1. Introduction
Retinoblastoma is the most common intraocular tumor of childhood. It develops from the inner layer of the eye (retina), where the optic nerves are located, and usually grows into the eyeball. Patients are diagnosed on average around the age of two. Approximately 80% of children are diagnosed before the age of three. As can be understood, retinoblastoma is usually a disease of young children. However, there is a risk of tumor development up to the age of six or seven [
1,
2]. The main goal of retinoblastoma treatment is to protect the patient’s life and then to save the eye and visual function. The treatment methods used today are approaching this goal, but they bring with them significant side effects and heavy costs to the patient and the healthcare system. Therefore, alternative treatment searches continue.
In recent years, natural products have been shown to have effective results against retinoblastoma, either in combination with standard drugs or alone [
3,
4]. Current treatment options for retinoblastoma are radiotherapy, chemotherapy, thermotherapy and brachytherapy [
5]. Many complications arise due to traditional treatments. Secondary cancers caused by traditional treatments also occur in younger people [
6]. The importance of plant-derived natural products in cancer treatment cannot be ignored. Many drugs used in current treatment such as Paclitaxel, camptothecin, etoposide, vinblastine and vincristine are plant-derived natural products [
7]. Effective results of the combination of plant-derived agents and Cisplatin have been reported in many cancer treatments. Boswellic acid, which contains many triterpenes, has been shown to have apoptotic, cytostatic and antiproliferative activities against different cancers both in vivo and in vitro [
8,
9]. It has also been reported that BA can increase apoptosis together with chemotherapeutic agents. It has been reported to prevent invasive cancers as a result of inhibition of
NF-κB-regulated gene expression [
10]. Moreover, it prevents mitochondrial apoptosis by activating activated
Caspase-8 to
Caspase-3 [
11].
NF-κB is an important transcription factor in cells and has an important role in the immune system through various cellular responses [
12]. Research confirms that inflammation is closely related to the formation and development of cancers, and
NF-κB, a key molecule linking chronic inflammation to cancer [
13], is an important subject of antitumor studies.
In this study, we investigate the impact of BA and its combination with Cis on Y79 retinoblastoma cells, focusing on cell viability, apoptosis induction, NF-κB modulation, and inflammatory cytokine levels. Our findings aim to provide insights into the therapeutic potential of BA in retinoblastoma management and contribute to the development of alternative treatment strategies.
2. Material and Methods
2.1. Cell Culture
Human retinoblastoma cell line Y79 was obtained from American Type Culture Collection (ATCC® HTB-18TM, Manassas, VA, USA). Y79 was used to determine the effects of boswellic acid and Cisplatin on inflammation, oxidative stress and apoptosis in retinoblastoma cancer. The Y79 cell line was commercially obtained (ATCC) and the medium used for the propagation of its cells was prepared to contain 10% (v/v) Fetal bovine serum (Gibco, ThermoFisher, Waltham, MA, USA), 100 µg/mL streptomycin (Invitrogen, ThermoFisher, USA) and RPMI-1640 (Sigma-Aldrich, Merck, St. Louis, MO, USA). After the applications to the cells, all incubations were carried out in a sterile environment with 5% CO2 and 37 °C incubator that maintained the balance.
2.2. Determination of Boswellic Acid and Cisplatin Concentration
Acetyl-11-Keto-Beta-Boswellic Acid (Santa Cruz, CA, USA) and CISPLATIN DBL (Hospira Australia Pty Ltd., Mulgrave-Victoria, Australia) were obtained commercially. Optimization experiments were performed for BA and Cis concentrations of 0.5 μM, 1 μM, 2 μM, 5 μM, 10 μM, 25 μM, 50 μM, 75 μM and 100 μM and the lethal dose of BA and Cis on human retinoblastoma cancer cells were determined. A total of 5 mg of BA in the stock was dissolved in DMSO and then divided into aliquots by adding distilled water. To determine the concentration, WST-1 assay was performed to determine the lethal dose of BA and Cis on human retinoblastoma cancer cells. WST-1 test was performed to determine half maximal inhibitory concentration (IC50) doses.
