Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Research Question
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Randomized Controlled Trials (RCTs).
- Trials with multiple study groups were included if the probiotic intervention arm could be isolated.
- Studies published between January 2000 and July 2024.
- Studies published in English or Spanish.
- Studies involving pediatric or adult populations with a diagnosis of CF with no restrictions regarding genotype, disease severity, or comorbidities, in which the effects of probiotics, prebiotics, or synbiotics were evaluated—regardless of treatment duration, regimen, or form of administration (capsule, food, fermented product, powder).
- Studies assessing at least one of the following outcomes: pulmonary exacerbations; hospitalizations; inflammatory biomarkers (intestinal, serological, or sputum) such as calprotectin, C-reactive protein, or cytokines; gastrointestinal symptoms; pulmonary function; quality of life; or adverse events.
2.3.2. Exclusion Criteria
- Letters to the editor in which the necessary information for this review could not be extracted.
- Pre-print articles.
- Studies published solely as conference abstracts.
- Studies not available in an accessible format.
- Studies that do not specify the strain of the probiotic administered to patients.
- Investigations in which it is difficult to discern how much probiotics, prebiotics, or synbiotics contribute to the outcome compared to other simultaneous or subsequent interventions.
2.4. Data Sources and Search Strategy
2.5. Selection and Extraction of Information
2.6. Risk of Bias Assessment
- (a)
- Random sequence generation
- (b)
- Allocation concealment
- (c)
- Blinding of participants and personnel
- (d)
- Blinding of outcome assessment
- (e)
- Incomplete outcome data
- (f)
- Selective reporting
2.7. Quality of Evidence Assessment
- Whether the study is randomized
- Whether the intervention is double-blinded
- Whether the study addresses and describes withdrawals
- Whether an appropriate randomization method is described
- Whether the inclusion and exclusion criteria are clearly described
2.8. Statistical Analysis
3. Results
3.1. Studies Identified for the Review
3.2. Characteristics of the Studies Included in the Review
3.3. Characteristics of the Patient and Intervention
3.4. Results of the Risk of Bias Assessment
3.4.1. Random Sequence Generation
3.4.2. Allocation Concealment
3.4.3. Blinding of Participants and Personnel
3.4.4. Blinding of Outcome Assessment
3.4.5. Incomplete Outcome Data
3.4.6. Selective Reporting
3.5. Qualitative Synthesis of Results
3.5.1. Hospitalizations
3.5.2. Inflammatory Biomarkers
3.5.3. Quality of Life
3.5.4. Gastrointestinal Symptoms
3.5.5. Adverse Events
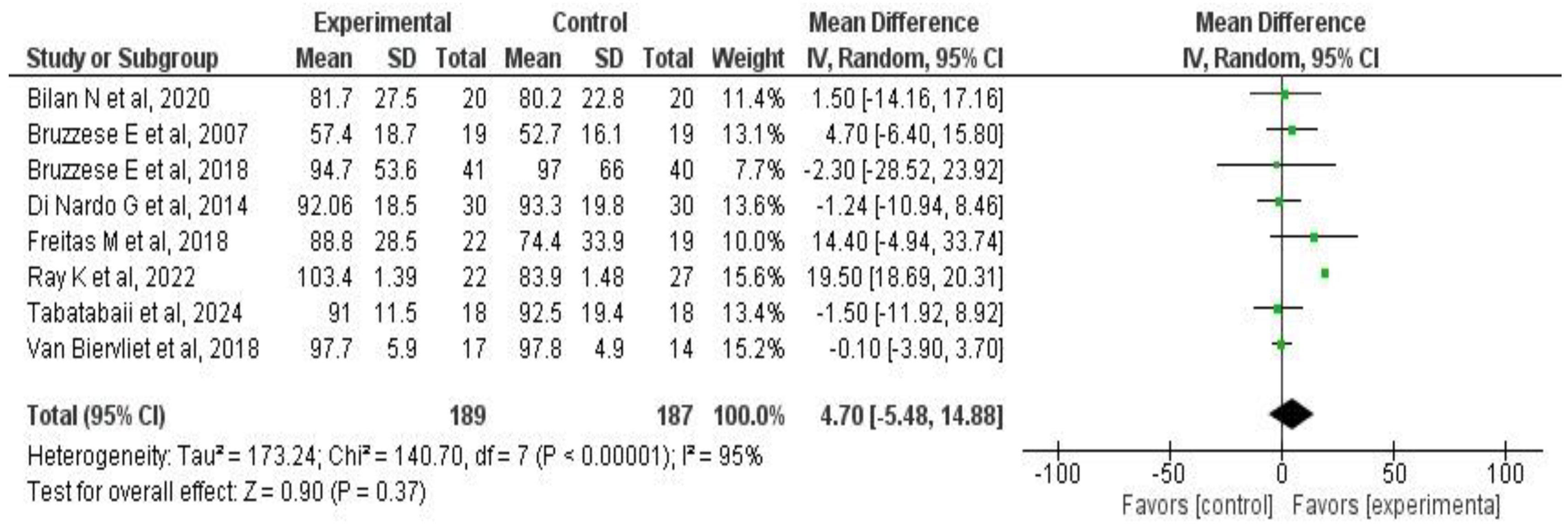
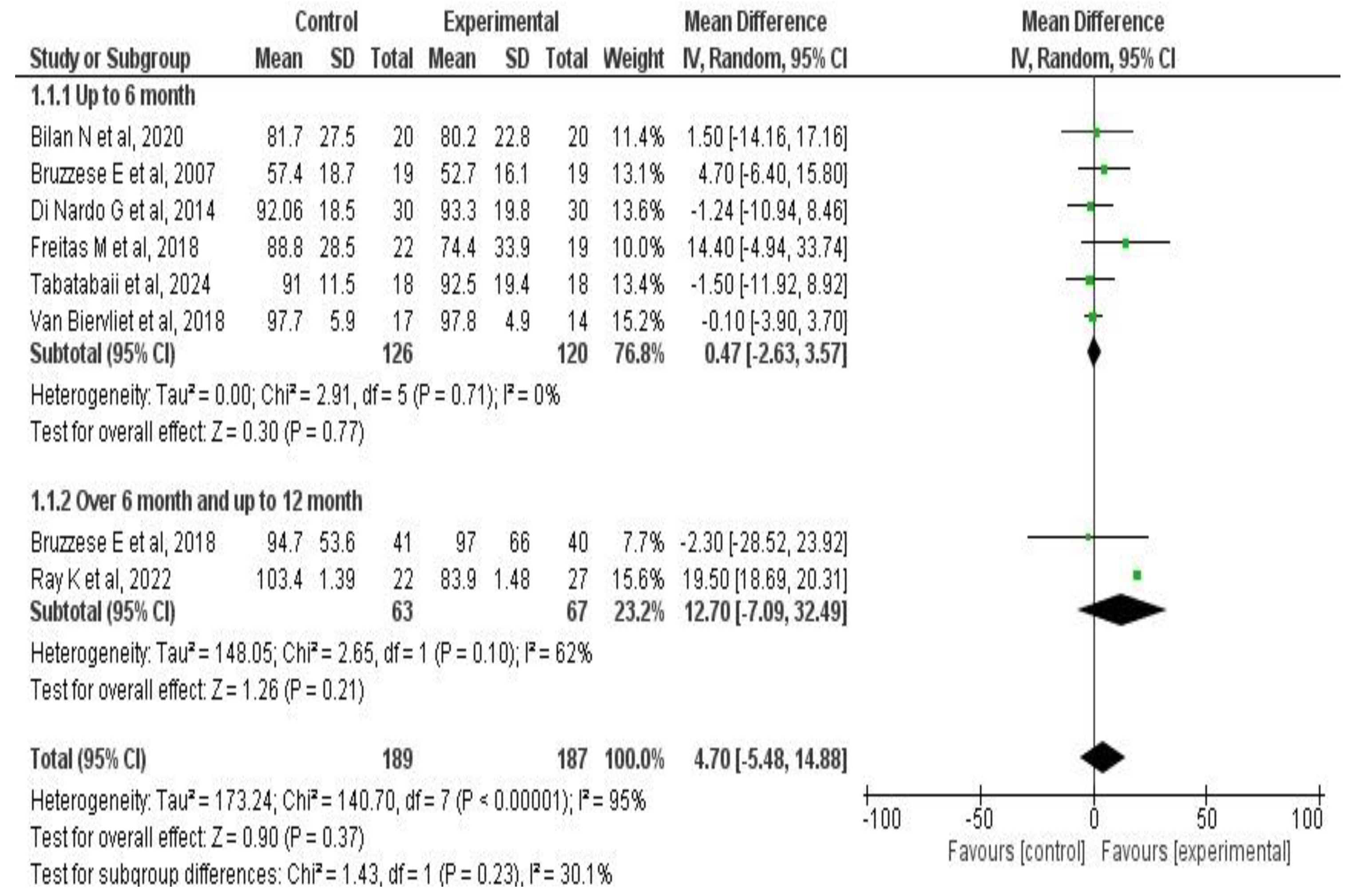
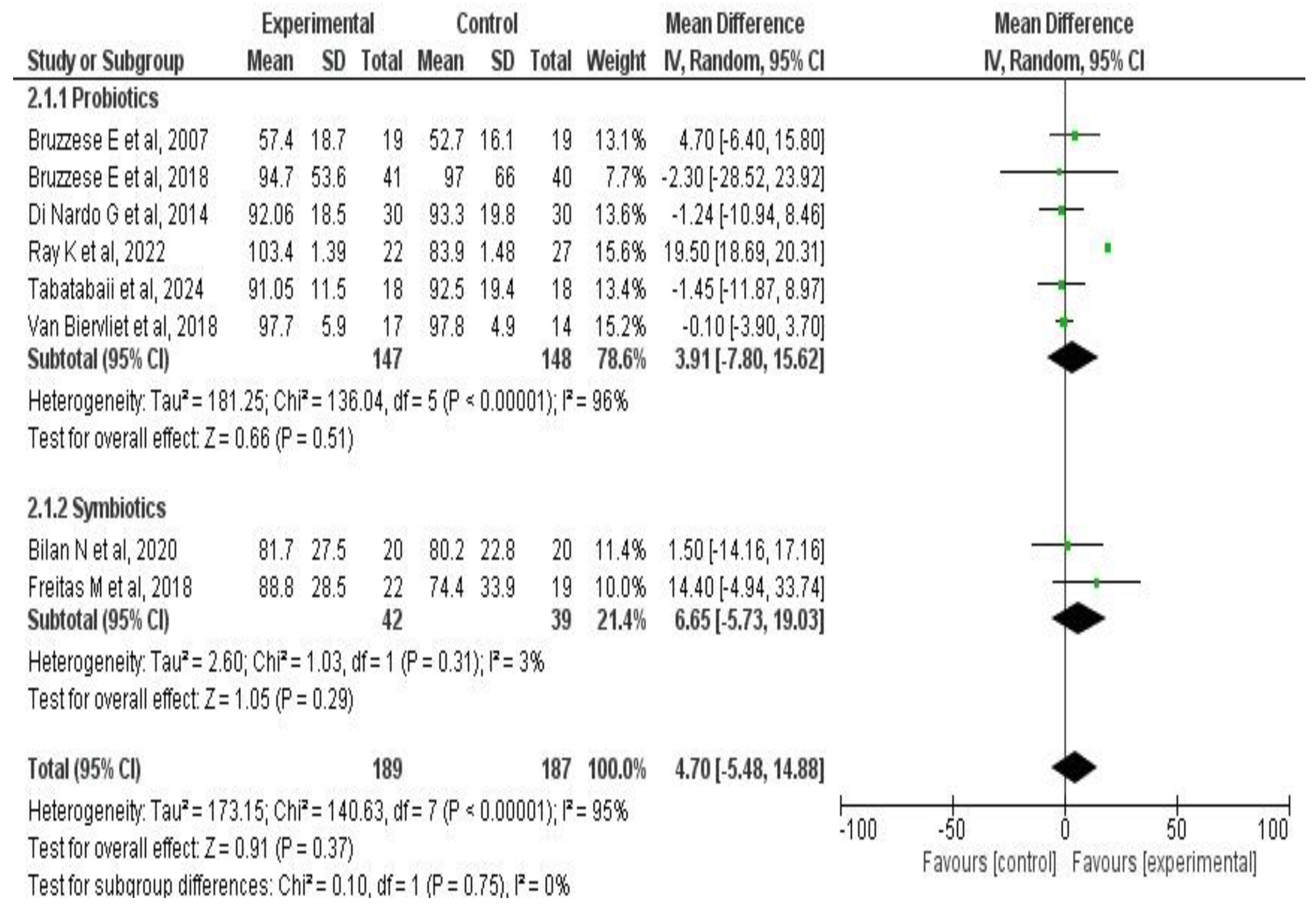
3.6. Meta-Analysis Results
3.6.1. Results of the Quality of Evidence Assessment
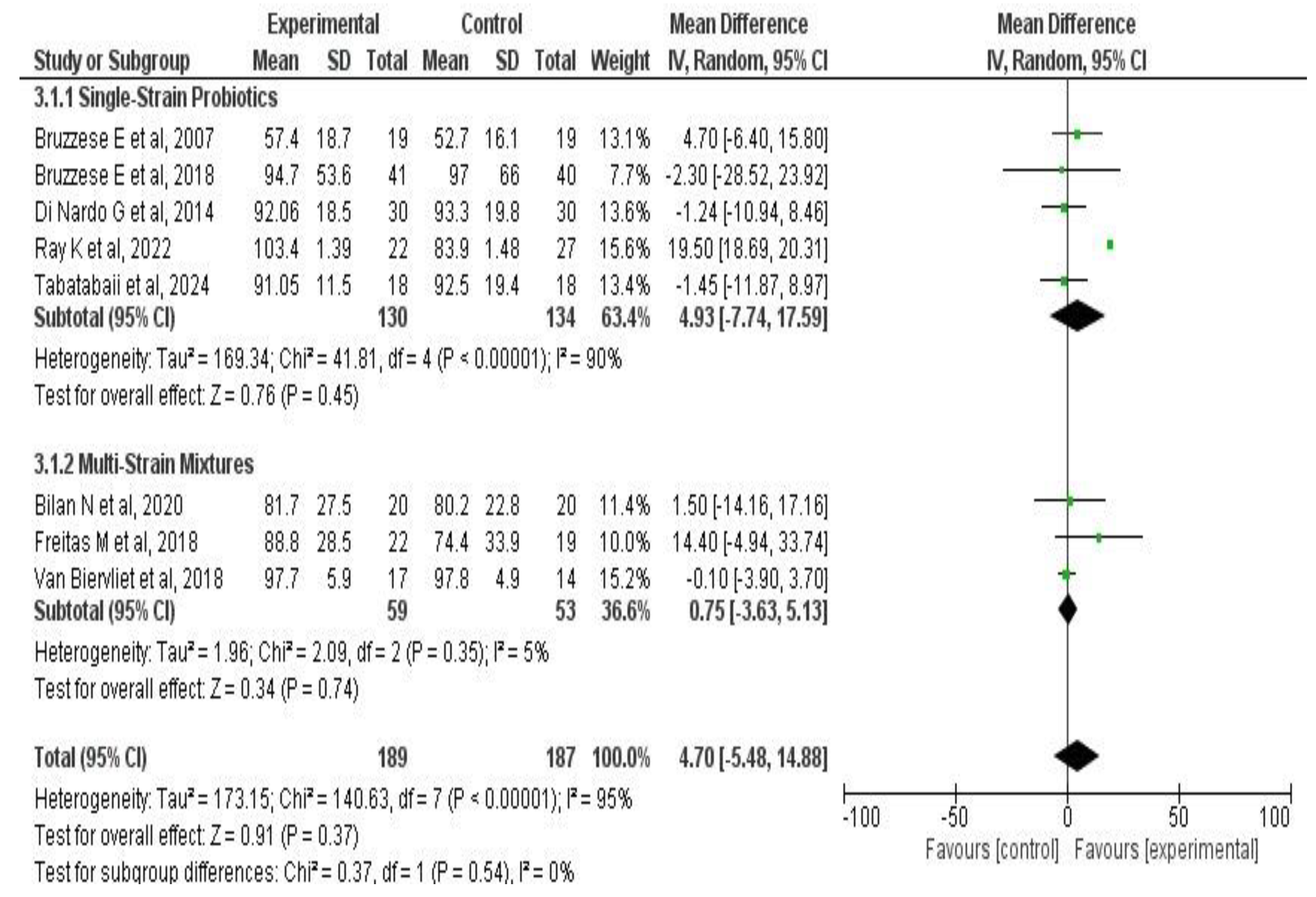
3.6.2. Effects on Pulmonary Function
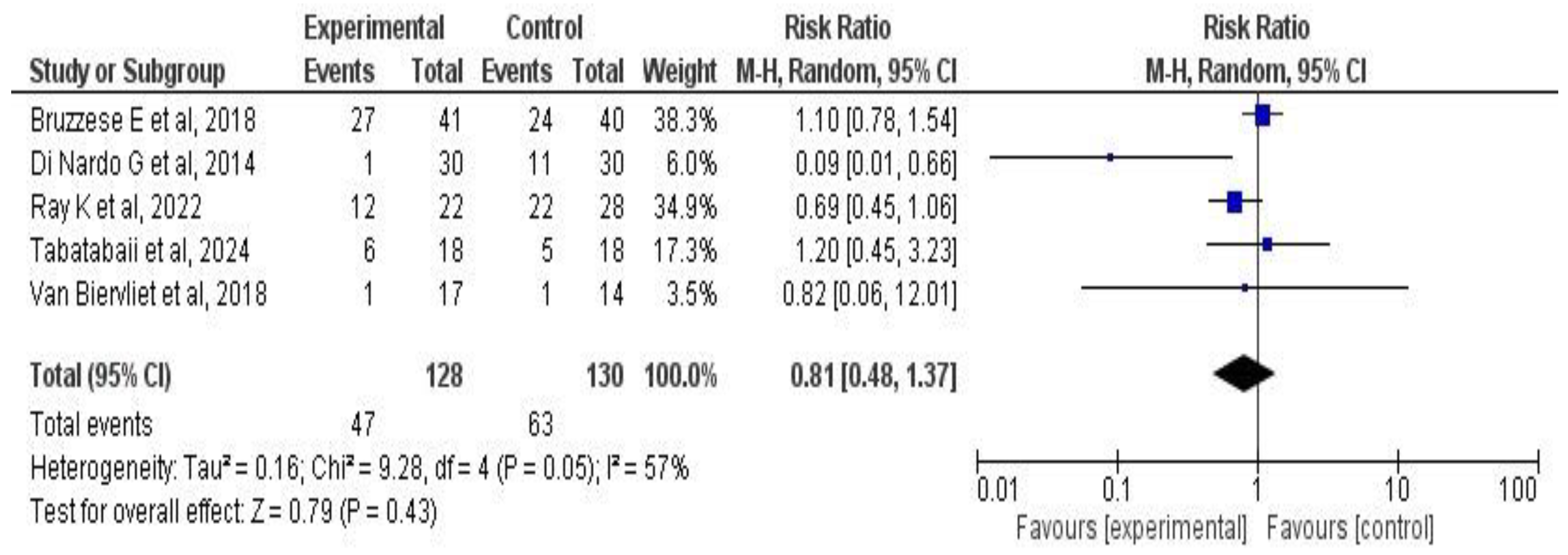
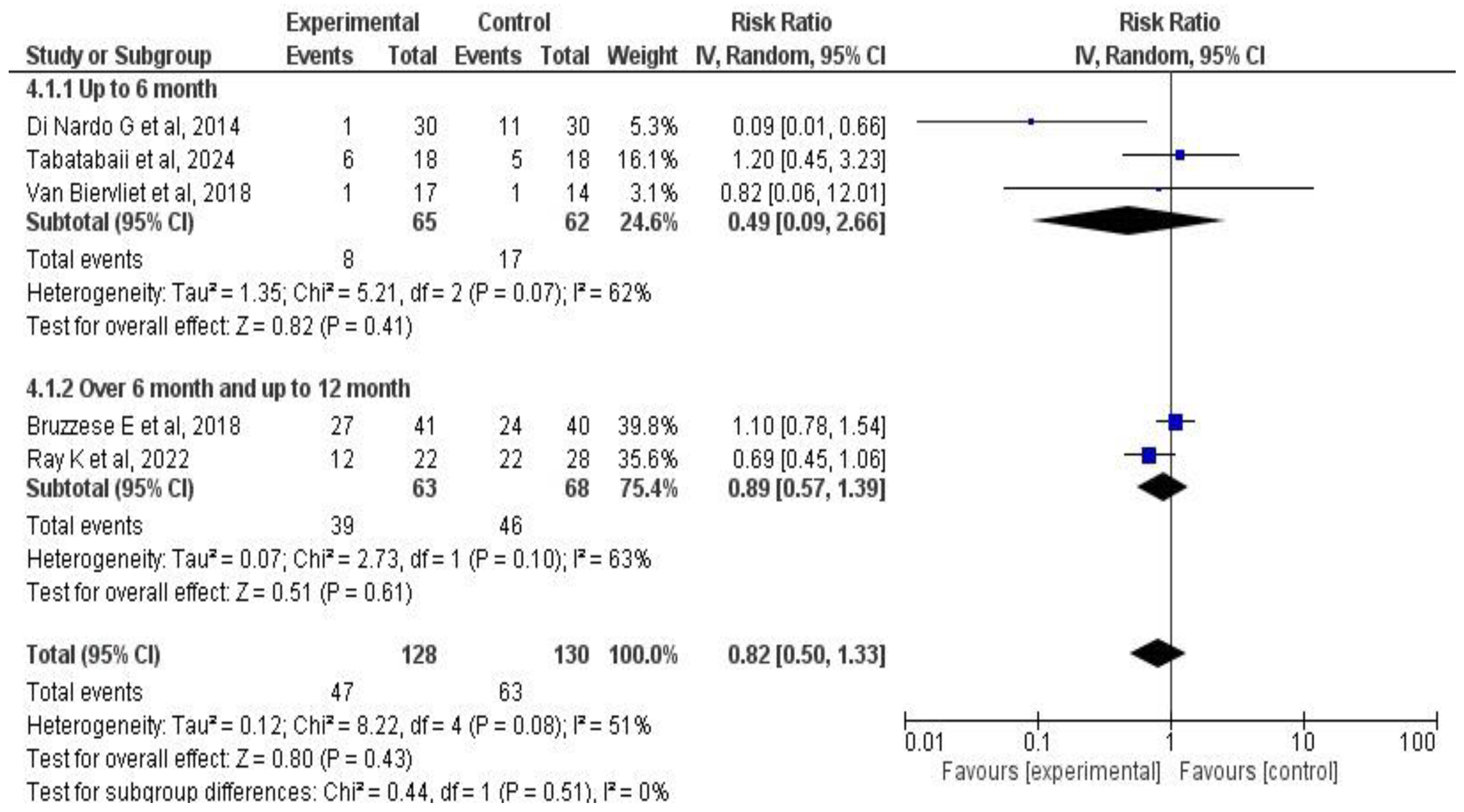
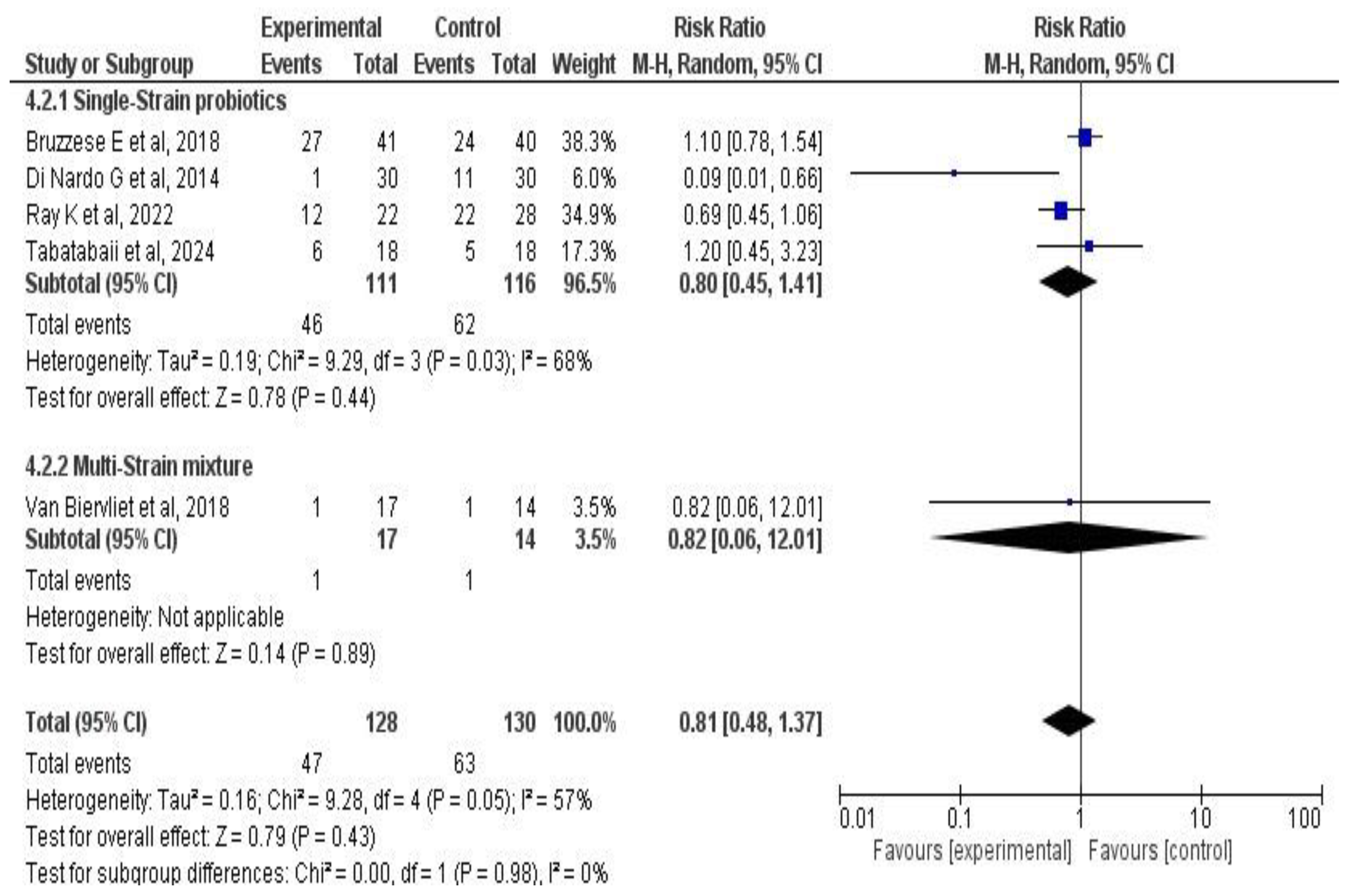
3.6.3. Effects on Pulmonary Exacerbations
3.6.4. Publication Bias
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Possible Mechanisms of Action of Probiotics and/or Synbiotics in CF
4.4. Limitations of the Included Studies
4.5. Limitations of the Review
4.6. Clinical Implications
4.7. Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CFU | Colony-Forming Units |
References
- Castellani, C.; Assael, B.M. Cystic Fibrosis: A Clinical View. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Bellis, G.; Olesen, H.V.; Viviani, L.; Zolin, A.; Blasi, F.; Elborn, J.S. Future Trends in Cystic Fibrosis Demography in 34 European Countries. Eur. Respir. J. 2015, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.; Ramsey, B.W. Cystic Fibrosis: A Review. JAMA 2023, 329, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Polgreen, P.M.; Comellas, A.P. Clinical Phenotypes of Cystic Fibrosis Carriers. Annu. Rev. Med. 2022, 73, 563–574. [Google Scholar] [CrossRef]
