Efficacy of Intravitreal Dexamethasone Implant (Ozurdex®) in Naïve and Refractory Patients with Different Morphological Subtypes of Diabetic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design—Participants
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. OCT Measurements Analysis
3.2. Visual Acuity Analysis
3.3. Intraocular Pressure Measurements and Re-Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, N.; Grigorian, R.A.; Tutela, A.; Zarbin, M.A. Diabetic macular edema: Pathogenesis and treatment. Surv. Ophthalmol. 2009, 54, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Antcliff, R.J.; Marshall, J. The pathogenesis of edema in diabetic maculopathy. Semin. Ophthalmol. 1999, 14, 223–232. [Google Scholar] [CrossRef]
- Grant, M.B.; Afzal, A.; Spoerri, P.; Pan, H.; Shaw, L.C.; Mames, R.N. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert. Opin. Investig. Drugs 2004, 13, 1275–1293. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- Funatsu, H.; Noma, H.; Mimura, T.; Eguchi, S.; Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.-E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef]
- Richter, B.; Kohner, E. Medical interventions for diabetic retinopathy. In Evidence-Based Ophthalmology; Wardnold, R., Smeeth, L., Henshaw, K., Eds.; BMJ Books: London, UK, 2004; pp. 331–340. [Google Scholar]
- Group ETDRS Research. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Rechtman, E.; Harris, A.; Garzozi, H.J.; Ciulla, T.A. Pharmacologic therapies for diabetic retinopathy and diabetic macular edema. Clin. Ophthalmol. 2007, 1, 383–391. [Google Scholar]
