Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of “Advanced Glaucoma”
2.2. Ophthalmic Examination
2.3. Surgical Procedure
2.4. Study Outcomes
2.5. Surgical Success
2.6. Statistical Analysis
3. Results
3.1. Patient Demographic and Clinical Data
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Tertiary Outcomes
3.5. Factors Influencing Postoperative Outcomes and Complications
4. Discussion
4.1. Comparison to Other Studies
4.2. Clinical Implications: GATT vs. Trabeculectomy
4.3. Complications and Safety Profile
4.4. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Bengtsson, B.; Oskarsdottir, S.E. Prevalence and severity of undetected manifest glaucoma: Results from the early manifest glaucoma trial screening. Ophthalmology 2013, 120, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Glaucoma Diagnosis and Management. NICE Guideline [NG81]. 2017. Available online: https://www.nice.org.uk/guidance/ng81 (accessed on 1 June 2019).
- Kastner, A.; King, A.J. Advanced glaucoma at diagnosis: Current perspectives. Eye 2020, 34, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Feuer, W.J.; Shi, W.; Lim, K.S.; Barton, K.; Goyal, S.; Ahmed, I.I.; Brandt, J.; Banitt, M.; Budenz, D.; et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology 2018, 125, 650–663. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Xin, C.; Sang, J.; Sun, Y.; Wang, H. Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: Two-year results. BMC Ophthalmol. 2023, 23, 89. [Google Scholar] [CrossRef]
- Dar, N.; Naftali Ben Haim, L.; Yehezkeli, V.; Sharon, T.; Belkin, A. Gonioscopy-assisted transluminal trabeculotomy in patients with advanced glaucoma. Indian J. Ophthalmol. 2023, 71, 3024–3030. [Google Scholar] [CrossRef]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; Montes de Oca, I.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [CrossRef]
- Grover, D.S.; Smith, O.; Fellman, R.L.; Godfrey, D.G.; Gupta, A.; Montes de Oca, I.; Feuer, W.J. Gonioscopy-assisted Transluminal Trabeculotomy: An Ab Interno Circumferential Trabeculotomy: 24 Months Follow-up. J Glaucoma. 2018, 27, 393–401. [Google Scholar] [CrossRef]