2.3. WST-1 Assay
In order to determine cell proliferation, 0.5 μM, 1 μM, 2 μM, 5 μM, 10 μM, 25 μM, 50 μM, 75 μM and 100 μM BA and Cis concentrations were added to Y79 cancer cells and incubated for 24, 48 and 72 h with 3 repetitions for each ratio. Then, WST-1 test was applied. Cells grown in 96-well culture dishes were completely removed from the culture medium to perform WST-1 assay and incubated with 10% WST-1 reagent in the dark for 4 h. After this incubation period, a 540 nM measurement was performed on a 96-well culture dish spectrophotometer (Biotek, EL
X800, SpectraLab, Lathamn, NY, USA) [
14].
2.4. Apoptotic Staining
Tali™ Apoptosis Kit - Annexin V Alexa Fluor™ 488 & Propidium Iodide (Invitrogen™, ThermoFisfer, USA) was used for apoptosis determination. In this method, cells were stained using Annexin V AlexaFluor® 488 and propidiumiodide. Apoptotic cells are green, dead cells are red, and live cells show little or no fluorescence. Application was made with the 48 h doses (BA (IC50), Cis (IC50) and BA (IC50)+Cis (IC50)) determined in the previous step and centrifugation was performed. Then, 200 µL of 1X Annexin binding buffer was added to the cells collected at the bottom of the tube. Next, 5 µL of Annexin V AlexaFluor® 488 (Component A) was added to 100 µL of the mixture and kept in the dark for 20 min. Centrifugation was performed again and 100 µL of 1X Annexin binding buffer was added to the cells that settled at the bottom and vortexed. Then, 1 μL of Tali® PropidiumIodide (PI, component B) solution was added to the final mixture and incubated in the dark for 5 min. Then, 25 μL of the mixture was taken and dropped onto special slides prepared for Tali and reading was performed with the analysis program. This was photographed using the Thermo EVOS® FL ImagingSystem using brightfield mode and fluorescence mode, using a DAPI filter at 20x objective magnification.
2.5. Inflammatory Cytokines Levels
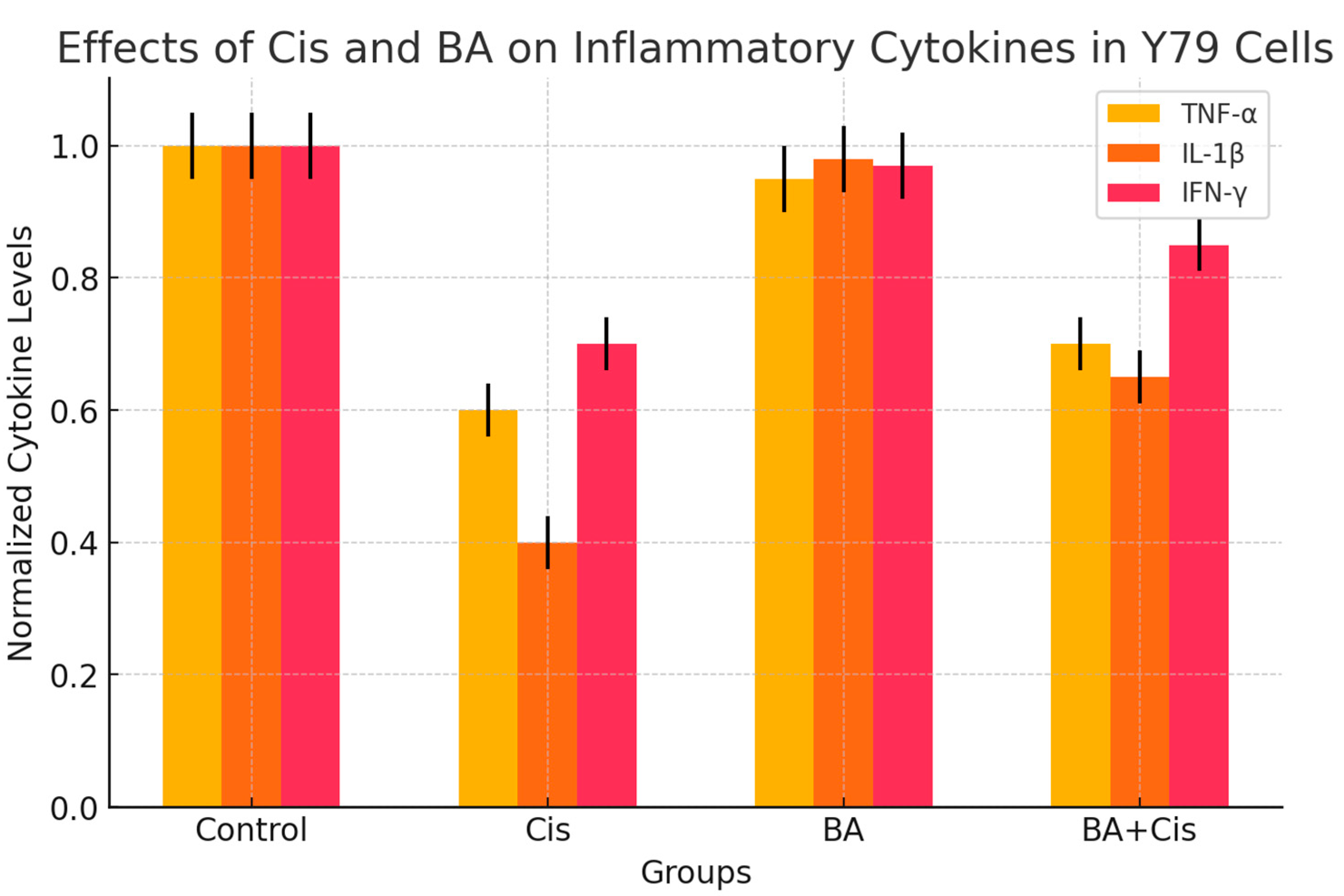
To determine the anti-inflammatory effect of BA (IC50), Cis (IC50) and BA (IC50)+Cis (IC50) in Y79 cells for 48 h, TNF-α (Invitrogen, Cat #13-7341-81), IL1-β (Invitrogen, Cat #PA1-84913) and IFN-γ (Invitrogen, Cat #14-7311-81) levels were determined in cell lysates using a multiplate reader device at 540 nm using specific commercial kits (ThermoFisher, USA ). The obtained data were divided by the total protein concentrations of the samples. Thus, normalized cytokine levels were expressed as ng/mg-protein or pg/mg-protein.