- Shimura, M.; Yasuda, K.; Yasuda, M.; Nakazawa, T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina 2013, 33, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Yu, S.Y.; Kim, M.; Kwak, H.W. Visual and morphologic outcomes of intravitreal ranibizumab for dıabetıc macular edema based on optıcal coherence tomography patterns. Retina 2016, 36, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Lai, C.H.; Chen, C.L.; Kuo, C.N. Optical coherence tomographic patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J. Ocul. Pharmacol. Ther. 2012, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.C.; Scott, I.U.; Kim, S.J.; Brown, G.C.; Brown, M.M.; Ip, M.S.; Recchia, F.M. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: A report by the American Academy of Ophthalmology. Ophthalmology 2012, 119, 2179–2188. [Google Scholar] [CrossRef]
- Grover, D.; Li, T.J.; Chong, C.C. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst. Rev. 2008, 1, CD005656. [Google Scholar] [CrossRef]
- Pacella, E.; Vestri, A.R.; Turchetti, P.; Muscella, R.; Carbotti, M.R.; Coi, L.; Castellucci, M.; Pacella, F. Preliminary results of an intravitreal dexamethasone implant (Ozurdex) in patients with persistent diabetic macular edema. Clin. Ophthalmol. 2013, 7, 1423–1428. [Google Scholar] [CrossRef]
- Chhablani, J.; Bansal, P.; Veritti, D.; Sambhana, S.; Sarao, V.; Pichi, F.; Carrai, P.; Massaro, D.; Lembo, A.; Mansour, A.M.; et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye 2016, 30, 426–430. [Google Scholar] [CrossRef]
- Malclès, A.; Dot, C.; Voirin, N.; Agard, É.; Vié, A.L.; Bellocq, D.; Denis, P.; Kodjikian, L. Real-life study in diabetic macular edema treated with dexamethasone implant: The Reldex Study. Retina 2017, 37, 753–760. [Google Scholar] [CrossRef]
- Murakami, T.; Yoshimura, N. Structural changes in individual retinal layers in diabetic macular edema. J. Diabetes Res. 2013, 2013, 920713. [Google Scholar] [CrossRef]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Otsuka, H.; Sonoda, Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina 2014, 34, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y.; Lee, S.J. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines based on optical coherence tomography patterns of diabetic macular edema. Indian. J. Ophthalmol. 2015, 63, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Bandyopadhyay, S.K.; Saha, M.; Sinha, A. Study of aqueous cytokines in patients with different patterns of diabetic macular edema based on optical coherence tomography. Int. Ophthalmol. 2018, 38, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Kaldırım, H.; Yazgan, S.; Atalay, K.; Gurez, C.; Savur, F. Intravitreal dexamethasone implantation in patients with different morphological diabetic macular edema having insufficient response to ranibizumab. Retina 2018, 38, 986–992. [Google Scholar] [CrossRef]
- Zur, D.; Iglicki, M.; Busch, C.; Invernizzi, A.; Mariussi, M.; Loewenstein, A.; International Retina Group. OCT Biomarkers as Functional Outcome Predictors in Diabetic Macular Edema Treated with Dexamethasone Implant. Ophthalmology 2018, 125, 267–275. [Google Scholar] [CrossRef]
- Castro-Navarro, V.; Cervera-Taulet, E.; Navarro-Palop, C.; Monferrer-Adsuara, C.; Hernández-Bel, L.; Montero-Hernández, J. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema. BMC Ophthalmol. 2019, 19, 15. [Google Scholar] [CrossRef]
- Ozsaygili, C.; Duru, N. Comparison of intravitreal dexamethasone implant and aflibercept in patients with treatment-naive diabetic macular edema with serous retinal detachment. Retina 2020, 40, 1044–1052. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Campbell, J.; Holekamp, N.M.; Kiss, S.; Loewenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: Analysis of protocol I data. Am. J. Ophthalmol. 2016, 172, 72–79. [Google Scholar] [CrossRef]
- Kim, B.Y.; Smith, S.D.; Kaiser, P.K. Optical coherence tomographic patterns of diabetic macular edema. Am. J. Ophthalmol. 2006, 142, 405–412. [Google Scholar] [CrossRef]