- Rahmatnejad, K.; Pruzan, N.L.; Amanullah, S.; Shaukat, B.A.; Resende, A.F.; Waisbourd, M.; Zhan, T.; Moster, M.R. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J. Glaucoma 2017, 26, 1137–1143. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases, Tenth Revision; Clinical Modification (ICD-10-CM); World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Wan, Y.; Cao, K.; Wang, J.; Sun, Y.; Du, R.; Wang, Z.; Zhang, J.; Wang, H.; Wang, N. Gonioscopy-assisted transluminal trabeculotomy (GATT) combined with phacoemulsification surgery: Outcomes at a 2-year follow-up. Eye 2022, 37, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Aktas, Z.; Ozmen, M.C.; Atalay, H.T.; Ucgul, A.Y. Evaluation of episcleral venous fluid wave during gonioscopy-assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye 2018, 33, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Aktas, Z.; Ucgul, A.Y.; Bektas, C.; Sahin Karamert, S. Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J. Glaucoma. 2019, 28, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Sharkawi, E.; Lindegger, D.J.; Artes, P.H.; Lehmann-Clarke, L.; El Wardani, M.; Misteli, M.; Pasquier, J.; Guarnieri, A. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br. J. Ophthalmol. 2021, 105, 977–982. [Google Scholar] [CrossRef]
- Guo, C.Y.; Qi, X.H.; Qi, J.M. Systematic review and meta-analysis of treating open-angle glaucoma with gonioscopy-assisted transluminal trabeculotomy. Int. J. Ophthalmol. 2020, 13, 317–324. [Google Scholar] [CrossRef]
- Ahuja, Y.; Ma Khin Pyi, S.; Malihi, M.; Hodge, D.O.; Sit, A.J. Clinical results of ab interno trabeculotomy using the Trabectome for open-angle glaucoma: The Mayo Clinic series in Rochester, Minnesota. Am. J. Ophthalmol. 2013, 156, 927–935.e2. [Google Scholar] [CrossRef]
- Jordan, J.F.; Wecker, T.; van Oterendorp, C.; Anton, A.; Reinhard, T.; Boehringer, D.; Neuburger, M. Trabectome surgery for primary and secondary open-angle glaucomas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2753–2760. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.E.; Quan, A.; Grajewski, A.; Hodapp, E.; Vanner, E.A.; Chang, T.C. Risk factors for complications and failure after gonioscopy- assisted transluminal trabeculotomy in a young cohort. Ophthalmol. Glaucoma 2020, 3, 190–195. [Google Scholar] [CrossRef]
