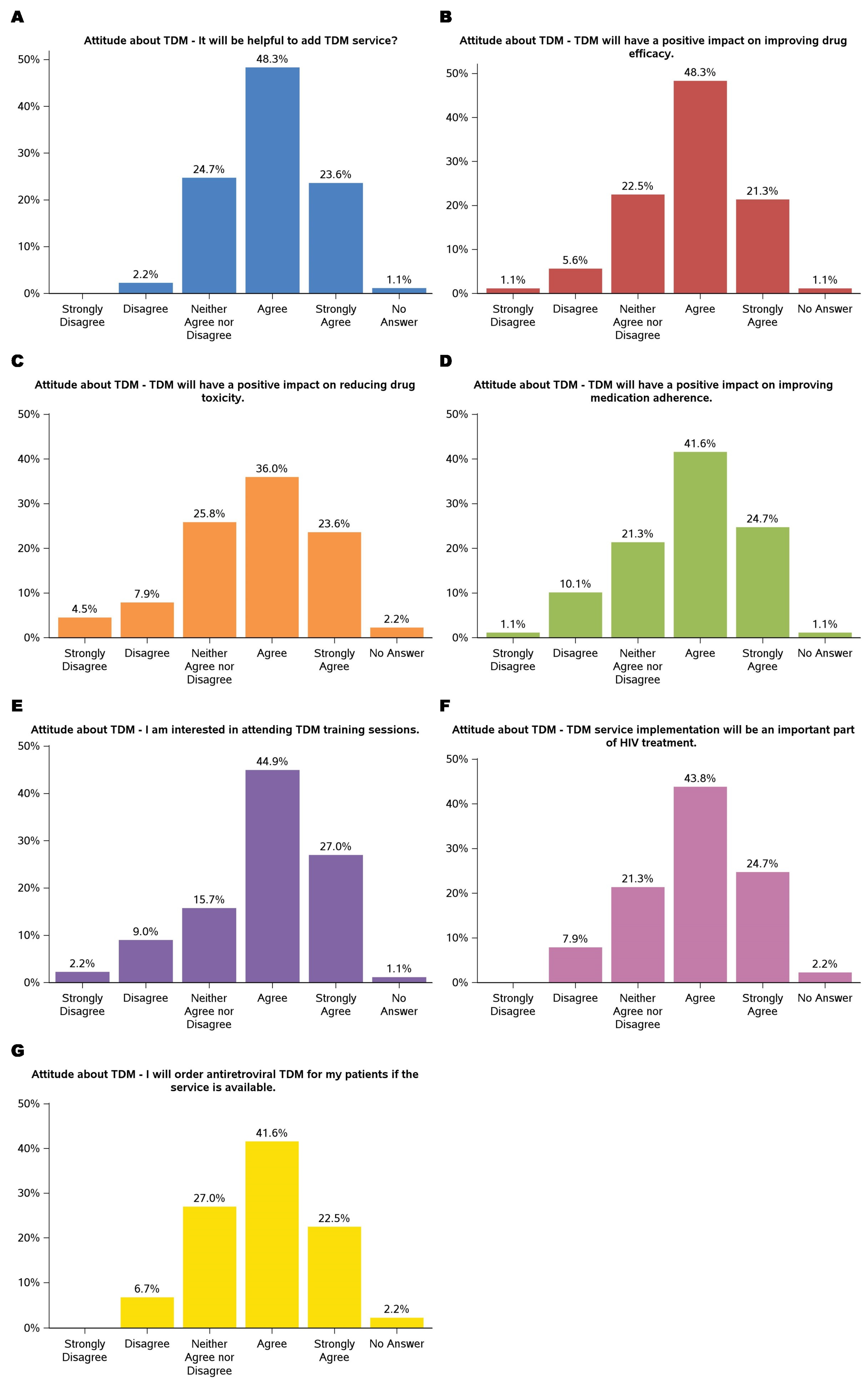
3.2. Attitude About TDM
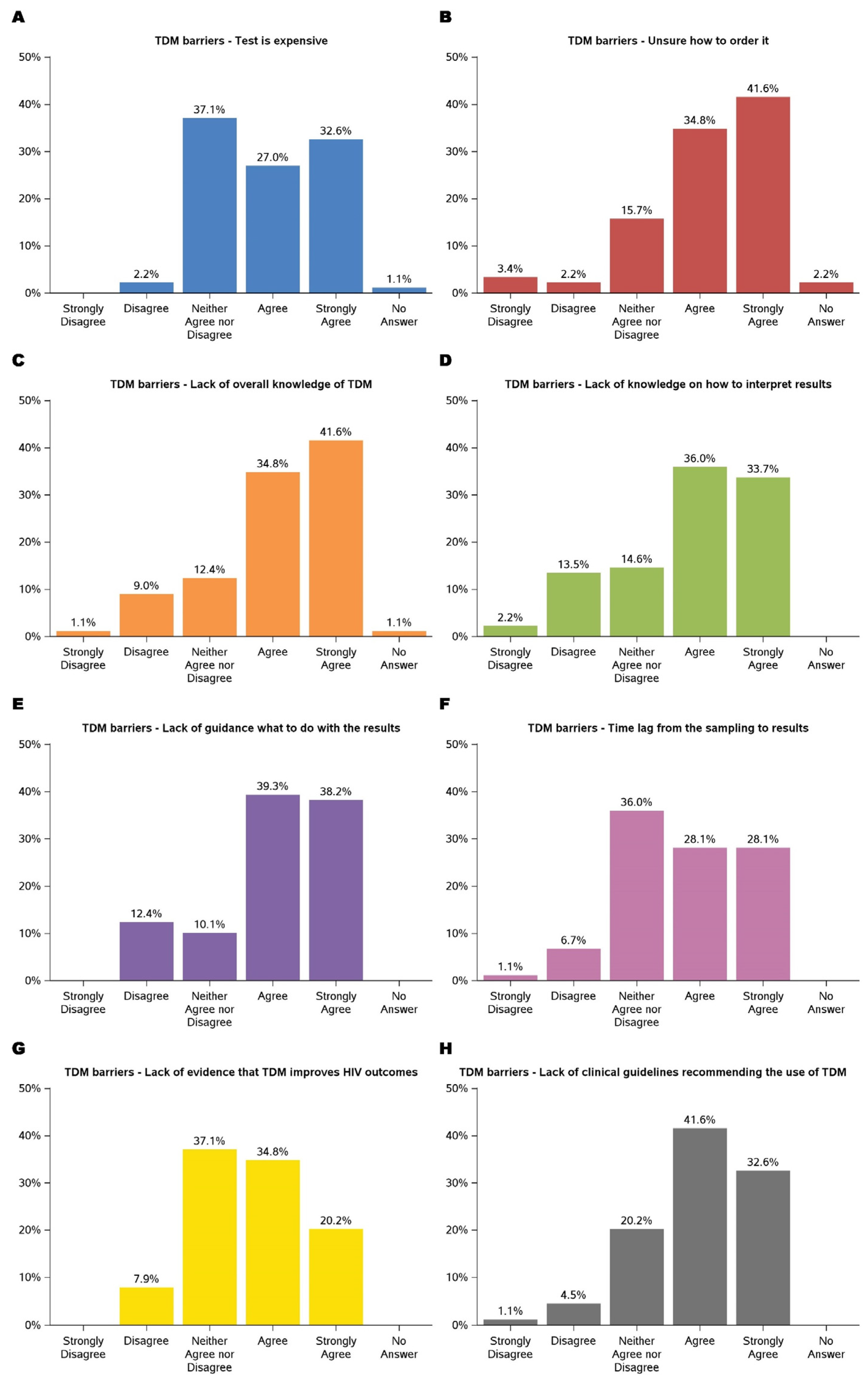
3.3. Barriers to TDM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HIV.gov. Global HIV/AIDS Overview. 17 December 2024. Available online: https://www.hiv.gov/ (accessed on 11 January 2025).
- UNAIDS. The Path That Ends AIDS: UNAIDS Global AIDS Update. July 2023. Available online: https://www.unaids.org/ (accessed on 11 January 2025).
