The Efficacy of Remineralizing Materials on Artificial Enamel Lesions: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Tooth Collection and Enamel Surface Evaluation
2.3. Sample Preparation and Processing
2.4. Sample Grouping
- Group 1: Control group (CG).
- Group 2: Self-assembling peptide P11-4 group (P11-4).
- Group 3: 22,600 ppm fluoride varnish group (FV).
- Group 4: 20% nano-hydroxyapatite varnish group (nano-HA).
2.5. Measurement of Enamel Surface Hardness
2.6. Phase of Artificial Enamel Lesions
2.7. Application of Remineralizing Agents
2.8. Statistical Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Bonchev, A.; Vasileva, R.; Dyulgerova, E.; Yantcheva, S. Self-assembling Peptide P11-4: A Biomimetic Agent for Enamel Remineralization. Int. J. Pept. Res. Ther. 2021, 27, 899–907. [Google Scholar] [CrossRef]
- Soliman, E.M.; Abdelfattah, W.M.; Mohamed, D.R.; Nagui, D.A.; Holiel, A.A. Topographic Evaluation of the Remineralizing Potential of Biomimetic Scaffolds on Enamel White Spot Lesions: An In Vitro Study. J. Esthet. Restor. Dent. 2024. ahead of print. [Google Scholar] [CrossRef]
- Kharbot, B.; Askar, H.; Gruber, D.; Paris, S. Biomimetic Remineralization of Artificial Caries Lesions with a Calcium Coacervate, Its Components and Self-Assembling Peptide P(11)-4 In Vitro. Bioengineering 2024, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Alkilzy, M.; Tarabaih, A.; Santamaria, R.M.; Splieth, C.H. Self-assembling Peptide P(11)-4 and Fluoride for Regenerating Enamel. J. Dent. Res. 2018, 97, 148–154. [Google Scholar] [CrossRef]
- Qvist, V. Longevity of restorations—“the death spiral”. In Dental Caries—The Disease and Its Clinical Management, 2nd ed.; University of Copenhagen: Copenhagen, UK, 2008; pp. 443–455. [Google Scholar]
- Garcia, R.I.; Gregorich, S.E.; Ramos-Gomez, F.; Braun, P.A.; Wilson, A.; Albino, J.; Tiwari, T.; Harper, M.; Batliner, T.S.; Rasmussen, M.; et al. Absence of Fluoride Varnish-Related Adverse Events in Caries Prevention Trials in Young Children, United States. Prev. Chronic Dis. 2017, 14, E17. [Google Scholar] [CrossRef]
- Baik, A.; Alamoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride Varnishes for Preventing Occlusal Dental Caries: A Review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef]