- Nazarali, S.; Damji, F.; Damji, K.F. What have we learned about exfoliation syndrome since its discovery by John Lindberg 100 years ago? Br. J. Ophthalmol. 2018, 102, 1342–1350. [Google Scholar] [CrossRef]
- Cubuk, M.O.; Ucgul, A.Y.; Unsal, E. Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. Int. Ophthalmol. 2020, 40, 1923–1930. [Google Scholar] [CrossRef]
- Elghonemy, H.; Dewedar, A.; Nader, M.; Shaban, N. Trabeculectomy outcomes for patients with advanced glaucoma. J. Med. Sci. Res. 2020, 3, 176. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L. Three-year follow-up of the Tube Versus Trabeculectomy study. Am. J. Ophthalmol. 2009, 148, 670–684. [Google Scholar] [CrossRef]
- Gedde, S.J.; Schiffman, J.C.; Feuer, W.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am. J. Ophthalmol. 2012, 153, 789–803.e2. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Kumar, A.; Gupta, S.; Grover, D.S.; Gupta, V. Outcomes of gonioscopy-assisted transluminal trabeculotomy in advanced pigmentary glaucoma. Br. J. Ophthalmol. 2025, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Cakir, I.; Balci, A.S.; Alagoz, N.; Cakir, G.Y.; Altan, C.; Yasar, T. Efficacy of gonioscopy-assisted transluminal trabeculotomy and trabeculectomy in patients with primary open-angle glaucoma and pseudoexfoliative glaucoma: A single surgeon’s experience. Indian J. Ophthalmol. 2024, 1, 821–826. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Wang, P.; Dai, C.; Kurmi, R.; Zhang, W.; Wu, J.; Guo, H. Comparison of efficacy and safety between gonioscopy-assisted transluminal trabeculotomy and trabeculectomy for primary open-angle glaucoma treatment: A retrospective cohort study. BMC Ophthalmol. 2024, 20, 533. [Google Scholar] [CrossRef]
- Moster, M.R.; Moster, M.L. Wipe-out: A complication of glaucoma surgery or just a blast from the past? Am. J. Ophthalmol. 2005, 140, 705–706. [Google Scholar] [CrossRef]
- Ramesh, P.V.; Senthil Kumar, N.K.; Ramesh, S.V.; Devadas, A.K. Commentary: Minimally invasive glaucoma surgery for a surgical take diversion: An economic perspective. Indian J. Ophthalmol. 2023, 71, 566–568. [Google Scholar] [CrossRef]
- Meshksar, A.; Razeghinejhad, M.R.; Azimi, A. Ab-interno trabeculotomy procedures: A review. J. Curr. Ophthalmol. 2023, 35, 110–124. [Google Scholar] [CrossRef]