2.6. Total RNA Isolation and cDNA Synthesis
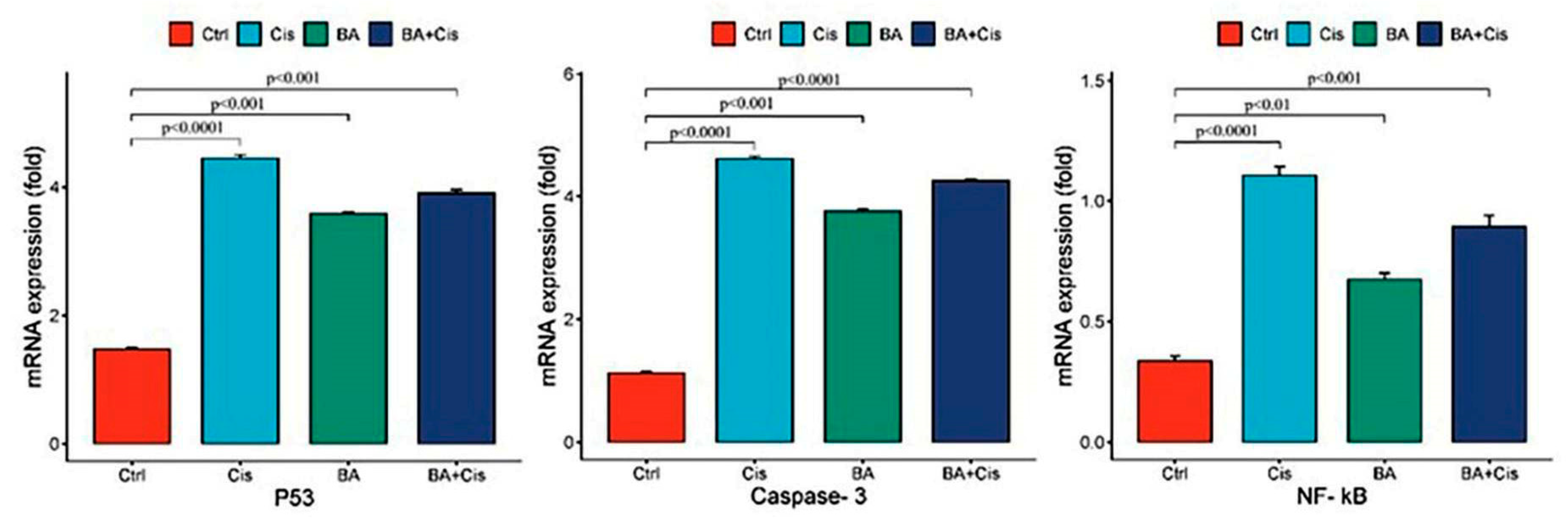
In order to detect changes in gene expression of cultured cells as a result of BA (IC50), Cis (IC50) and BA (IC50)+Cis (IC50) treatment for 48 h, RNAs of cancer cells were purified with RNA isolation kit (ThermoFisher, USA). After isolation, RNA concentration was measured with a spectrophotometer and converted to cDNA with cDNA synthesis kit (Life Technologies, ThermoFisher, USA) as 1 μg total RNA. Amplification of target genes was performed with SYBR green dye in a LightCycler 480-II (ThermoFisher, USA ) real-time quantitative PCR device. Target gene and appropriately selected reference genes were amplified in the same panel. PCR conditions were applied as 10 min incubation at 95 °C, followed by 45 cycles of 15 s denaturation at 95 °C, 1 min annealing and extension at 60 °C. The Ct values of the peaks obtained during the amplification process were used to determine gene expressions and gene expressions were calculated with the 2−∆∆Ct method. The primers used to investigate changes in the expression of these genes are given below in 5′-3′ order.