- Medina-Baena, M.; Cejudo-Corbalán, O.; García-Pulido, J.I.; Huertos-Carrillo, M.J.; Girela-López, E. Intravitreal dexamethasone implant in naïve and previously treated patients with diabetic macular edema: A retrospective study. Int. J. Ophthalmol. 2020, 13, 1597–1605. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Barranco, J.J.; Pina-Marín, B.; Fernández-Bonet, M. Dexamethasone Implants in Patients with Naïve or Refractory Diffuse Diabetic Macular Edema. Ophthalmologica. 2015, 233, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Cervera-Taulet, E.; Navarro-Palop, C.; Hernández-Bel, L.; Monferrer-Adsuara, C.; Mata-Moret, L.; Montero-Hernández, J. Analysis of anatomical biomarkers in subtypes of diabetic macular edema refractory to anti-vascular endothelial growth factor treated with dexamethasone implant. Eur. J. Ophthalmol. 2020, 30, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Callanan, D.G.; Gupta, S.; Boyer, D.S.; Ciulla, T.A.; Singer, M.A.; Kuppermann, B.D.; Liu, C.C.; Li, X.Y.; Hollander, D.A.; Schiffman, R.M.; et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013, 120, 1843–1851. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R., Jr.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Aknin, I.; Melki, L. Longitudinal Study of Sustained-Release Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmologica 2016, 235, 187–188. [Google Scholar] [CrossRef]
- Iglicki, M.; Busch, C.; Zur, D.; Okada, M.; Mariussi, M.; Chhablani, J.K.; Cebeci, Z.; Fraser-Bell, S.; Chaikitmongkol, V.; Couturier, A.; et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: The International Retina Group Real-Life 24-Month Multicenter Study. The IRGREL-DEX Study. Retina 2019, 39, 44–51. [Google Scholar] [CrossRef]
- Lazic, R.; Lukic, M.; Boras, I.; Draca, N.; Vlasic, M.; Gabric, N.; Tomic, Z. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina 2014, 34, 719–724. [Google Scholar] [CrossRef]