- Cubuk, M.O.; Unsal, E. One-year results of gonioscopy-assisted transluminal trabeculotomy: Evaluation of prognostic factors. Eur. J. Ophthalmol. 2021, 31, 460–468. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.; Oatts, J.T.; Xin, C.; Yin, P.; Zhang, L.; Tian, J.; Zhang, Y.; Cao, K.; Han, Y.; et al. A Prospective Study of Intraocular Pressure Spike and Failure After Gonioscopy-Assisted Transluminal Trabeculotomy in Juvenile Open-Angle Glaucoma: A Prospective Study of GATT in JOAG. Am. J. Ophthalmol. 2022, 236, 79–88. [Google Scholar] [CrossRef]
- Salimi, A.; Nithianandan, H.; Al Farsi, H.; Harasymowycz, P.; Saheb, H. Gonioscopy-assisted transluminal trabeculotomy in younger to middle-aged adults: One-year outcomes. Ophthalmol. Glaucoma 2021, 4, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Smith, O.; Fellman, R.L.; Godfrey, D.G.; Butler, M.R.; Montes de Oca, I.; Feuer, W.J. Gonioscopy assisted transluminal trabeculotomy: An ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br. J. Ophthalmol. 2015, 99, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Dada, T.; Midha, N.; Bhartiya, S. Minimally invasive glaucoma surgery—To remove or preserve the trabecular meshwork: That is the question? J. Curr. Glaucoma Pract. 2021, 15, 47–51. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, J.; Shin, J.W.; Lee, J.; Kook, M.S. Alterations of the Foveal Avascular Zone Measured by Optical Coherence Tomography Angiography in Glaucoma Patients With Central Visual Field Defects. Investig. Ophthalmol. Vis Sci. 2017, 1, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Sung, K.R.; Shin, J.W. Changes in Peripapillary and Macular Vessel Densities and Their Relationship with Visual Field Progression after Trabeculectomy. J. Clin. Med. 2021, 14, 5862. [Google Scholar] [CrossRef]
- Ozmen, M.C.; Acar, B.; Uysal, B.S. Wipe-out following gonioscopy-assisted transluminal trabeculotomy combined with phacoemulsification. J. Glaucoma 2023, 32, 147–152. [Google Scholar] [CrossRef]
| Parameter | Value | Percentage (%) | Number of Patients |
|---|---|---|---|
| Total Number of Patients | 44 | ||
| Gender | |||
| -Male | 84.1 | 37 | |
| -Female | 15.9 | 7 | |
| Laterality | |||
| -Right Eye | 51.1 | 23 | |
| -Left Eye | 48.9 | 21 | |