- Giordano, T.P.; Suarez-Almazor, M.E.; Grimes, R.M. The population effectiveness of highly active antiretroviral therapy: Are good drugs good enough? Curr. HIV/AIDS Rep. 2005, 2, 177–183. [Google Scholar]
- Bangsberg, D.R.; Perry, S.; Charlebois, E.D.; Clark, R.A.; Roberston, M.; Zolopa, A.R.; Moss, A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001, 15, 1181–1183. [Google Scholar] [PubMed]
- Parienti, J.; Fournier, A.L.; Cotte, L.; Schneider, M.; Etienne, M.; Unal, G.; Perré, P.; Dutheil, J.; Morilland-Lecoq, E.; Chaillot, F.; et al. Forgiveness of Dolutegravir-Based Triple Therapy Compared with Older Antiretroviral Regimens: A Prospective Multicenter Cohort of Adherence Patterns and HIV-RNA Replication. Open Forum Infect. Dis. 2021, 8, ofab316. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Manalel, J.A.; Kaufman, J.E.; Wu, Y.; Fusaris, E.; Correa, A.; Ernst, J.; Brennan-Ing, M. Association of ART regimen and adherence to viral suppression: An observational study of a clinical population of people with HIV. AIDS Res. Ther. 2024, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Komandt, M.; Canfield, S.; Lengel, M.; Gilmore, V.; Kilcrease, C. Correlation between medication adherence using proportion of days covered and achieving viral suppression in patients living with HIV. JMCP 2023, 29, 1129–1137. [Google Scholar] [CrossRef]
- Burton, K.L.; Ritchwood, T.D.; Metzger, I.W. Structural Racism and Racial Trauma Among African Americans at Elevated Risk for HIV Infection. Am. J. Public Health 2023, 113 (Suppl. S2), S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Murdock, C.J.; Laurencin, L.; Christensen, D.M. HIV/AIDS and the African-American Community 2018: A Decade Call to Action. J. Racial Ethn. Health Disparities 2018, 5, 449–458. [Google Scholar]
- Goodlet, K.J.; Zmarlicka, M.T.; Peckham, A.M. Drug–drug interactions and clinical considerations with co-administration of antiretrovirals and psychotropic drugs. CNS Spectr. 2019, 24, 287–312. [Google Scholar] [CrossRef]
- Nhean, S.; Tseng, A.; Back, D. The intersection of drug interactions and adverse reactions in contemporary antiretroviral therapy. Curr. Opin. HIV AIDS 2021, 16, 292–302. [Google Scholar] [CrossRef]
- Lim, S.Y.; Pettit, R.S. Pharmacokinetic considerations in pediatric pharmacotherapy. Am. J. Health-Syst. Pharm. 2019, 76, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Collins, I.J.; Turkova, A. A step closer to optimal ART for all children. Lancet HIV 2023, 10, e487–e489. [Google Scholar] [CrossRef]
- Jhajra, S.; Singh, S.; Holm, K.A.; Faqi, A.S.; Sahi, J. Influence of changes in physiology, transporters, and enzyme expression on disposition and metabolism of drugs during pregnancy and clinical implications. In Encyclopedia of Drug Metabolism and Interactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–29. [Google Scholar] [CrossRef]
- Kim, J.; Lee, E.; Park, B.; Bang, J.H.; Lee, J.Y. Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: A nationwide study. Sci. Rep. 2018, 8, 3133. [Google Scholar] [CrossRef]
- Ekstrand, M.L.; Shet, A.; Chandy, S.; Singh, G.; Shamsundar, R.; Madhavan, V.; Saravanan, S.; Heylen, E.; Kumarasamy, N. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int. Health 2011, 3, 27–34. [Google Scholar] [PubMed]
- Scibona, P.; Cruz, C.V.; Lopardo, G.; Gimenez, M.I.; Frecha, C.; Rodriguez, L.F.; Rolon, M.J.; Cecchini, D.; Cordova, E.; Abela, C.; et al. Individualized Antiretroviral Therapy. Impact of pharmacogenetic and therapeutic drug monitoring in the safety and efficacy of first line antirretroviral therapy in patients with HIV infection. Preliminary report. Int. J. Infect. Dis. 2018, 73, 243–244. [Google Scholar] [CrossRef]
- Aarnoutse, R.E.; Schapiro, J.M.; Boucher, C.A.B.; Hekster, Y.A.; Burger, D.M. Therapeutic Drug Monitoring. Drugs 2003, 63, 741–753. [Google Scholar] [CrossRef]
- Burger, D.M. Criteria for Therapeutic Drug Monitoring in HIV. 2002. Available online: https://hospitalpharmacyeurope.com/news/editors-pick/criteria-for-therapeutic-drug-monitoring-in-hiv/ (accessed on 21 December 2024).