- Zur, D.; Iglicki, M.; Sala-Puigdollers, A.; Chhablani, J.; Lupidi, M.; Fraser-Bell, S.; Mendes, T.S.; Chaikitmongkol, V.; Cebeci, Z.; Dollberg, D.; et al. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020, 98, e217–e223. [Google Scholar] [CrossRef]
- Zarranz-Ventura, J.; Romero-Núñez, B.; Bernal-Morales, C.; Velazquez-Villoria, D.; Sala-Puigdollers, A.; Figueras-Roca, M.; Copete, S.; Distefano, L.; Boixadera, A.; García-Arumi, J.; et al. Differential response to intravitreal dexamethasone implant in naïve and previously treated diabetic macular edema eyes. BMC Ophthalmol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Wang, J.K.; Huang, T.L.; Hsu, Y.R.; Chang, P.Y. Effect of dexamethasone intravitreal implant for refractory and treatment-naive diabetic macular edema in Taiwanese patients. J. Chin. Med. Assoc. 2021, 84, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Jee, D.; Kwon, J.W. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS ONE 2019, 14, e0222364. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Bennett, P.H.; Ballintine, E.J. Increased incidence of retinopathy in diabetics with elevated blood pressure. A six-year follow-up study in Pima Indians. N. Engl. J. Med. 1980, 302, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E. Blood pressure control and diabetic retinopathy. Br. J. Ophthalmol. 2002, 86, 365–367. [Google Scholar] [CrossRef]
- Sophie, R.; Lu, N.; Campochiaro, P.A. Predictors of Functional and Anatomic Outcomes in Patients with Diabetic Macular Edema Treated with Ranibizumab. Ophthalmology 2015, 122, 1395–1401. [Google Scholar] [CrossRef]
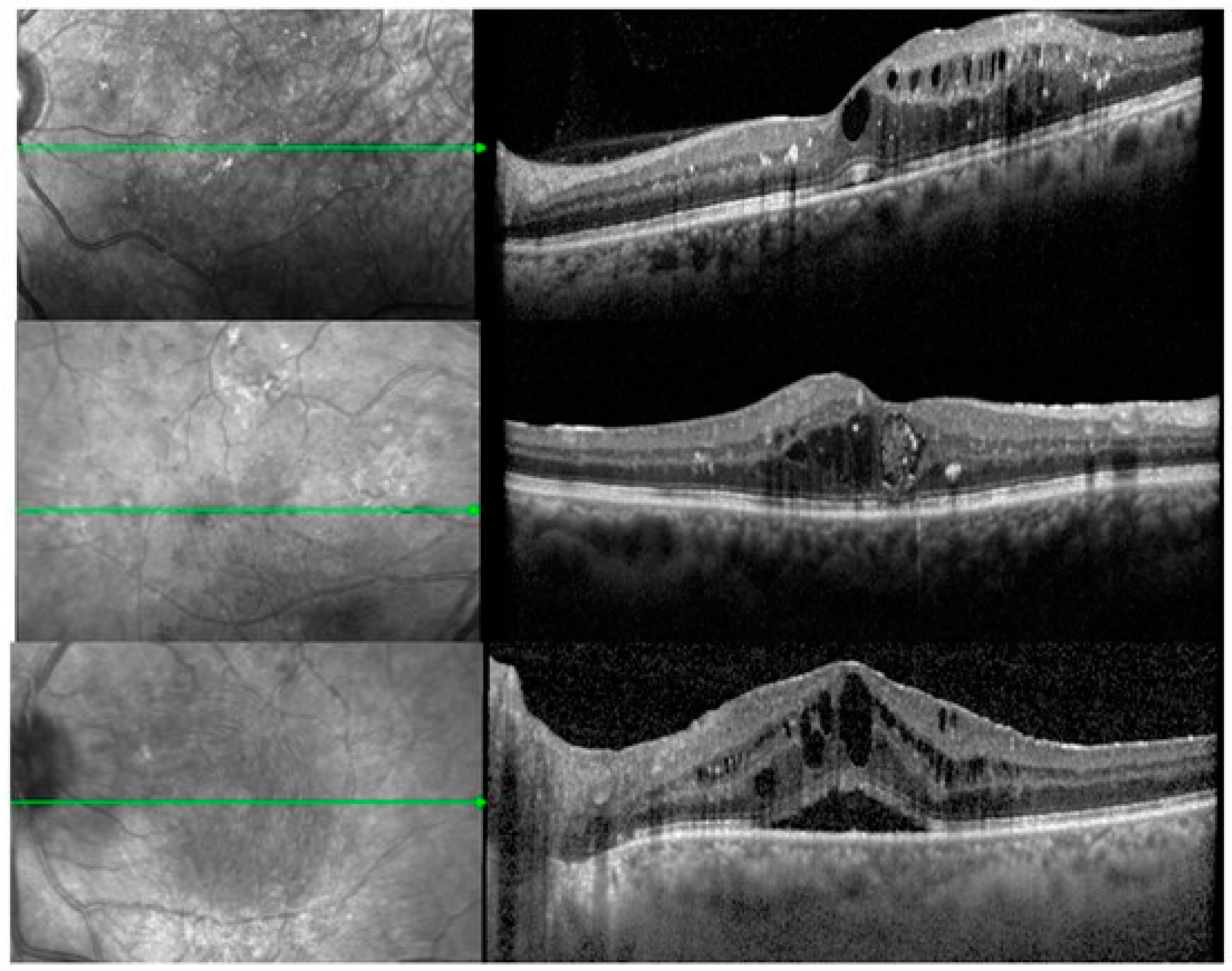
| Variables | Total N = 84 Eyes | CME Subtype N = 48 | DRT Subtype N = 15 | SRD Subtype N = 21 | TN N = 27 | PT N = 57 |
|---|---|---|---|---|---|---|