NF-κB: F: GCG CAT CCA GAC CAA CAA TAA C, R: GCC GAA GCT GCA TGG ACA CT
p53: F: CACGAGCGCTGCTCAGATAGC, R: ACAGGCACAAACACGCACAAA
Caspase-3: F: GGTATTGAGACAGACAGTGG, R: CATGGGATCTGTTTCTTTGC
β-Actin: F: CCTCTGAACCCTAAGGCCAAC, R: TGCCACAGGATTCCATACCC
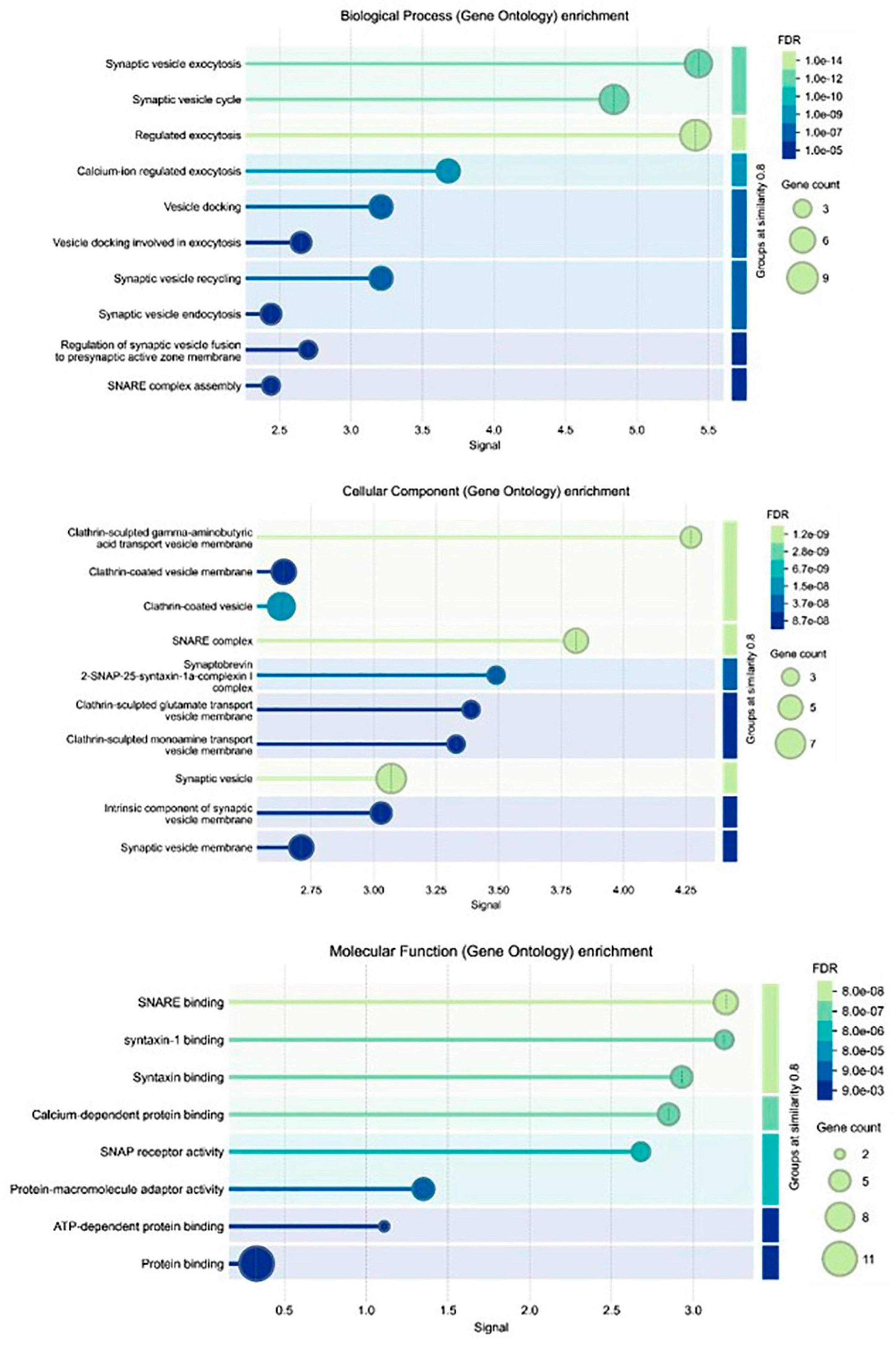
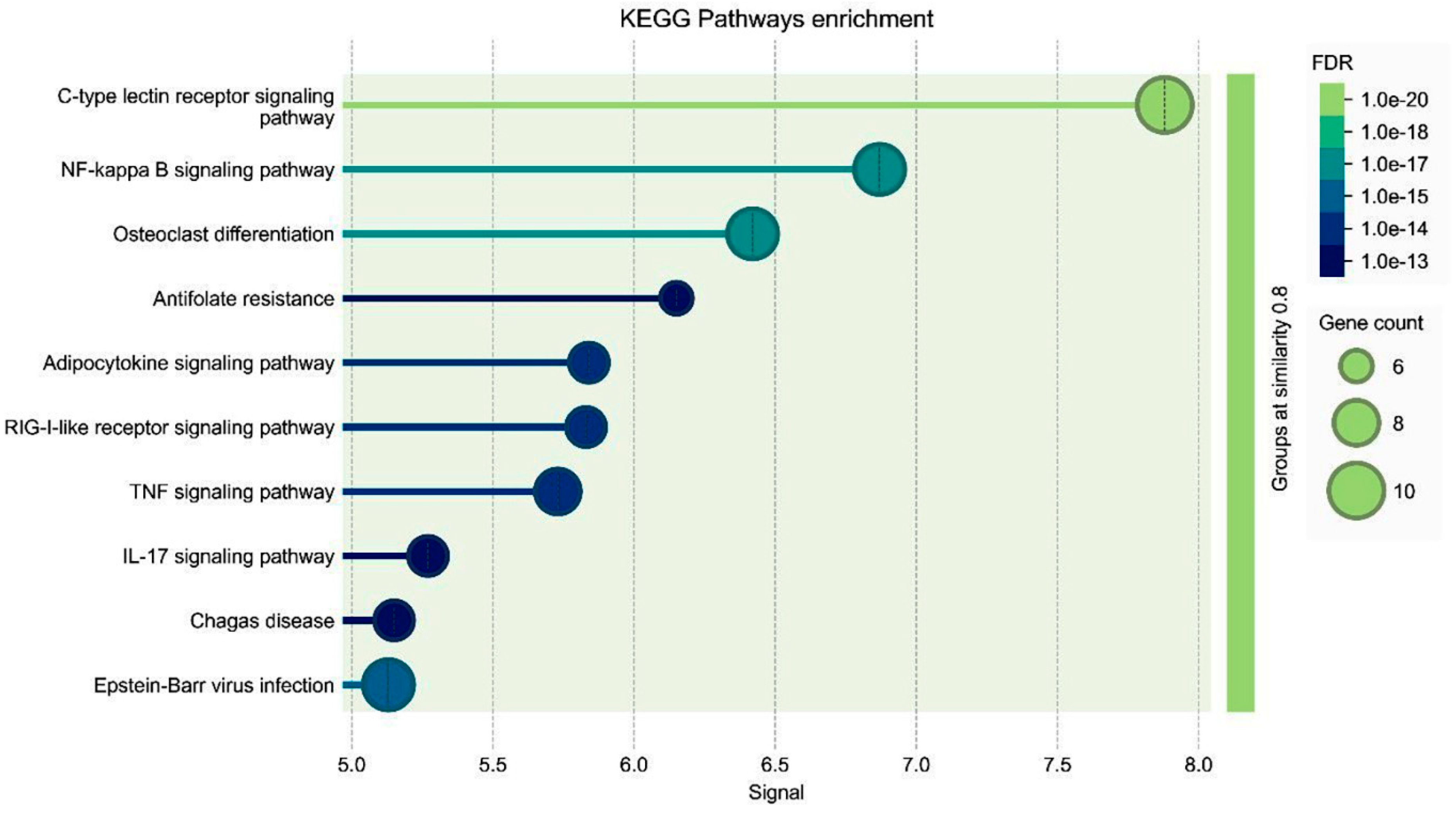
2.7. Gene Ontologies (GO)
Three types of gene ontologies (GOs) were performed on possible target genes: cellular component, biological process and molecular function. The STRİNG program was used to evaluate these data. The rationale behind performing GO analysis in our study was to comprehensively characterize the biological processes, molecular functions and cellular components associated with the differentially expressed genes upon boswellic acid treatment in retinoblastoma cells. By conducting GO analysis, we aimed to identify key biological pathways, understand the functional relevance of differentially expressed genes and strengthen data interpretation. Instead of focusing solely on individual genes, GO analysis allows us to identify broader biological trends, helping to elucidate the molecular mechanisms underlying boswellic acid’s inhibitory effects, particularly in relation to NF-κB suppression. This approach provides an unbiased validation of our findings by linking gene expression changes to known tumor-suppressive or apoptotic pathways.