- Farinha, C.M.; Callebaut, I. Molecular Mechanisms of Cystic Fibrosis—How Mutations Lead to Misfunction and Guide Therapy. Biosci. Rep. 2022, 42, BSR20212006. [Google Scholar] [CrossRef]
- López-Valdez, J.A.; Aguilar-Alonso, L.A.; Gándara-Quezada, V.; Ruiz-Rico, G.E.; Ávila-Soledad, J.M.; Reyes, A.A.; Pedroza-Jiménez, F.D. Cystic fibrosis: Current concepts. Bol. Méd. Hosp. Infant. México 2021, 78, 6536. [Google Scholar] [CrossRef]
- De Boeck, K.; Vermeulen, F.; Dupont, L. The Diagnosis of Cystic Fibrosis. Presse Médicale 2017, 46, e97–e108. [Google Scholar] [CrossRef]
- Bierlaagh, M.C.; Muilwijk, D.; Beekman, J.M.; van der Ent, C.K. A New Era for People with Cystic Fibrosis. Eur. J. Pediatr. 2021, 180, 2731–2739. Available online: https://link.springer.com/article/10.1007/s00431-021-04168-y (accessed on 12 December 2024). [CrossRef]
- Stephenson, A.L.; Sykes, J.; Stanojevic, S.; Quon, B.S.; Marshall, B.C.; Petren, K.; Ostrenga, J.; Fink, A.K.; Elbert, A.; Goss, C.H. Survival Comparison of Patients with Cystic Fibrosis in Canada and the United States. Ann. Intern. Med. 2017, 166, 537–546. [Google Scholar] [CrossRef]
- Infante, C.S.; Behar, R.R.; Carpenter, L.T.R.; Paredes, B.B.; López, C.R.; Gámez, H.C.; Acuña, M.C. Fibrosis quística en niños y su seguimiento durante 40 años (1977–2017). Rev. Cuba. Pediatría 2019, 91, 1–15. [Google Scholar]
- Spoonhower, K.A.; Davis, P.B. Epidemiology of Cystic Fibrosis. Clin. Chest Med. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Silva Filho, L.V.R.F.; Castaños, C.; Ruíz, H.H. Cystic Fibrosis in Latin America—Improving the Awareness. J. Cyst. Fibros. 2016, 15, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.; Ren, C.L. Review of Cystic Fibrosis. Pediatr. Ann. 2019, 48, e154–e161. [Google Scholar] [CrossRef] [PubMed]
- Norkina, O.; Burnett, T.G.; De Lisle, R.C. Bacterial Overgrowth in the Cystic Fibrosis Transmembrane Conductance Regulator Null Mouse Small Intestine. Infect. Immun. 2004, 72, 6040–6049. [Google Scholar] [CrossRef]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Buckling, A.; Kong, W.; Wild, Y.; Lynch, S.V.; Harrison, F. Gut Dysbiosis in Cystic Fibrosis. J. Cyst. Fibros. 2012, 11, 454–455. [Google Scholar] [CrossRef]
- Kristensen, M.; Prevaes, S.M.P.J.; Kalkman, G.; Tramper-Stranders, G.A.; Hasrat, R.; de Winter-de Groot, K.M.; Janssens, H.M.; Tiddens, H.A.; van Westreenen, M.; Sanders, E.A.M.; et al. Development of the Gut Microbiota in Early Life: The Impact of Cystic Fibrosis and Antibiotic Treatment. J. Cyst. Fibros. 2020, 19, 553–561. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota Regulates Immune Defense against Respiratory Tract Influenza A Virus Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Samuelson, D.R.; Welsh, D.A.; Shellito, J.E. Regulation of Lung Immunity and Host Defense by the Intestinal Microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar] [CrossRef]
- Taylor-Robinson, D.; Whitehead, M.; Diderichsen, F.; Olesen, H.V.; Pressler, T.; Smyth, R.L.; Diggle, P. Understanding the Natural Progression in %FEV1 Decline in Patients with Cystic Fibrosis: A Longitudinal Study. Thorax 2012, 67, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.L.; Boutin, S.; Dittrich, S.A.; Graeber, S.Y.; Stahl, M.; Wege, S.; Herth, F.J.F.; Sommerburg, O.; Schultz, C.; Mall, M.A.; et al. Relationship between Airway Dysbiosis, Inflammation and Lung Function in Adults with Cystic Fibrosis. J. Cyst. Fibros. 2021, 20, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.L.; Bridson, C.; Dittrich, S.; Graeber, S.Y.; Stahl, M.; Wege, S.; Herth, F.; Sommerburg, O.; Schultz, C.; Dalpke, A.; et al. Changes in Microbiome Dominance Are Associated with Declining Lung Function and Fluctuating Inflammation in People with Cystic Fibrosis. Front. Microbiol. 2022, 13, 885822. [Google Scholar] [CrossRef]