- Haugejorden, O.; Magne Birkeland, J. Ecological time-trend analysis of caries experience at 12 years of age and caries incidence from age 12 to 18 years: Norway 1985-2004. Acta Odontol. Scand. 2006, 64, 368–375. [Google Scholar] [CrossRef]
- Weatherell, J.A.; Deutsch, D.; Robinson, C.; Hallsworth, A.S. Assimilation of fluoride by enamel throughout the life of the tooth. Caries Res. 1977, 11 (Suppl. 1), 85–115. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Bell, M.; Boden, N.; Carrick, L.M.; Strong, A.E. Self-Assembling Peptide Polyelectrolyte β-Sheet Complexes Form Nematic Hydrogels. Angew. Chem. Int. Ed. 2003, 42, 5603–5606. [Google Scholar] [CrossRef]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Heinzel-Gutenbrunner, M. Efficacy of the self-assembling peptide P11-4 in constructing a remineralization scaffold on artificially-induced enamel lesions on smooth surfaces. J. Orofac. Orthop. 2014, 75, 175–190. [Google Scholar] [CrossRef]
- Brunton, P.A.; Davies, R.P.; Burke, J.L.; Smith, A.; Aggeli, A.; Brookes, S.J.; Kirkham, J. Treatment of early caries lesions using biomimetic self-assembling peptides--a clinical safety trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised clinical trial investigating self-assembling peptide P(11)-4 in the treatment of early caries. Clin. Oral. Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef]
- Sedlakova Kondelova, P.; Mannaa, A.; Bommer, C.; Abdelaziz, M.; Daeniker, L.; di Bella, E.; Krejci, I. Efficacy of P(11)-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 2020, 10, 20211. [Google Scholar] [CrossRef]
- Gözetici, B.; Öztürk-Bozkurt, F.; Toz-Akalın, T. Comparative Evaluation of Resin Infiltration and Remineralisation of Noncavitated Smooth Surface Caries Lesions: 6-month Results. Oral. Health Prev. Dent. 2019, 17, 99–106. [Google Scholar] [CrossRef]
- Doberdoli, D.; Bommer, C.; Begzati, A.; Haliti, F.; Heinzel-Gutenbrunner, M.; Juric, H. Randomized Clinical Trial investigating Self-Assembling Peptide P(11)-4 for Treatment of Early Occlusal Caries. Sci. Rep. 2020, 10, 4195. [Google Scholar] [CrossRef] [PubMed]
- Welk, A.; Ratzmann, A.; Reich, M.; Krey, K.F.; Schwahn, C. Effect of self-assembling peptide P(11)-4 on orthodontic treatment-induced carious lesions. Sci. Rep. 2020, 10, 6819. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Nothelfer, R.; Morawietz, M.; Kiesow, A.; Korbmacher-Steiner, H. Impact of self-assembling peptides in remineralisation of artificial early enamel lesions adjacent to orthodontic brackets. Sci. Rep. 2020, 10, 15132. [Google Scholar] [CrossRef]