2.8. Statistical Analysis
The raw data compiled as a result of laboratory analyses were defined as mean ± standard deviation. SPSS 20.0 software was preferred for statistical evaluations. In statistical evaluation, whether there was a statistical difference in parameters showing normal distribution was determined with the ANOVA test. Whether there was a statistical difference between groups in parameters not showing normal distribution was determined with the Kruskal–Wallis test. The groups between which the difference occurred were determined with the Mann–Whitney U test. Expression levels of genes showing statistical differences were indicated with (*) in the graphs.
4. Discussion
The results of this study demonstrate that BA, in combination with Cis, effectively inhibits the growth of Y79 retinoblastoma cells by inducing apoptosis and modulating NF-κB signaling. WST-1 analysis revealed that both BA and Cis exhibited dose- and time-dependent cytotoxic effects on Y79 cells, with IC50 values decreasing over time. The BA + Cis combination showed the strongest inhibitory effect on cell viability, suggesting a potential synergistic interaction between the two agents. Our findings further show that BA enhances the expression of apoptotic markers p53 and Caspase-3, supporting its pro-apoptotic role in retinoblastoma cells. However, NF-κB mRNA expression was upregulated in all treatment groups, with the highest levels observed in the Cis-treated group, while BA treatment alone exhibited relatively lower NF-κB expression. This suggests that although BA does not directly suppress NF-κB transcription, it may modulate its functional activity through post-translational mechanisms such as phosphorylation, nuclear translocation, or interaction with inhibitory proteins. The BA + Cis combination resulted in an intermediate NF-κB expression level, implying a potential regulatory effect rather than direct inhibition. Given the complexity of NF-κB signaling, further research is required to investigate NF-κB protein levels, phosphorylation status, and nuclear translocation to determine the exact mechanism by which BA influences this pathway in retinoblastoma cells.
New drugs are being developed by utilizing natural resources to treat many diseases. Although it is more expensive and laborious to discover and develop new therapeutic agents in natural resources, it provides an abundant source for new drugs. Natural resources continue to be a rich and abundant source of potential new drugs. One of these sources is boswellic acid. BA has very strong biological and pharmacological potentials and contains pentacyclic triterpenoids that attract the attention of synthetic and medicinal chemistry. It shows that natural BA and its synthetic derivatives can play an important role in the treatment of many inflammatory diseases, especially cancer. Moreover, BA have been used in the treatment of various diseases in formulations with active compounds [
15]. Particular combination ideas are extremely important in reducing toxicity and resistance. Moreover, the interactions of BA with other anticancer drugs should be fully investigated, because they are expected to increase synergism with anticancer and anti-inflammatory effects. The most important challenge in drug discovery is to eliminate drug resistance and to ensure the development of molecules with a new target. Therefore, we investigated the effects of the combination of BA with a potent anticancer agent Cis and the obtained results confirm the synergism. While apoptotic gene expressions increased, an increase in anti-inflammatory biochemical parameters was also observed. In other words, while killing cancer cells, it creates a microenvironment that will allow healthy cells to survive.