- Esposito, S.; Testa, I.; Mariotti Zani, E.; Cunico, D.; Torelli, L.; Grandinetti, R.; Fainardi, V.; Pisi, G.; Principi, N. Probiotics Administration in Cystic Fibrosis: What Is the Evidence? Nutrients 2022, 14, 3160. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Declercq, D.; Somerset, S. Clinical Effects of Probiotics in Cystic Fibrosis Patients: A Systematic Review. Clin. Nutr. ESPEN 2017, 18, 37–43. [Google Scholar] [CrossRef]
- Weiss, B.; Bujanover, Y.; Yahav, Y.; Vilozni, D.; Fireman, E.; Efrati, O. Probiotic Supplementation Affects Pulmonary Exacerbations in Patients with Cystic Fibrosis: A Pilot Study. Pediatr. Pulmonol. 2010, 45, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Tierney, A.C.; Miles, C.; Kotsimbos, T.; King, S.J. Probiotic Use in Adults with Cystic Fibrosis Is Common and Influenced by Gastrointestinal Health Needs: A Cross-sectional Survey Study. J. Hum. Nutr. Diet. 2022, 35, 444–454. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and Publication Bias. J. Cranio-Maxillofac. Surg. 2011, 39, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaii, S.A.; Khanbabaee, G.; Sadr, S.; Farahbakhsh, N.; Modarresi, S.Z.; Pourghasem, M.; Hajipour, M. The Effect of Lactobacillus reuteri on Pulmonary Function Test and Growth of Cystic Fibrosis Patients. J. Sci. Food Agric. 2024, 104, 9056–9061. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.J.; Santee, C.; McCauley, K.; Panzer, A.R.; Lynch, S.V. Gut Bifidobacteria Enrichment Following Oral Lactobacillus-Supplementation Is Associated with Clinical Improvements in Children with Cystic Fibrosis. BMC Pulm. Med. 2022, 22, 287. [Google Scholar] [CrossRef]
- Bilan, N.; Marefat, E.; Nikniaz, L.; Abbasalizad Farhangi, M.; Nikniaz, Z. Does Synbiotic Supplementation Affect the Quality of Life in Children with Cystic Fibrosis? A Pilot Randomized Controlled Clinical Trial. BMC Nutr. 2020, 6, 44. [Google Scholar] [CrossRef]
- Bilan, N.; Marefat, E.; Nouri-Vaskeh, M.; Nikniaz, L.; Nikniaz, Z. Effects of Synbiotic Supplementation on the Pulmonary Manifestations and Anthropometric Measurements in Children with Cystic Fibrosis- a Randomized Clinical Trial. Eur. J. Integr. Med. 2020, 33, 101027. [Google Scholar] [CrossRef]
- De Freitas, M.B.; Moreira, E.A.M.; Oliveira, D.D.L.; Tomio, C.; Da Rosa, J.S.; Moreno, Y.M.F.; Barbosa, E.; Ludwig Neto, N.; Buccigrossi, V.; Guarino, A.; et al. Effect of Synbiotic Supplementation in Children and Adolescents with Cystic Fibrosis: A Randomized Controlled Clinical Trial. Eur. J. Clin. Nutr. 2018, 72, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Raia, V.; Ruberto, E.; Scotto, R.; Giannattasio, A.; Bruzzese, D.; Cavicchi, M.C.; Francalanci, M.; Colombo, C.; Faelli, N.; et al. Lack of Efficacy of Lactobacillus GG in Reducing Pulmonary Exacerbations and Hospital Admissions in Children with Cystic Fibrosis: A Randomised Placebo Controlled Trial. J. Cyst. Fibros. 2018, 17, 375–382. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Hauser, B.; Verhulst, S.; Stepman, H.; Delanghe, J.; Warzee, J.-P.; Pot, B.; Vandewiele, T.; Wilschanski, M. Probiotics in Cystic Fibrosis Patients: A Double Blind Crossover Placebo Controlled Study. Clin. Nutr. ESPEN 2018, 27, 59–65. [Google Scholar] [CrossRef]
- Del Campo, R.; Garriga, M.; Pérez-Aragón, A.; Guallarte, P.; Lamas, A.; Máiz, L.; Bayón, C.; Roy, G.; Cantón, R.; Zamora, J.; et al. Improvement of Digestive Health and Reduction in Proteobacterial Populations in the Gut Microbiota of Cystic Fibrosis Patients Using a Lactobacillus Reuteri Probiotic Preparation: A Double Blind Prospective Study. J. Cyst. Fibros. 2014, 13, 716–722. [Google Scholar] [CrossRef]