- Güven, E.; Eden, E.; Attin, R.; Fırıncıoğulları, E.C. Remineralization of post-orthodontic white spot lesions with a fluoride varnish and a self-assembling P 11 − 4 peptides: A prospective in-vivo-study. Clinical Oral. Investig. 2024, 28, 464. [Google Scholar] [CrossRef]
- Enax, J.; Fabritius, H.O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of Action and Clinical Efficacy of Particulate Hydroxyapatite in Preventive Oral Health Care—State of the Art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Ramis, J.; Coelho, C.; Córdoba, A.; Quadros, P.; Monjo, M. Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics 2018, 5, 53. [Google Scholar] [CrossRef]
- Savas, S.; Kucukyilmaz, E.; Celik, E.U. Effects of Remineralization Agents on Artificial Carious Lesions. Pediatr. Dent. 2016, 38, 511–518. [Google Scholar]
- Buskes, J.A.; Christoffersen, J.; Arends, J. Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res. 1985, 19, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Tellez, M.; Pitts, N.B.; Ekstrand, K.R.; Ricketts, D.; Longbottom, C.; Eggertsson, H.; Deery, C.; Fisher, J.; Young, D.A.; et al. Caries management pathways preserve dental tissues and promote oral health. Community Dent. Oral. Epidemiol. 2013, 41, e12–e40. [Google Scholar] [CrossRef]
- Desai, H.; Stewart, C.A.; Finer, Y. Minimally Invasive Therapies for the Management of Dental Caries-A Literature Review. Dent. J. 2021, 9, 147. [Google Scholar] [CrossRef]
- Rehder Neto, F.C.; Maeda, F.A.; Turssi, C.P.; Serra, M.C. Potential agents to control enamel caries-like lesions. J. Dent. 2009, 37, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Kresak, M.; Zahradnik, R.T. Physicochemical aspects of fluoride-apatite systems relevant to the study of dental caries. Caries Res. 1977, 11 (Suppl. 1), 142–171. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Gulzar, R.A.; Ajitha, P.; Subbaiyan, H. Self Assembling Peptide P11-4 for Enamel Remineralization: A Biomimetic Approach. J. Pharm. Res. Int. 2020, 32, 83–89. [Google Scholar] [CrossRef]