| Lens Status | |||
| -Phakic | 79.5 | 35 | |
| -Pseudophakic | 20.5 | 9 | |
| Age (Mean ± SD) Range: 17–83 years | 60.29 ± 15.15 | ||
| Central Corneal Thickness | 523.40 ± 34.49 µm | ||
| Follow-up Duration | 2.69 ± 0.60 years | ||
| Patients with Additional Ocular Pathology | 11.4 | 5 | |
| Patients Without Any Progression | 66.0 | 29 | |
| Glaucoma Etiologies | |||
| -Pseudoexfoliation (PEX) Glaucoma | 61.4 | 27 | |
| -Primary Open-Angle Glaucoma (POAG) | 20.5 | 9 | |
| -Pigment Dispersion Syndrome (PDS) | 6.8 | 3 | |
| -Juvenile Glaucoma | 6.8 | 3 | |
| -Secondary to Fuchs’ Uveitis | 2.3 | 1 | |
| -Post-traumatic Angle Recession | 2.3 | 1 | |
| GATT Procedure Extent | |||
| -360 degrees | 79.5 | 35 | |
| -180 degrees | 20.5 | 9 | |
| Complications | |||
| -No complication | 56.8 | 25 | |
| -Hyphema (no intervention required) | 34.1 | 15 | |
| -Hyphema (anterior chamber washout) | 4.5 | 2 | |
| -Permanent vision loss | 4.5 | 2 | |
| -Urrets-Zavalia syndrome | 2.3 | 1 | |
| -Hypotonic maculopathy with choroidal folds | 2.3 | 1 | |
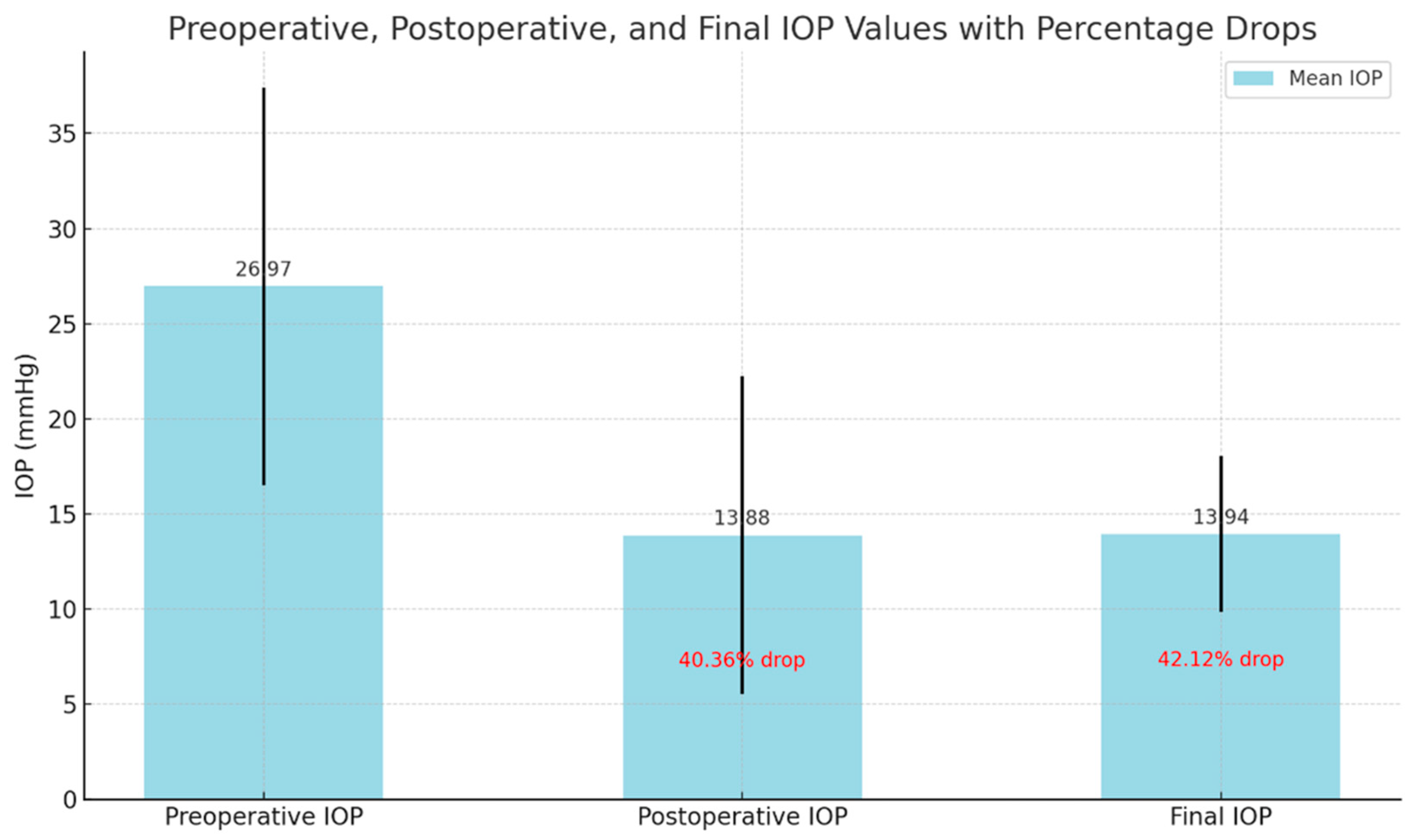
| Parameter | Preoperative (Mean ± SD) | Postoperative 3rd Month (Mean ± SD) | Final (Mean ± SD) | p-Value (Pre-3rd) | p-Value (Pre-Final) |
|---|---|---|---|---|---|
| Visual Acuity (LogMAR) | 0.61 ± 0.36 | 0.41 ± 0.33 | 0.59 ± 0.35 | 0.011 | 1.00 |
| Intraocular Pressure (IOP) (mmHg) | 26.9 ± 10.4 | 13.8 ± 8.3 | 13.9 ± 4.0 | <0.001 | <0.001 |
| Mean Deviation (MD) (dB) | −19.79 ± 9.11 | −19.03 ± 9.70 | −19.06 ± 9.27 | 1.000 | 1.000 |
| Visual Field Index (VFI) | 52.25 ± 3.51 | 50.32 ± 2.11 | 51.28 ± 2.90 | 1.000 | 1.000 |
| Mean RNFL (µm) | 51.97 ± 13.09 | 52.76 ± 13.51 | 49.94 ± 12.37 | 1.000 | 1.000 |
| Variable | No Complications (n = 19) | Complications (n = 25) | Test Statistic | Test Used | p-Value |
|---|---|---|---|---|---|
| Gender | Female: 3 (15.8%) Male: 16 (84.2%) | Female: 4 (16.0%) Male: 21 (84.0%) | χ2 = 0.0 | Chi-Square Test | 1.000 |
| GATT Procedure | 360 degrees: 16 (84.2%) 180 degrees: 3 (15.8%) | 360 degrees: 19 (76.0%) 180 degrees: 6 (24.0%) | χ2 = 0.085 | Chi-Square Test | 0.093 |
| Lens Status | Phakic: 15 (78.9%) Pseudophakic: 4 (21.1%) | Phakic: 20 (80.0%) Pseudophakic: 5 (20.0%) | χ2 = 0.0 | Chi-Square Test | 1.000 |