| Age (mean years ± SD, range) | 73.1 ± 7.1 (53–86) | 74.8 ± 6.0 (62–86) | 70.0 ± 11.2 (53–82) | 71.4 ± 4.5 (65–79) | 72.6 ± 7.3 (62–82) | 73.3 ± 7.0 (53–86) |
| Gender (M/F) | 60/24 | 36/12 | 6/9 | 18/3 | 21/6 | 39/18 |
| IOP mmHg (SD) | 14.6 (2.7) | 14.6 (2.9) | 16.0 (1.2) | 13.7 (2.8) | 15.6 (1.07) | 14.1 (3.1) |
| Number of anti-VEGF treatments before DEX, mean (SD) | 5.1 (5.7) | 4.4 (3.7) | 11.6 (8.5) | 2.1 (3.4) | - | 7.5 (5.5) |
| CST, mean (SD) μm | 513.3 (177.8) | 501.3 (93.0) | 359.6 (111.3) | 650.3 (253.1) | 541.2 (245.6) | 500.2 (135.3) |
| CSTmax, mean (SD) μm | 583.0 (201.5) | 570.7 (89.0) | 427.2 (119.6) | 722.2 (322.7) | 623.1 (301.4) | 564.0 (129.8) |
| BCVA LogMAR (SD) | 0.82 (0.5) | 0.81 (0.6) | 0.90 (0.2) | 0.79 (0.6) | 0.81 (0.6) | 0.82 (0.5) |
| Total | CME Subtype | DRT Subtype | SRD Subtype | TN | PT | |
|---|---|---|---|---|---|---|
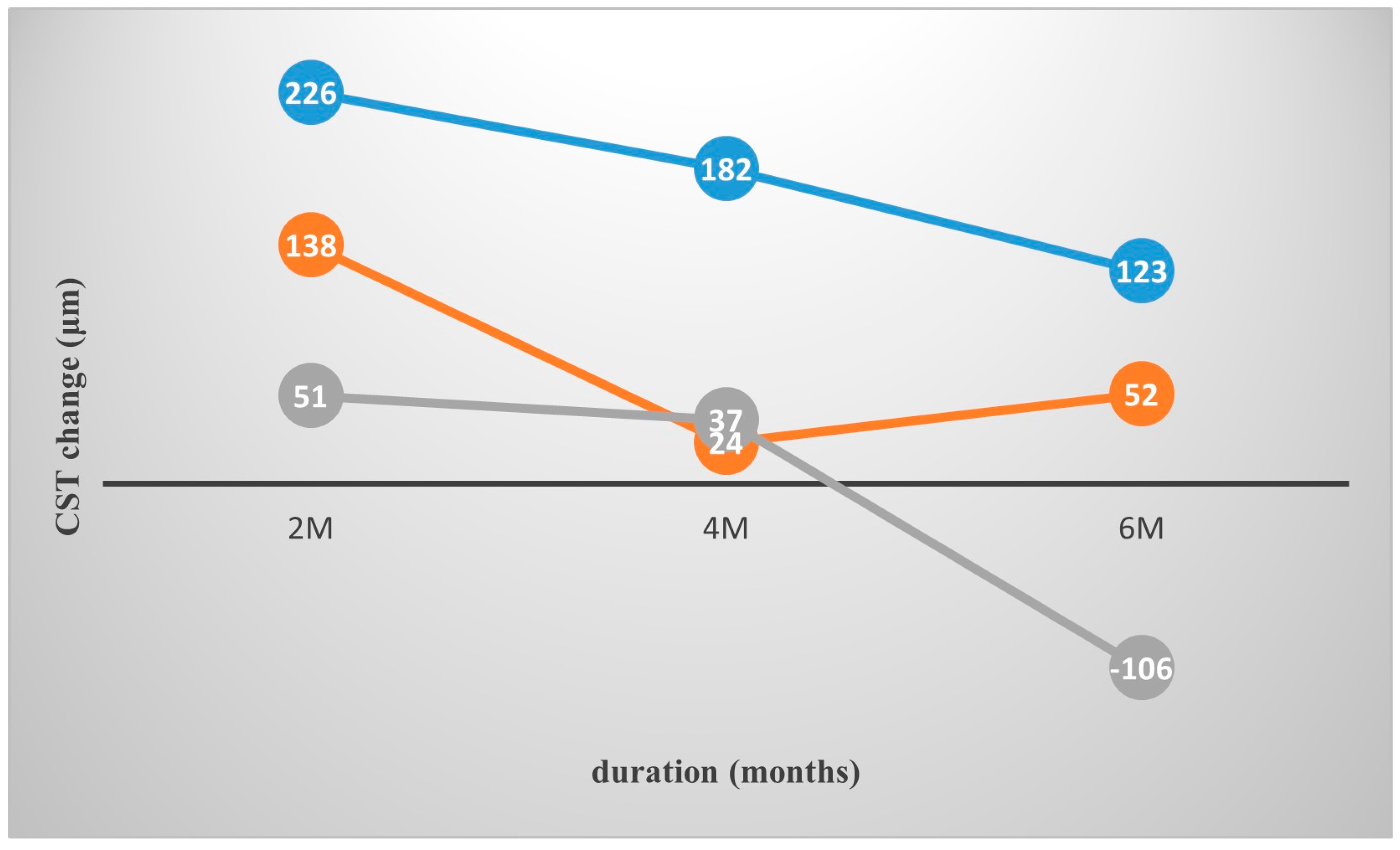
| CST baseline | 513.3 (177.8) | 501.3 (93.0) | 359.6 (111.3) | 650.3 (253.1) | 541.2 (245.6) | 500.2 (135.3) |
| CST 2 month | 368.2 (125.2) | 362.5 (109.3) | 308.4 (98.4) | 424.1 (151.4) | 378.5 (119.9) | 363.3 (142.2) |
| CST 4 month | 447.2 (139.7) | 477.3 (116.2) | 322.0 (91.1) | 468.4 (168.4) | 452.1 (107.0) | 445.0 (153.6) |
| CST 6 month | 471.5 (152.4) | 448.8 (102.3) | 465.8 (98.4) | 527.2 (246.2) | 525.8 (196.6) | 445.7 (119.9) |
| CSTmax baseline | 583.0 (201.5) | 570.7 (89.0) | 427.2 (119.6) | 722.2 (322.7) | 623.1 (301.4) | 564.0 (129.8) |
| CSTmax 2 month | 426.6 (122.0) | 419.9 (113.8) | 367.0 (82.8) | 484.7 (142.5) | 428.5 (93.7) | 425.7 (134.1) |
| CSTmax 4 month | 523.3 (131.4) | 549.4 (115.2) | 395.0 (97.5) | 555.7 (135.1) | 535.8 (102.6) | 517.4 (143.5) |
| CSTmax 6 month | 558.1 (212.7) | 511.1 (102.4) | 643.2 (199.3) | 604.7 (295.6) | 608.6 (248.8) | 534.2 (191.0) |
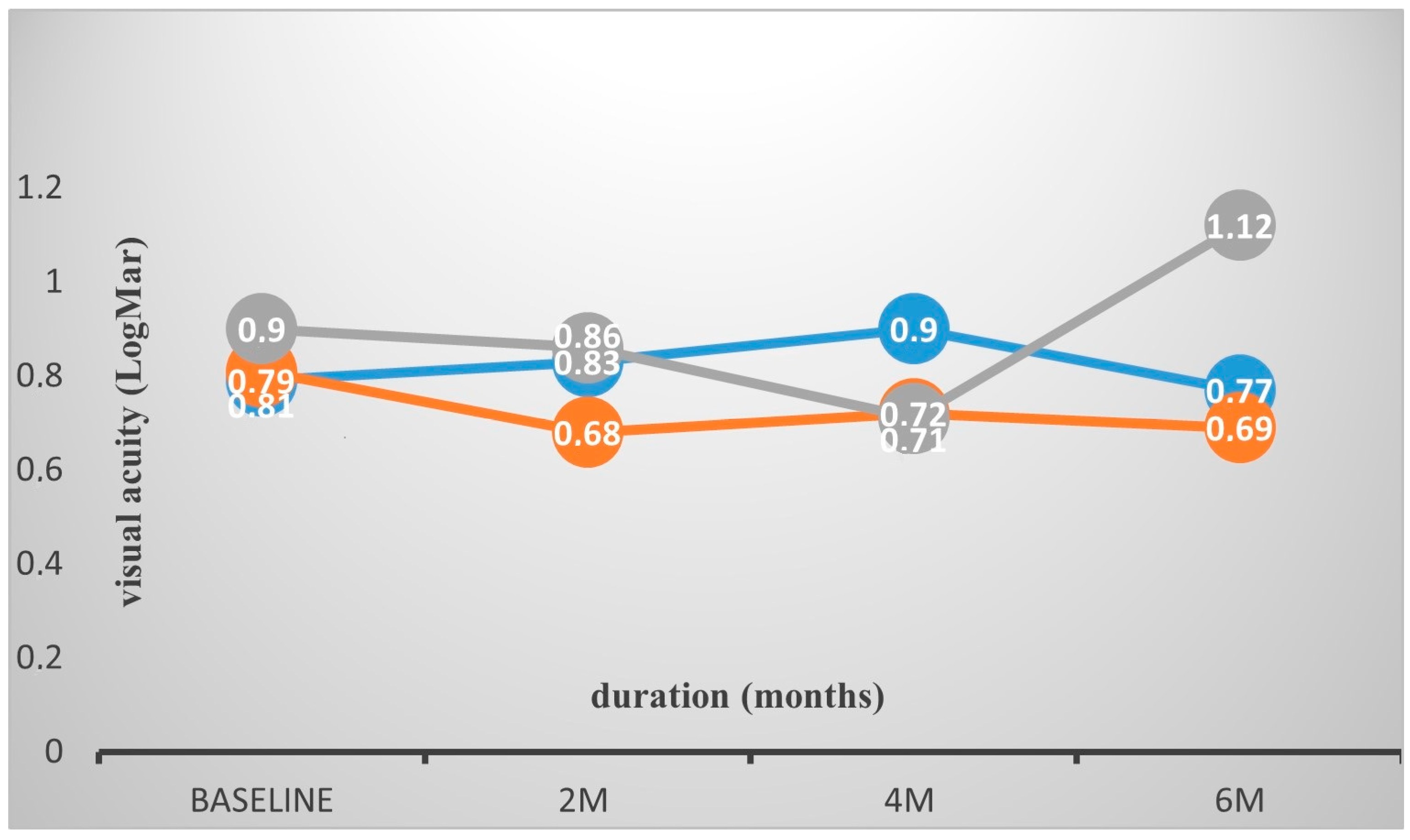