- Perry, C. Monitoring Drug Therapy: Importance, Methods, and Challenges. J. Dev. Drugs 2023, 12, 1–2. Available online: https://www.longdom.org/ (accessed on 11 January 2025).
- Milone, M.C.; Shaw, L.M. Breaking the Therapeutic Drug Monitoring Logistics Barrier. Clin. Chem. 2014, 60, 1471–1472. [Google Scholar] [CrossRef]
- Chatjaroenpat, S.; Chuenmueang, C.; Jaisue, S. A cross-sectional study of the current situation with therapeutic drug monitoring in Thailand: Requirements, challenges and the role of educational institutions. Pharm. Educ. 2024, 24, 154–163. [Google Scholar] [CrossRef]
- Bjørlykke, K.H.; Jahnsen, J.; Brynskov, J.; Molander, P.; Eberhardson, M.; Davidsdottir, L.G.; Sipponen, T.; Hjortswang, H.; Goll, G.L.; Syversen, S.W.; et al. Therapeutic drug monitoring in inflammatory bowel disease: Implementation, utilization, and barriers in clinical practice in Scandinavia. Scand. J. Gastroenterol. 2023, 58, 25–33. [Google Scholar] [CrossRef]
- DeArmond, P.D.; Bunch, D.R. Chapter 10—Therapeutic drug monitoring of antiretroviral drugs for the management of human immunodeficiency infection. In Therapeutic Drug Monitoring, 2nd ed.; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 241–264. [Google Scholar] [CrossRef]
- Preskorn, S.H. Charting and Handling Therapeutic Drug Monitoring Results: How they Differ from Most Laboratory Results. J. Psychiatr. Pract. 2021, 27, 283. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, I. Therapeutic Drug Monitoring (TDM): An Aspect of Clinical Pharmacology and Pharmacy Practice. Res. Rev. J. Pharmacol. 2015, 5, 27–34. [Google Scholar]
- Selinger, C.; Carbonell, J.; Kane, J.; Omer, M.; Ford, A.C. Acceptability of a ‘treat to target’ approach in inflammatory bowel disease to patients in clinical remission. Frontline Gastroenterol. 2021, 12, 30–38. [Google Scholar] [CrossRef]
- Grossberg, L.B.; Papamichael, K.; Feuerstein, J.D.; Siegel, C.A.; Ullman, T.A.; Cheifetz, A.S. A Survey Study of Gastroenterologists’ Attitudes and Barriers Toward Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Qualtrics in Academic Research. 2018. Available online: https://www.qualtrics.com/blog/citing-qualtrics/ (accessed on 8 January 2025).
- Archibald, T.L.; Murrell, D.E.; Brown, S.D. Chromatographic methods in HIV medicine: Application to therapeutic drug monitoring. Biomed. Chromatogr. 2018, 32, e4170. [Google Scholar] [CrossRef]
- van Gelder, T. The Appropriately Designed TDM Clinical Trial: Endpoints, Pitfalls, and Perspectives. Ther. Drug Monit. 2023, 45, 6. [Google Scholar] [CrossRef]
- Buzibye, A.; Musaazi, J.; von Braun, A.; Nanzigu, S.; Sekaggya-Wiltshire, C.; Kambugu, A.; Fehr, J.; Lamorde, M.; Gutteck, U.; Muller, D.; et al. Antiretroviral concentration measurements as an additional tool to manage virologic failure in resource limited settings: A case control study. AIDS Res. Ther. 2019, 16, 39. [Google Scholar] [CrossRef]
- Li, N.; Zheng, H.; He, W.; He, X.; Li, R.; Cui, W.; Yang, W.; Dong, X.; Shen, Z.; Zheng, Y. Treatment outcomes amongst older people with HIV infection receiving antiretroviral therapy. AIDS 2024, 38, 803–812. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. 2021. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 11 January 2025).