- Sindhura, V.; Uloopi, K.S.; Vinay, C.; Chandrasekhar, R. Evaluation of enamel remineralizing potential of self-assembling peptide P(11)-4 on artificially induced enamel lesions in vitro. J. Indian. Soc. Pedod. Prev. Dent. 2018, 36, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.; Hassanein, H.; Elkassas, D.; Hamza, H. Complementary remineralizing effect of self-assembling peptide (P11-4) with CPP-ACPF or fluoride: An in vitro study. J. Clin. Exp. Dent. 2020, 12, e161–e168. [Google Scholar] [CrossRef]
- Soares, R.; De Ataide, I.N.; Fernandes, M.; Lambor, R. Assessment of Enamel Remineralisation After Treatment with Four Different Remineralising Agents: A Scanning Electron Microscopy (SEM) Study. J. Clin. Diagn. Res. 2017, 11, Zc136–Zc141. [Google Scholar] [CrossRef]
- Tripathi, P.; Mengi, R.; Gajare, S.M.; Nanda, S.S.; Wani, S.A.; Kochhar, A.S. Evaluation of Remineralizing Capacity of P11-4, CPP-ACP, Silver Diamine Fluoride, and NovaMin: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 357–360. [Google Scholar] [CrossRef]
- Aşık, A.; Önçağ, Ö. Evaluation of the effect of self-assembling peptide and fluoride varnish, alone or in combination with laser irradiation, on artificial enamel caries: A SEM/EDS and Micro-CT study. Clin. Oral. Investig. 2024, 28, 503. [Google Scholar] [CrossRef]
- Krishnamoorthi, A.; Shanbhog, R.S.; Godhi, B.S.; Sundaravadivelu, M. Efficacy of Self-assembling Peptide P11-4 in Remineralizing In Vitro Caries-like Lesions in Primary Enamel Samples in Combination with Calcium Phosphate-based Remineralization Agents. Int. J. Clin. Pediatr. Dent. 2024, 17, 552–557. [Google Scholar] [CrossRef]
- Kucukyilmaz, E.; Savas, S. Measuring the remineralization potential of different agents with quantitative light-induced fluorescence digital Biluminator. J. Appl. Biomater. Funct. Mater. 2017, 15, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Memarpour, M.; Razmjouei, F.; Rafiee, A.; Vossoughi, M. Remineralization effects of self-assembling peptide P(11) -4 associated with three materials on early enamel carious lesions: An in vitro study. Microsc. Res. Tech. 2022, 85, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Jablonski-Momeni, A.; Korbmacher-Steiner, H.; Heinzel-Gutenbrunner, M.; Jablonski, B.; Jaquet, W.; Bottenberg, P. Randomised in situ clinical trial investigating self-assembling peptide matrix P11-4 in the prevention of artificial caries lesions. Sci. Rep. 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Skucha-Nowak, M.; Fischer, M.; Tanasiewicz, M.; Machorowska Pieniążek, A.; Skaba, D.; Morawiec, T.; Kielbassa, A. Attempt to modify the chemical model of enamel demineralization used in microinvasive dentistry. J. Stomatol. 2019, 72, 249–314. [Google Scholar] [CrossRef]