| BCVA baseline | 0.82 (0.56) | 0.81 (0.60) | 0.90 (0.25) | 0.79 (0.64) | 0.81 (0.64) | 0.82 (0.52) |
| BCVA 2 month | 0.75 (0.51) | 0.68 (0.56) | 0.86 (0.19) | 0.83 (0.56) | 0.82 (0.56) | 0.72 (0.49) |
| BCVA 4 month | 0.76 (0.54) | 0.72 (0.60) | 0.712 (0.22) | 0.90 (0.56) | 0.83 (0.57) | 0.73 (0.53) |
| BCVA 6 month | 0.84 (0.61) | 0.69 (0.56) | 1.12 (0.50) | 0.97 (0.72) | 1.07 (0.70) | 0.73 (0.49) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrakas, P.; Christou, E.E.; Nasikas, V.; Koutsiouki, C.; Vakalis, A.; Asteriadis, S.; Panos, G.D.; Tranos, P. Efficacy of Intravitreal Dexamethasone Implant (Ozurdex®) in Naïve and Refractory Patients with Different Morphological Subtypes of Diabetic Macular Edema. Medicina 2025, 61, 488. https://doi.org/10.3390/medicina61030488
Stavrakas P, Christou EE, Nasikas V, Koutsiouki C, Vakalis A, Asteriadis S, Panos GD, Tranos P. Efficacy of Intravitreal Dexamethasone Implant (Ozurdex®) in Naïve and Refractory Patients with Different Morphological Subtypes of Diabetic Macular Edema. Medicina. 2025; 61(3):488. https://doi.org/10.3390/medicina61030488
Chicago/Turabian StyleStavrakas, Panagiotis, Evita Evangelia Christou, Vasileios Nasikas, Chrysoula Koutsiouki, Athanasios Vakalis, Solon Asteriadis, Georgios D. Panos, and Paris Tranos. 2025. "Efficacy of Intravitreal Dexamethasone Implant (Ozurdex®) in Naïve and Refractory Patients with Different Morphological Subtypes of Diabetic Macular Edema" Medicina 61, no. 3: 488. https://doi.org/10.3390/medicina61030488
APA StyleStavrakas, P., Christou, E. E., Nasikas, V., Koutsiouki, C., Vakalis, A., Asteriadis, S., Panos, G. D., & Tranos, P. (2025). Efficacy of Intravitreal Dexamethasone Implant (Ozurdex®) in Naïve and Refractory Patients with Different Morphological Subtypes of Diabetic Macular Edema. Medicina, 61(3), 488. https://doi.org/10.3390/medicina61030488