- HIV.gov. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2024. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new (accessed on 11 January 2025).
- European AIDS Clinical Society (EACS). EACS Guidelines for the Management of People Living with HIV in Europe. 2023. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 11 January 2025).
- Rindi, L.V.; Zaçe, D.; Compagno, M.; Colagrossi, L.; Santoro, M.M.; Andreoni, M.; Perno, C.F.; Sarmati, L. Management of low-level HIV viremia during antiretroviral therapy: Delphi consensus statement and appraisal of the evidence. Sex. Transm. Infect. 2024, 100, 442–449. [Google Scholar] [CrossRef]

| Characteristics | Number (Percent) | |
|---|---|---|
| Gender | Male | 32 (36.0%) |
| Female | 56 (62.9%) | |
| Age | ≤34 years | 47 (53.4%) |
| 35–49 years | 34 (38.6%) | |
| 50–64 years | 6 (6.8%) | |
| ≥65 years | 1 (1.1%) | |
| Employment | Hospital | 46 (52.3%) |
| Clinic | 31 (35.2%) | |
| Public health department | 2 (2.3%) | |
| Academia | 9 (10.2%) | |
| Clinical role | Physician | 44 (50%) |
| Physical assistant | 3 (3.4%) | |
| Nurse practitioner | 6 (6.8%) | |
| Pharmacist | 32 (36.4%) | |
| Other | 3 (3.41%) | |
| Years in infectious disease practice | Still training | 31 (34.8%) |
| 0–5 years | 32 (36.0%) | |
| 6–10 years | 10 (11.2%) | |
| 11–19 years | 9 (10.1%) | |
| ≥20 years | 7 (7.9%) | |
| % of patients with HIV | <10% | 32 (36.0%) |
| 11–25% | 18 (20.2%) | |
| 26–50% | 8 (9.0%) | |
| >50% | 24 (27.0%) | |
| Unsure | 7 (7.9%) | |
| number of HIV patients/month | <5 | 28 (31.5%) |
| 5–10 | 10 (11.2%) | |
| 11–20 | 14 (15.7%) | |
| 20–30 | 11 (12.4%) | |
| >30 | 19 (21.4%) | |
| % of patients with HIV achieving viral suppression on antiretrovirals | <50% | 11 (12.4%) |
| 50–75% | 12 (13.5%) | |
| 76–85% | 24 (27.0%) | |
| 86–95% | 23 (25.8%) | |
| >95% | 19 (21.4%) | |
| Able to order TDM of antiretroviral drugs | No | 60 (67.4%) |
| Yes | 29 (32.6%) | |
| TDM orders within 1-year period for antiretroviral drugs (N = 29) | <1 time per year | 16 (55.2%) |
| 1–3 times per year | 7 (24.1%) | |
| 3–5 times per year | 1 (3.5%) | |
| 5–10 times per year | - | |
| >10 times per year | 5 (17.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Torres, C.M.; Giordano, T.P.; Dang, B.N.; Liang, D. Attitudes and Barriers Toward Antiretroviral Therapeutic Drug Monitoring Among Infectious Disease Providers. Medicina 2025, 61, 544. https://doi.org/10.3390/medicina61030544
Wang H, Torres CM, Giordano TP, Dang BN, Liang D. Attitudes and Barriers Toward Antiretroviral Therapeutic Drug Monitoring Among Infectious Disease Providers. Medicina. 2025; 61(3):544. https://doi.org/10.3390/medicina61030544
Chicago/Turabian StyleWang, Hongmei, Cecilia M. Torres, Thomas P. Giordano, Bich N. Dang, and Dong Liang. 2025. "Attitudes and Barriers Toward Antiretroviral Therapeutic Drug Monitoring Among Infectious Disease Providers" Medicina 61, no. 3: 544. https://doi.org/10.3390/medicina61030544
APA StyleWang, H., Torres, C. M., Giordano, T. P., Dang, B. N., & Liang, D. (2025). Attitudes and Barriers Toward Antiretroviral Therapeutic Drug Monitoring Among Infectious Disease Providers. Medicina, 61(3), 544. https://doi.org/10.3390/medicina61030544