- Bruzzese, E.; Callegari, M.L.; Raia, V.; Viscovo, S.; Scotto, R.; Ferrari, S.; Morelli, L.; Buccigrossi, V.; Lo Vecchio, A.; Ruberto, E.; et al. Disrupted Intestinal Microbiota and Intestinal Inflammation in Children with Cystic Fibrosis and Its Restoration with Lactobacillus GG: A Randomised Clinical Trial. PLoS ONE 2014, 9, e87796. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Oliva, S.; Menichella, A.; Pistelli, R.; De Biase, R.V.; Patriarchi, F.; Cucchiara, S.; Stronati, L. Lactobacillus Reuteri ATCC55730 in Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.-A.; Mehdizadeh-Hakkak, A.; Kianifar, H.-R.; Hebrani, P.; Ahanchian, H.; Abbasnejad, E. Effects of Probiotics on Quality of Life in Children with Cystic Fibrosis; a Randomized Controlled Trial. Iran. J. Pediatr. 2013, 23, 669–674. [Google Scholar]
- Fallahi, G.; Motamed, F.; Yousefi, A.; Shafieyoun, A.; Najafi, M.; Khodadad, A.; Farhmand, F.; Ahmadvand, A.; Rezaei, N. The Effect of Probiotics on Fecal Calprotectin in Patients with Cystic Fibrosis. Turk. J. Pediatr. 2013, 55, 475–478. [Google Scholar] [PubMed]
- Bruzzese, E.; Raia, V.; Spagnuolo, M.I.; Volpicelli, M.; De Marco, G.; Maiuri, L.; Guarino, A. Effect of Lactobacillus GG Supplementation on Pulmonary Exacerbations in Patients with Cystic Fibrosis: A Pilot Study. Clin. Nutr. 2007, 26, 322–328. [Google Scholar] [CrossRef]
- Neri, L.D.C.L.; Taminato, M.; Silva Filho, L.V.R.F.D. Systematic Review of Probiotics for Cystic Fibrosis Patients: Moving Forward. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 394–399. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Van Dorst, J.M.; Tam, R.Y.; Ooi, C.Y. What Do We Know about the Microbiome in Cystic Fibrosis? Is There a Role for Probiotics and Prebiotics? Nutrients 2022, 14, 480. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.; Gil-Campos, M.; Gil, A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Kaya, E.; Esin, S. Lung-Directed Bacteriotherapy in Cystic Fibrosis: Could It Be an Option? Antibiotics 2022, 11, 326. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A. Use of Synbiotics for Ulcerative Colitis Treatment. Curr. Clin. Pharmacol. 2020, 15, 174–182. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2020, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, F.E.C.; Lizcano Martinez, S.; Liscano, Y. Effectiveness of Psychobiotics in the Treatment of Psychiatric and Cognitive Disorders: A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 1352. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef]
- Liscano, Y.; Sanchez-Palacio, N. A Critical Look at Omega-3 Supplementation: A Thematic Review. Healthcare 2023, 11, 3065. [Google Scholar] [CrossRef] [PubMed]
- Price, C.E.; Valls, R.A.; Ramsey, A.R.; Loeven, N.A.; Jones, J.T.; Barrack, K.E.; Schwartzman, J.D.; Royce, D.B.; Cramer, R.A.; Madan, J.C.; et al. Intestinal Bacteroides Modulates Inflammation, Systemic Cytokines, and Microbial Ecology via Propionate in a Mouse Model of Cystic Fibrosis. mBio 2024, 15, e03144-23. [Google Scholar] [CrossRef] [PubMed]
- Gur, M.; Zuckerman-Levin, N.; Masarweh, K.; Hanna, M.; Laghi, L.; Marazzato, M.; Levanon, S.; Hakim, F.; Bar–Yoseph, R.; Wilschanski, M.; et al. The Effect of Probiotic Administration on Metabolomics and Glucose Metabolism in CF Patients. Pediatr. Pulmonol. 2022, 57, 2335–2343. [Google Scholar] [CrossRef]
- Viteri-Echeverría, J.; Andrés, A.; Calvo-Lerma, J.; Heredia, A.; García-Hernández, J.; Asensio-Grau, A. In Vitro Screening of the Impact of Dietary Prebiotic Components, Probiotic Strains, and Their Symbiotic Combinations on Colonic Microbiota in Children with Cystic Fibrosis. Food Funct. 2024, 15, 6512–6522. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Ferriz-Jordán, M.; García-Hernández, J.; Heredia, A.; Andrés, A. Effect of Lactobacillaceae Probiotics on Colonic Microbiota and Metabolite Production in Cystic Fibrosis: A Comparative In Vitro Study. Nutrients 2023, 15, 3846. [Google Scholar] [CrossRef]