| Postoperative Progression | No Progression: 16 (84.2%) Progression: 3 (15.8%) | No Progression: 13 (48.1%) Progression: 14 (51.9%) | χ2 = 4.77 | Chi-Square Test | 0.029 |
| Age (years) (median, min-max) | 73 (55–83) | 65 (17–78) | U = 206.5 | Mann–Whitney U Test | 0.274 |
| Central Corneal Thickness (CCT) (µm) (median, min-max) | 533 (486–581) | 510 (477–538) | U = 205 | Mann–Whitney U Test | 0.397 |
| Preoperative Visual Acuity (logMAR) (median, min-max) | 0.22 (0–0.3) | 0.05 (0–0.4) | U = 203.5 | Mann–Whitney U Test | 0.410 |
| Preoperative IOP (mmHg) (median, min-max) | 30 (18–43) | 26 (12–40) | U = 207 | Mann–Whitney U Test | 0.679 |
| Preoperative MD (dB) (median, IQR) | −27.38; IQR: 10.63 | −18.92; IQR: 18.11 | U = 206.5 | Mann–Whitney U Test | 0.421 |
| Preoperative PSD (dB) (median, IQR) | 6.44; IQR: 4.46 | 8.45; IQR: 4.45 | U = 205.5 | Mann–Whitney U Test | 0.429 |
| Preoperative RNFL (µm) (median, min-max) | 50 (36–67) | 48 (38–51) | U = 203 | Mann–Whitney U Test | 0.432 |
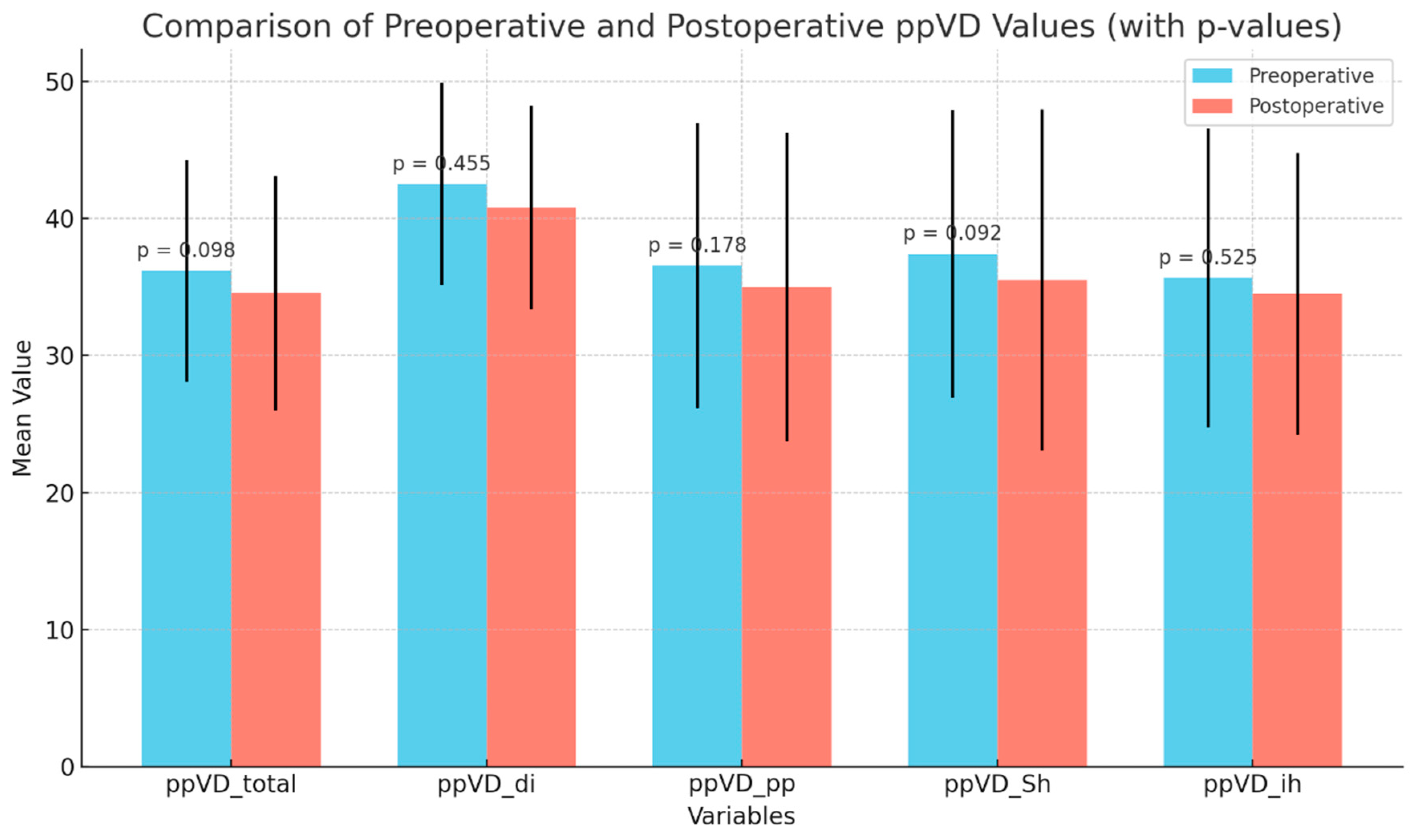
| ppVD_total_preop (%) (median, min-max) | 34(26–48) | 30 (27–40) | U = 204 | Mann–Whitney U Test | 0.431 |
| Categorical Variables | ||||
|---|---|---|---|---|
| Variable | No Progression (n = 29) | Progression (n = 15) | Test Statistic | p-Value |
| Gender | ||||
| -Female | 5 (17.2%) | 2(13.3%) | χ2 = 0.0 | 1.000 |
| -Male | 24 (82.8%) | 13 (86.7%) | ||
| GATT Procedure | ||||
| -360 degrees | 27 (93.1%) | 8 (53.3%) | χ2 = 7.32 | 0.006 |
| -180 degrees | 2 (6.9%) | 7 (46.7%) | ||
| Lens Status | ||||
| -Phakic | 25 (86.2%) | 10 (66.7%) | χ2 = 1.27 | 0.259 |
| -Pseudophakic | 4 (13.8%) | 5 (33.3%) | ||
| Continuous Variables | ||||
| Variables | Median (min–max) | Median (min–max) | Mann–Whitney U | p-value |
| Age (years) (median, min–max) | 60 (17–83) | 64 (42–78) | 102.0 | 0.899 |
| Central Corneal Thickness (CCT) (µm) (median, min–max) | 512 (477–581) | 524 (510–538) | 15.0 | 0.602 |
| Preoperative VA (logMAR) (median, min–max) | 0.22 (0.0–1) | 0.15 (0.0–1) | 108.0 | 0.353 |
| Preoperative IOP (mmHg) (median, min–max) | 27 (11–60) | 23 (12–40) | 118.0 | 0.443 |
| Preoperative MD (dB) (median, IQR) | −29.2; IQR: 5.95 | −18.40; IQR: 17.11 | 50.0 | 0.250 |