- White, D.J. The application of in vitro models to research on demineralization and remineralization of the teeth. Adv. Dent. Res. 1995, 9, 175–193; discussion 177–194. [Google Scholar] [CrossRef]




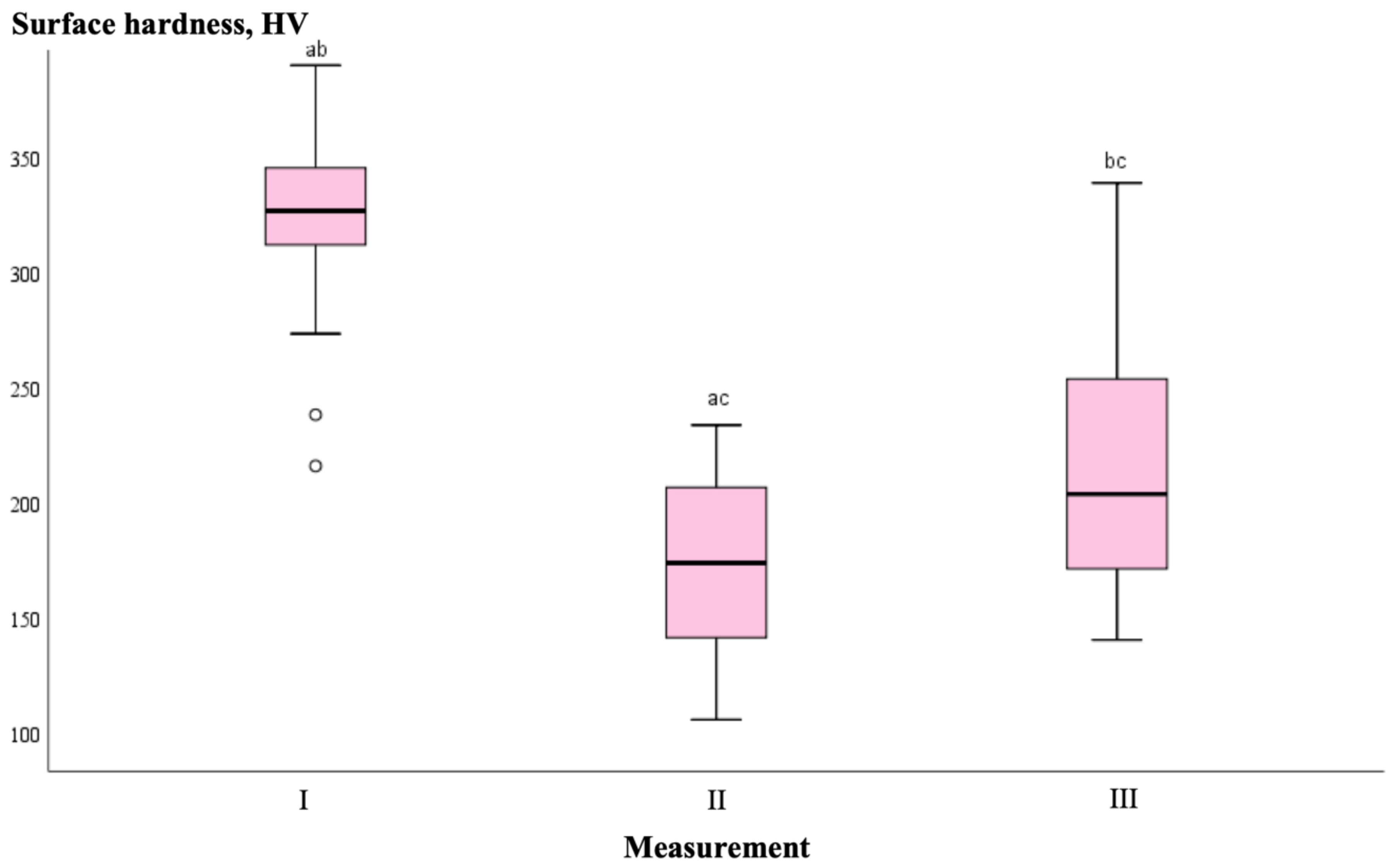

| Measurement No. | Group | n | Median Surface Hardness Values, HV | p-Value |
|---|---|---|---|---|
| I | CG | 9 | 328.60 | 0.758 |
| P11-4 | 9 | 320.87 | ||
| FV | 9 | 331.03 | ||
| Nano-HA | 9 | 324.90 | ||
| Total | 36 | 326.55 | ||
| II | CG | 9 | 174.83 | 0.211 |
| P11-4 | 9 | 163.47 | ||
| FV | 9 | 172.73 | ||
| Nano-HA | 9 | 178.93 | ||
| Total | 36 | 173.78 | ||
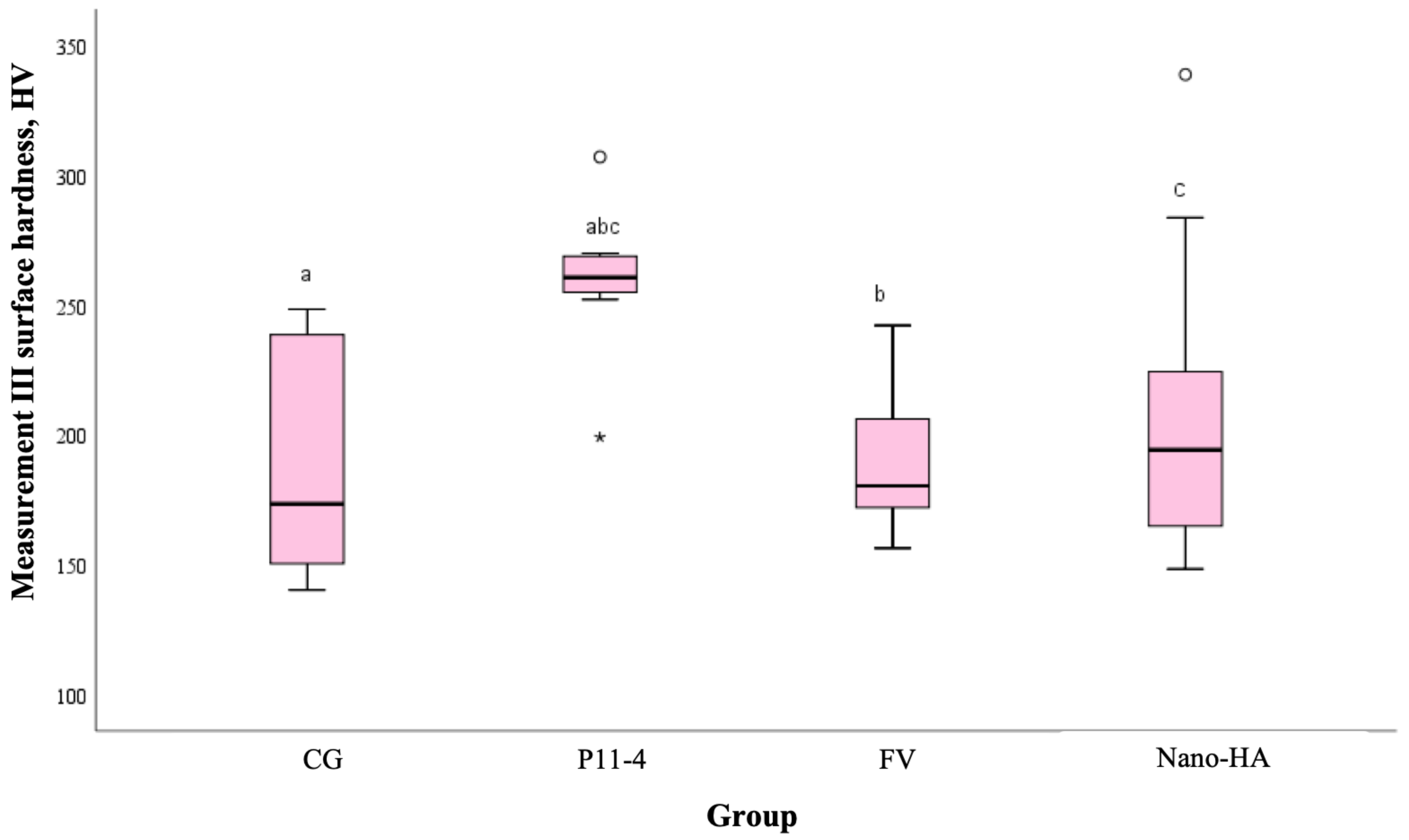
| III | CG | 9 | 173.43 | 0.036 |
| P11-4 | 9 | 260.60 | ||
| FV | 9 | 180.40 | ||
| Nano-HA | 9 | 194.23 | ||
| Total | 36 | 203.57 |
| Comparative Measurements | Group | |||
|---|---|---|---|---|
| 1 (CG) | 2 (P11-4) | 3 (FV) | 4 (Nano-HA) | |
| Average Difference (SD), HV | ||||
| I–II | 150.00 (48.02) * | 157.11 (40.62) * | 149.17 (62.54) * | 150.82 (33.45) * |
| I–III | 134.03 (59.60) * | 69.81 (43.58) * | 127.99 (46.61) * | 111.10 (74.28) * |
| II–III | 15.97 (41.89) | 87.30 (36.14) * | 21.18 (52.32) | 39.72 (92.85) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimaitė, G.; Vasiliauskas, A.; Grinkevičius, P.; Grinkevičienė, D.; Šapalas, D. The Efficacy of Remineralizing Materials on Artificial Enamel Lesions: An In Vitro Study. Medicina 2025, 61, 462. https://doi.org/10.3390/medicina61030462
Klimaitė G, Vasiliauskas A, Grinkevičius P, Grinkevičienė D, Šapalas D. The Efficacy of Remineralizing Materials on Artificial Enamel Lesions: An In Vitro Study. Medicina. 2025; 61(3):462. https://doi.org/10.3390/medicina61030462
Chicago/Turabian StyleKlimaitė, Gustė, Arūnas Vasiliauskas, Pranas Grinkevičius, Dominyka Grinkevičienė, and Deivydas Šapalas. 2025. "The Efficacy of Remineralizing Materials on Artificial Enamel Lesions: An In Vitro Study" Medicina 61, no. 3: 462. https://doi.org/10.3390/medicina61030462
APA StyleKlimaitė, G., Vasiliauskas, A., Grinkevičius, P., Grinkevičienė, D., & Šapalas, D. (2025). The Efficacy of Remineralizing Materials on Artificial Enamel Lesions: An In Vitro Study. Medicina, 61(3), 462. https://doi.org/10.3390/medicina61030462