- Rodríguez-Sojo, M.J.; Ruiz-Malagón, A.J.; Rodríguez-Cabezas, M.E.; Gálvez, J.; Rodríguez-Nogales, A. Limosilactobacillus Fermentum CECT5716: Mechanisms and Therapeutic Insights. Nutrients 2021, 13, 1016. [Google Scholar] [CrossRef]
- Pompilio, A.; Kaya, E.; Lupetti, V.; Catelli, E.; Bianchi, M.; Maisetta, G.; Esin, S.; Di Bonaventura, G.; Batoni, G. Cell-Free Supernatants from Lactobacillus Strains Exert Antibacterial, Antibiofilm, and Antivirulence Activity against Pseudomonas Aeruginosa from Cystic Fibrosis Patients. Microbes Infect. 2024, 26, 105301. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.C.; Koestler, D.C.; Stanton, B.A.; Davidson, L.; Moulton, L.A.; Housman, M.L.; Moore, J.H.; Guill, M.F.; Morrison, H.G.; Sogin, M.L.; et al. Serial Analysis of the Gut and Respiratory Microbiome in Cystic Fibrosis in Infancy: Interaction between Intestinal and Respiratory Tracts and Impact of Nutritional Exposures. mBio 2012, 3, 10-1128. [Google Scholar] [CrossRef]
- Hoen, A.G.; Li, J.; Moulton, L.A.; O’Toole, G.A.; Housman, M.L.; Koestler, D.C.; Guill, M.F.; Moore, J.H.; Hibberd, P.L.; Morrison, H.G.; et al. Associations between Gut Microbial Colonization in Early Life and Respiratory Outcomes in Cystic Fibrosis. J. Pediatr. 2015, 167, 138–147.e3. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Moreira, E.A.M.; Tomio, C.; Moreno, Y.M.F.; Daltoe, F.P.; Barbosa, E.; Ludwig Neto, N.; Buccigrossi, V.; Guarino, A. Altered Intestinal Microbiota Composition, Antibiotic Therapy and Intestinal Inflammation in Children and Adolescents with Cystic Fibrosis. PLoS ONE 2018, 13, e0198457. [Google Scholar] [CrossRef]
- Munir, I.; Yen, H.-W.; Geller, D.H.; Torbati, D.; Bierden, R.M.; Weitsman, S.R.; Agarwal, S.K.; Magoffin, D.A. Insulin Augmentation of 17alpha-Hydroxylase Activity Is Mediated by Phosphatidyl Inositol 3-Kinase but Not Extracellular Signal-Regulated Kinase-1/2 in Human Ovarian Theca Cells. Endocrinology 2004, 145, 175–183. [Google Scholar] [CrossRef]
- Price, C.E.; O’Toole, G.A. The Gut-Lung Axis in Cystic Fibrosis. J. Bacteriol. 2021, 203, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Lussac-Sorton, F.; Charpentier, É.; Imbert, S.; Lefranc, M.; Bui, S.; Fayon, M.; Berger, P.; Enaud, R.; Delhaes, L. The Gut-Lung Axis in the CFTR Modulator Era. Front. Cell. Infect. Microbiol. 2023, 13, 1271117. [Google Scholar] [CrossRef]
- Wrigley-Carr, H.E.; Van Dorst, J.M.; Ooi, C.Y. Intestinal Dysbiosis and Inflammation in Cystic Fibrosis Impacts Gut and Multi-Organ Axes. Med. Microecol. 2022, 13, 100057. [Google Scholar] [CrossRef]
- Davison, J.M.; Wischmeyer, P. Probiotic and Synbiotic Therapy in the Critically Ill: State of the Art. Nutrition 2019, 59, 29–36. [Google Scholar] [CrossRef]
- Jain, N. The Need for Personalized Approaches to Microbiome Modulation. Front. Public Health 2020, 8, 144. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.A.; Dias, I.P.; Maria de Lara, L.; Nunes, R.F. Probióticos e Suas Aplicações Clínicas: Uma Abordagem Multidisciplinar. Rev. Saude Multidiscip. 2020, 7, 101–107. [Google Scholar]
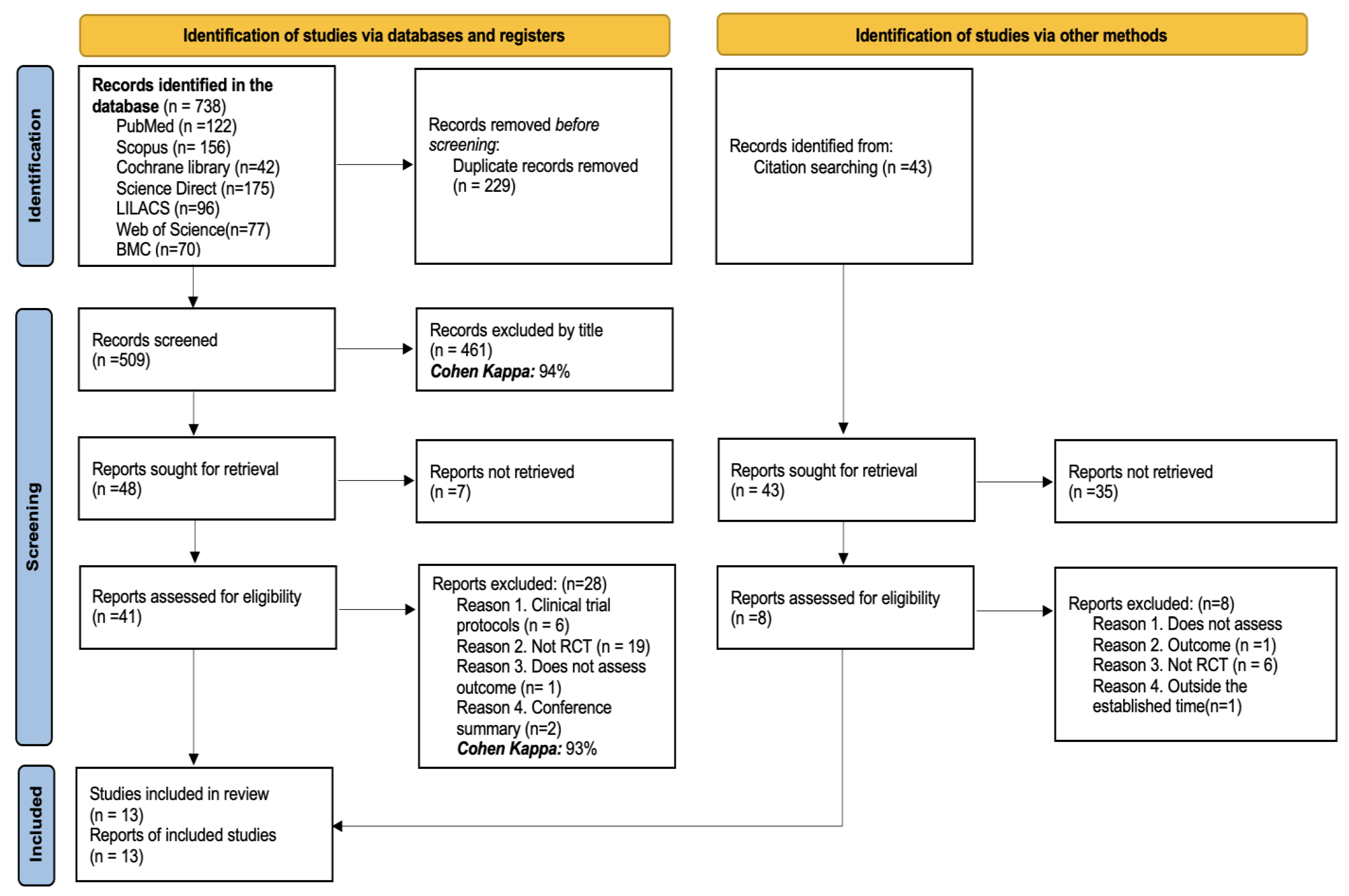

| Author, Year | Country | Disease | Population Included | Number of Patients | Outcomes Evaluated |
|---|---|---|---|---|---|
| Tabatabai S. et al., 2024 [33] | Iran | CF | Children and adults | 40 | Pulmonary exacerbations, hospitalizations, pulmonary function, and composition of the gastrointestinal microbiota. |
| Ray KJ. et al., 2022 [34] | Italy | CF | Children | 49 | Pulmonary exacerbations, hospitalizations, pulmonary function, inflammatory biomarkers, and intestinal microbiota composition. |
| Bilan N. et al., 2020 [35] | Iran | CF | Children | 40 | Health-related quality of life. |
| Bilan N. et al., 2020 [36] | Iran | CF | Children | 36 | Pulmonary exacerbations, hospitalizations, and pulmonary function. |