| Preoperative PSD (dB) (median, IQR) | 6.4; IQR: 3.33 | 7.9; IQR: 6.69 | 52.0 | 0.304 |
| Preoperative RNFL (µm) (median, min–max) | 48 (26–68) | 50 (35–87) | 134.0 | 0.909 |
| ppVD_total_preop (%) (median, min–max) | 35 (26–46) | 32 (27–48) | 10.0 | 0.117 |
| Study | Glaucoma Type | N | Mean Preop IOP (mmHg) | IOP at 3 M (mmHg) | % Reduction at 3M | IOP at 24 M (mmHg) | % Reduction at 24 M | Success Rate (24 M) |
|---|---|---|---|---|---|---|---|---|
| Current Study | Advanced OAG | 44 | 26.9 ± 10.4 | 13.8 ± 8.3 | 40.36% | 13.9 ± 4.0 | 42.12% | 76.5% |
| Grover et al. [10] (2018) | POAG | 157 | 23.2 ± 7.1 | Not Reported | Not Reported | 14.5 ± 3.6 | 37.3% | 75.3% |
| Wan et al. [13] (2022) | POAG + PEXG | 66 | 25.4 ± 5.1 | Not Reported | Not Reported | 14.3 ± 4.7 | 43.7% | 84.8% (with meds) |
| Wang et al. [7] (2023) | OAG w/failed surgery | 49 | 28.3 ± 5.2 | 16.0 ± 4.7 | 43.5% | 16.7 ± 5.1 | 41.1% | 71.4% |
| Dar et al. [8] (2023) | Advanced OAG | 41 | 27.1 ± 6.3 | 15.6 ± 3.8 | 42.4% | 15.2 ± 4.2 | 44.1% | 72.0% |
| Rahmatnejad et al. [11] (2017) | POAG + PEXG | 85 | 24.5 ± 6.2 | 15.2 ± 4.9 | 38.0% | Not Reported | Not Reported | 63.0% (12M) |
| Sharkawi et al. [16] (2021) | PEXG | 60 | 27.8 ± 5.3 | 16.2 ± 5.1 | 41.7% | 16.5 ± 5.4 | 40.6% | 78.2% |
| Aktaş et al. [15] (2018) | Advanced OAG | 35 | 27.3 ± 5.9 | 15.1 ± 4.6 | 44.7% | 15.5 ± 4.1 | 43.2% | 79.3% |
| Aktaş et al. [14] (2019) | Moderate–Advanced OAG | 42 | 26.7 ± 6.4 | 14.9 ± 4.8 | 44.2% | 15.1 ± 4.5 | 43.0% | 77.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyugelen, G.; Güvenç, U.; Burcu, A. Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis. Medicina 2025, 61, 444. https://doi.org/10.3390/medicina61030444
Soyugelen G, Güvenç U, Burcu A. Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis. Medicina. 2025; 61(3):444. https://doi.org/10.3390/medicina61030444
Chicago/Turabian StyleSoyugelen, Gülizar, Umay Güvenç, and Ayşe Burcu. 2025. "Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis" Medicina 61, no. 3: 444. https://doi.org/10.3390/medicina61030444
APA StyleSoyugelen, G., Güvenç, U., & Burcu, A. (2025). Outcomes of Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) in Advanced Glaucoma: A Retrospective Analysis. Medicina, 61(3), 444. https://doi.org/10.3390/medicina61030444