In vitro studies on different cancer cell lines have highlighted the strong anticancer properties of BA. It has been reported that BA treatment in the colon cancer cell line HCT-116 caused a decrease in cyclin-dependent kinases such as cyclin D, cyclin E, CDK2 and CDK4 [
16]. In another study, Acetyl-beta boswellic acid and BA stopped the activity of I-κB kinase in chemotherapeutic androgen-independent PC-3 prostate cancer cells in vitro and in vivo, inhibiting activated
NF-κB signaling, reducing proliferation and leading to cell death [
17]. It has been shown that apoptosis induced by TNF and chemotherapeutic agents is more effective and suppresses TNF-dependent invasion. In pancreatic cancer cell lines, BA treatment has been shown to inhibit the expression of
NF-κB, and thus suppress COX-2 and MMP-9 genes. In addition, in an experimental adenomatous polyposis model in which BA and aspirin were applied, it was shown that BA caused regression in adenomatous polyps by modulating the Wnt/β-catenin pathway and the
NF-κB/COX-2 pathway in mice [
18]. It was found that inhibition of
NF-κB with an extract containing Boswellia decreased the expressions of VEGF, TNF-α and MCP-1 and improved mouse liver granuloma [
19]. It was shown that apoptosis was induced and tumor regression was triggered by the reduction in
NF-κB in cancers treated with 3-α-Butyryloxy-β-boswellic acid, a semi-synthetic analog of BA [
20]. Colorectal cancer is one of the most common cancers. In HT-29 human colon cancer cells, Boswellia serrata (B. serrata) has anticancer activity. A significant difference was observed in B. serrata-treated HT-29 compared to the control group [
21].
In the presented study, it was determined that oxidative stress and, accordingly, apoptotic genes
p53 and
Caspase-3 mRNA expression levels were stimulated due to the increase in IL1-β and IFN-gamma levels in the group to which BA was applied at a 48 h IC
50 dose (
Figure 4). Indeed, studies have indicated that cytosolic and mitochondrial reactive oxygen species, which can also play an active role in
p53 stimulation, can be effective in the induction of apoptosis [
22]. However, a difference was observed in the experimental group to which BA+Cis was applied compared to the control group in terms of oxidative stress and inflammation, and apoptosis was observed to be stimulated. In addition, the presented study is the first study in which natural antioxidant and anticancer agent and a strong chemotherapy agent Cis were applied together. In previous studies, it has been reported that natural sources such as gallic acid and rosmarinic acid exhibit effective anticancer interactions with doxorubicin [
23,
24]. When we compare the reports of these studies with our results, we see that combination therapy can eliminate secondary effects in many cancer treatments. In addition, cytotoxicity and results that can directly affect survival are seen.
While this study provides valuable insights into the potential therapeutic effects of BA in combination with Cis on Y79 retinoblastoma cells, it has several limitations. First, NF-κB activity was assessed only at the mRNA level, and functional validation through protein expression, phosphorylation status, and nuclear translocation assays was not performed. Future studies should include Western blot and immunofluorescence analysis to confirm whether NF-κB activation is modulated post-translationally. Second, the study was conducted in vitro, and while the results suggest a promising anticancer effect, in vivo models are necessary to evaluate the pharmacokinetics, bioavailability, and systemic effects of BA in retinoblastoma treatment. Lastly, potential off-target effects and interactions with other cellular pathways remain unexplored, requiring further mechanistic studies to fully understand BA’s role in NF-κB signaling and apoptosis regulation.
Future research should focus on elucidating the precise molecular mechanisms by which BA modulates NF-κB signaling, particularly at the protein level. Since our study demonstrated NF-κB mRNA upregulation but lacked functional validation, subsequent investigations should employ Western blot analysis, phosphorylation assays and nuclear translocation studies to determine whether BA affects NF-κB activation post-translationally. Additionally, in vivo studies are crucial to evaluate the bioavailability, pharmacokinetics and systemic efficacy of BA in retinoblastoma models. Further research should also explore potential synergistic or antagonistic effects of BA with other chemotherapeutic agents to optimize combination therapies. Finally, given NF-κB’s dual role in inflammation and apoptosis, future studies should investigate how BA influences NF-κB-dependent gene expression networks and downstream pathways, ensuring a comprehensive understanding of its therapeutic potential in retinoblastoma treatment.