| Freitas D. et al., 2018 [37] | Brazil | CF | Children | 41 | Pulmonary function and inflammatory biomarkers. |
| Bruzzese E. et al., 2018 [38] | Italy | CF | Children | 81 | Pulmonary exacerbations, hospitalizations, and pulmonary function. |
| Van Biervliet. et al., 2018 [39] | Belgium | CF | Children | 31 | Pulmonary function, pulmonary exacerbations, and inflammatory biomarkers. |
| Del campo R et al., 2014 [40] | Spain | CF | Children and adults | 30 | Inflammatory biomarkers, intestinal microbiota composition, quality of life, and adverse effects. |
| Bruzzese E. et al., 2014 [41] | Italy | CF | Children | 22 | Inflammatory biomarkers and intestinal microbiota composition. |
| Di Nardo G. et al., 2014 [42] | Italy | CF | Children and adults | 60 | Pulmonary exacerbations, hospitalizations, inflammatory biomarkers, and pulmonary function. |
| Jafari S. et al., 2013 [43] | Iran | CF | Children | 37 | Pulmonary exacerbations and health-related quality of life. |
| Fallahi G. et al., 2013 [44] | Iran | CF | Children | 47 | Inflammatory biomarkers. |
| Bruzzese E. et al., 2007 [45] | Italy | CF | Children and adults | 38 | Pulmonary exacerbations, hospitalizations, and pulmonary function. |
| Author, Year | Patients (I/C) | Male (%) | Age Range (Years) | Mean Age | Product (Type, Formulation, Dose, Administration Route) | Placebo | Treatment Duration (Months) | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Tabatabai S. et al., 2024 [33] | n = 36 I: 18 C: 18 | 42.5 | 6–20 | 12 | Probiotic. Lactobacillus reuteri (8 × 108 CFU). One sachet per day, orally. | NE | 4 | No significant changes were observed in forced expiratory volume in one second (FEV1), forced expiratory flow at 25–75%, or forced vital capacity between the two groups after the treatment period. |
| Ray K.J. et al., 2022 [34] | n = 49 I: 22 C: 27 | 52 | 2–16 | 7.4 * | Probiotic. Lactobacillus rhamnosus GG. Daily supplementation orally. | NE | 12 | Patients receiving probiotic supplementation achieved better clinical outcomes compared to the control group. |
| Bilan N. et al., 2020 [35] | n = 36 I: 18 C: 18 | 55 | 5–12 | 8.75 | Synbiotic. L. casei (3.5 × 109 CFU/g), L. acidophilus (1 × 109 CFU/g), L. rhamnosus (7.5 × 108 CFU/g), L. bulgaricus (108 CFU/g), Streptococcus thermophilus (1 × 108 CFU/g), B. breve (1 × 1010 CFU/g), B. longum (3.5 × 109 CFU/g), and 38.5 mg of fructooligosaccharides. Two capsules daily, orally. | Maltodextrin | 6 | The probiotic blend did not reduce pulmonary exacerbations, did not significantly improve pulmonary function, and did not reduce the number or duration of hospitalizations. |
| Bilan N. et al., 2020 [36] | n = 40 I: 20 C: 20 | 52 | 5–12 | 8.72 | Synbiotic. L. casei (3.5 × 109 CFU/g), L. acidophilus (1 × 109 CFU/g), L. rhamnosus (7.5 × 108 CFU/g), L. bulgaricus (108 CFU/g), S. thermophilus (1 × 108 CFU/g), B. breve (1 × 1010 CFU/g), B. longum (3.5 × 109 CFU/g), and 38.5 mg of fructooligosaccharides. Two capsules daily, orally. | Maltodextrin | 6 | After six months of synbiotic supplementation, there was no significant effect on health-related quality of life in CF patients. |
| Freitas et al., 2018 [37] | n = 41 I: 22 C: 19 | 53.6 | 1–16 | 10.1 | Synbiotic. Fructooligosaccharides 5.5 g/day; L. paracasei, L. rhamnosus, L. acidophilus, and B. lactis (108–109 CFU/day each strain). Powder, orally. | Maltodextrin | 3 | Synbiotic supplementation showed promise in reducing the pro-inflammatory markers IL-6 and IL-8. No differences were found between groups in FEV1 levels or nutritional status. |
| Bruzzese E. et al., 2018 [38] | n = 81 I: 41 C: 40 | 50.6 | 2–16 | 8.5 | Probiotic. Lactobacillus rhamnosus GG (6 × 109 CFU/day). Capsule, orally. | Maltodextrin | 12 | Lactobacillus rhamnosus GG supplementation did not significantly improve respiratory or nutritional outcomes in the studied CF population. |
| Van Biervliet et al., 2018 [39] | n = 31 I: 17 C: 14 | 42 | 6.9–12 | 9.3 | Probiotic. Lactobacillus rhamnosus SP1 (DSM 21690) and Bifidobacterium animalis spp. BLC1 (LGM23512), 1010 CFU/day, orally. | NE | 4 | Probiotic supplementation did not influence fecal calprotectin, pulmonary function, pulmonary exacerbations, or the microbiome. |
| Del campo R. et al, 2014 [40] | n = 30 I: 15 C: 15 | 70 | 8–44 | 17.7 * | Probiotic. Lactobacillus reuteri DSM17938 (108 CFU). Chewable tablet, 1 tablet/day, orally. | NE | 6 | Probiotics may improve CF-related gut microbiota dysbiosis characterized by a high density of Proteobacteria. L. reuteri reduces intestinal inflammation and improves digestive comfort. |
| Bruzzese E. et al., 2014 [41] | n = 22 I: 10 C: 12 | 59 | 2–9 | 7 * | Probiotic. Lactobacillus rhamnosus GG (6 × 109 CFU/day). Capsule, orally. | Maltodextrin | 1 | L. rhamnosus GG administration can partially restore a healthy gut microbiota, favoring communities that limit intestinal inflammation and improving the disease course. |
| Di Nardo G. et al., 2014 [42] | n = 60 I: 30 C: 30 | 65 | 6–29 | 17.5 | Probiotic. Lactobacillus reuteri ATCC55730 (1010 CFU). Five drops/day, orally. | NE | 6 | L. reuteri reduces pulmonary exacerbations and upper respiratory tract infections in CF patients with mild to moderate pulmonary disease. Its administration may exert a beneficial effect on the course of CF. |
| Jafari S. et al., 2013 [43] | n = 37 I: 20 C: 10 | 35 | 2–12 | 5.3 | Probiotics. L. casei, L. rhamnosus, S. thermophilus, B. breve, B. infantis, and L. bulgaricus (each at 109 CFU). Two capsules per day, orally. | NE | 1 | Probiotics may help reduce the number of pulmonary exacerbations and improve quality of life in CF patients, though these quality-of-life improvements appear to be temporary. |
| Fallahi G. et al., 2013 [44] | n = 47 I: 24 C: 23 | NE | NE | 8.56 | Synbiotic. Fructooligosaccharide plus a bacterial mix at 1 × 109 CFU per sachet, including L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, and L. bulgaricus. One sachet daily, orally. | Maltodextrin | 1 | About two-thirds of the patients had intestinal inflammation. Probiotic administration was shown to reduce calprotectin levels and thereby decrease intestinal inflammation in CF patients. |
| Bruzzese E. et al., 2007 [45] | n = 38 I: 19 C:19 | 42 | 5–23 | 13.2 | Probiotic. Lactobacillus rhamnosus GG (6 × 109 CFU/day). Solution, orally. | ORS | 6 | L. rhamnosus GG reduced pulmonary exacerbations and hospital admissions in CF patients, suggesting that probiotics may delay respiratory failure and that a connection exists between intestinal and pulmonary inflammation. |
| Author, Year | Patients (I/C) | Product (Formulation and Dose) | Evaluated Biomarkers | Sample Type | Results on Inflammatory Biomarkers |
|---|---|---|---|---|---|
| Ray K.J. et al., 2022 [34] | n = 49 I: 22 C: 27 | Probiotic. Lactobacillus rhamnosus GG. Daily supplementation, oral route. | Calprotectin | Fecal | The use of Lactobacillus rhamnosus GG was associated with a higher frequency of Bifidobacterium-dominant microbiota, which led to lower fecal calprotectin levels. |
| Freitas et al., 2018 [37] | n = 41 I: 22 C: 19 | Synbiotic; 5.5 g/day fructooligosaccharides (FOSs); L. paracasei, L. rhamnosus, L. acidophilus, B. lactis (108–109 CFU/day per strain). Powder, oral route. | IL-12, TNF-α, IL-10, IL-6, IL-1β, IL-8, MPO, NOx | Serum | Significant differences in NOx levels were found after supplementation with the product. In the group that received synbiotics with positive bacteriological results, reductions in IL-6 (p = 0.033) and IL-8 (p = 0.009) were observed. |
| Van Biervliet et al., 2018 [39] | n = 31 I: 17 C: 14 | Probiotic. Lactobacillus rhamnosus SP1 (DSM 21690) and Bifidobacterium animalis ssp. BLC1 (LGM23512), 1010 CFU/day, oral route. | Calprotectin | Fecal | Although 61% of the patients initially showed abnormal calprotectin values, there were no statistically significant changes in this marker following probiotic supplementation. |
| Del campo R. et al., 2014 [40] | n = 30 I: 15 C: 15 | Probiotic. Lactobacillus reuteri DSM17938 (108 CFU). Chewable tablet, 1 tablet/day, oral route. | Calprotectin, IL-8, IL-1β, IL-6, IL-10, TNF-α, IL-12 | Fecal | CF patients who received probiotic supplementation showed lower calprotectin levels compared to the placebo group (20.3 vs. 33.8 μg/mL; p = 0.003). No differences were noted in other biomarkers. |
| Bruzzee E. et al., 2014 [41] | n = 22 I: 10 C: 12 | Probiotic. Lactobacillus rhamnosus GG (6 × 109 CFU/day). Capsule, oral route. | Calprotectin, rNO | Fecal | Children with CF had elevated fecal calprotectin and rectal nitric oxide levels compared to healthy controls. Lactobacillus GG administration reduced fecal calprotectin levels and partially restored the gut microbiota in CF patients. |
| Di Nardo G. et al., 2014 [42] | n = 60 I: 30 C: 30 | Probiotic. Lactobacillus reuteri ATCC55730 (1010 CFU). Five drops/day, oral route. | Calprotectin, TNF-α, IL-8 | Fecal, Serum | No statistically significant differences were found between the groups in fecal calprotectin or the measured cytokines (TNF-α, IL-8). |
| Fallahi G. et al., 2013 [44] | n = 47 I: 24 C: 23 | Synbiotic. Fructooligosaccharides plus a bacterial mix at 1 × 109 CFU per sachet, containing L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus. One sachet/day, oral route. | Calprotectin | Fecal | At baseline, patients in the probiotic group had an average fecal calprotectin level of 101 μg/g, whereas those in the placebo group had 70.22 μg/g. After probiotic supplementation, levels in the intervention arm were significantly lower than in the placebo group (56.2 μg/g vs. 182.1 μg/g; p = 0.031). |
| Author | The Study Is Randomized | The Intervention Is Double-Blind | Study Withdrawals Are Accounted for and Described | The Randomization Procedure Is Adequate | Selection Criteria | Score |
|---|---|---|---|---|---|---|
| Tabatabai S. 2024 [33] | 1 | 1 | 1 | 1 | 1 | 5 |
| Ray K. 2022 [34] | 1 | 1 | 0 | 0 | 1 | 3 |
| Freitas M. 2018 [37] | 1 | 1 | 0 | 1 | 1 | 4 |
| Bruzzese E. 2018 [38] | 1 | 1 | 1 | 1 | 1 | 5 |
| Jafari S. 2013 [43] | 1 | 0 | 1 | 0 | 1 | 3 |
| Del Campo R. 2014 [40] | 1 | 1 | 1 | 0 | 1 | 4 |
| Bruzzese E. 2014 [41] | 1 | 1 | 0 | 0 | 1 | 3 |
| Fallahi G. 2013 [44] | 1 | 1 | 1 | 0 | 1 | 4 |
| Di Nardo G. 2014 [42] | 1 | 1 | 1 | 1 | 1 | 5 |
| Bruzzese E. 2007 [45] | 1 | 1 | 1 | 1 | 1 | 5 |
| Bilan N. 2020 [36] | 1 | 1 | 1 | 0 | 1 | 4 |
| Bilan N. 2020 [35] | 1 | 1 | 1 | 0 | 1 | 4 |
| Van Biervliet S. 2018 [39] | 1 | 1 | 1 | 0 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Mosquera, F.E.; Perlaza, C.L.; Naranjo Rojas, A.; Murillo Rios, S.; Carrero Gallego, A.; Fischersworring, S.I.; Rodríguez, J.S.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2025, 61, 489. https://doi.org/10.3390/medicina61030489
Cruz Mosquera FE, Perlaza CL, Naranjo Rojas A, Murillo Rios S, Carrero Gallego A, Fischersworring SI, Rodríguez JS, Liscano Y. Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina. 2025; 61(3):489. https://doi.org/10.3390/medicina61030489
Chicago/Turabian StyleCruz Mosquera, Freiser Eceomo, Claudia Lorena Perlaza, Anisbed Naranjo Rojas, Saray Murillo Rios, Alejandra Carrero Gallego, Sara Isabel Fischersworring, Juan Sebastián Rodríguez, and Yamil Liscano. 2025. "Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials" Medicina 61, no. 3: 489. https://doi.org/10.3390/medicina61030489
APA StyleCruz Mosquera, F. E., Perlaza, C. L., Naranjo Rojas, A., Murillo Rios, S., Carrero Gallego, A., Fischersworring, S. I., Rodríguez, J. S., & Liscano, Y. (2025). Effectiveness of Probiotics, Prebiotics, and Symbiotic Supplementation in Cystic Fibrosis Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina, 61(3), 489. https://doi.org/10.3390/medicina61030489






