Vitamin D Deficiency in Kazakhstani Children: Insights from a Systematic Review and Meta-Analysis
Abstract
1. Introduction
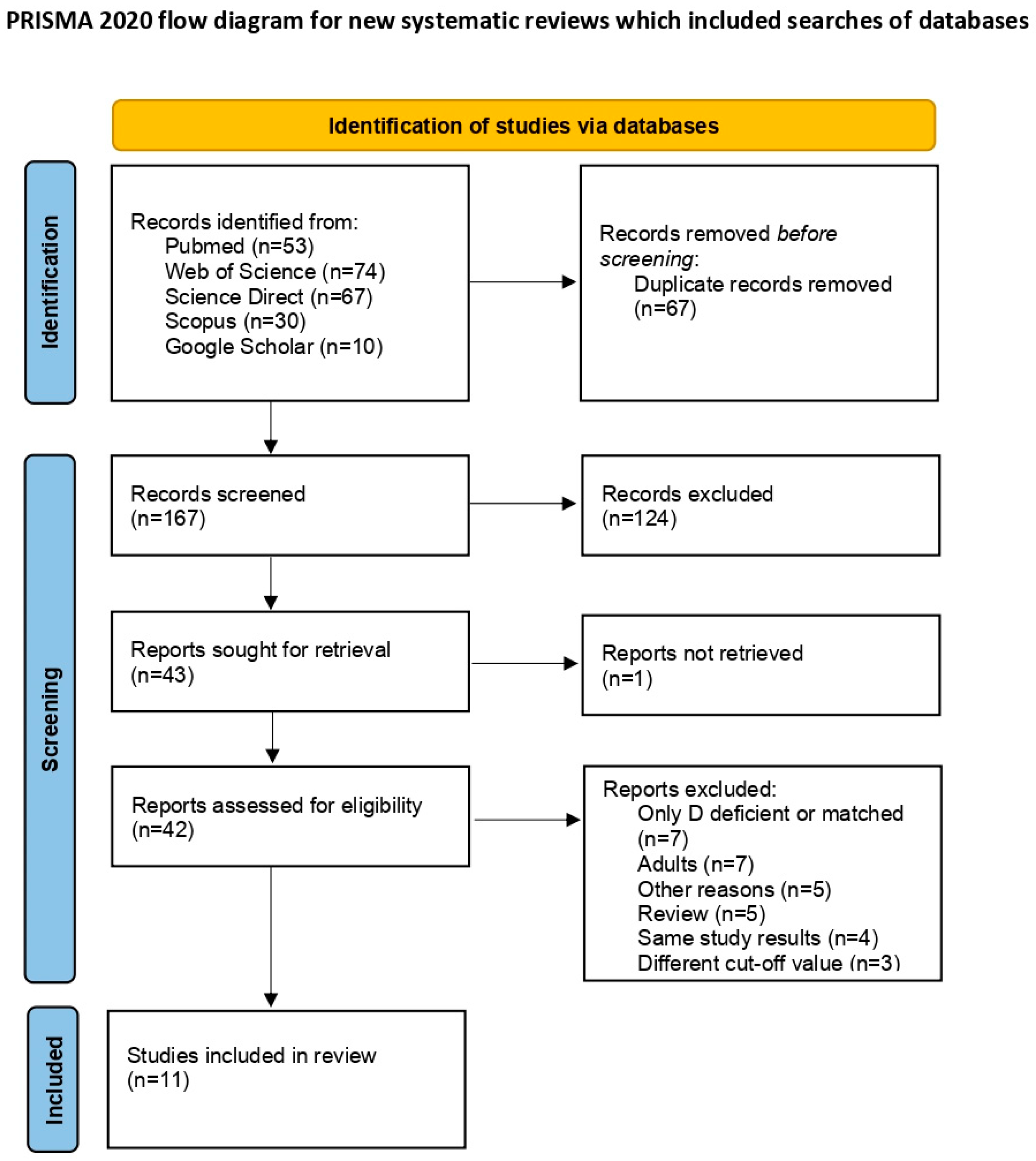
2. Materials and Methods
2.1. Search Strategy Framework
2.2. Inclusion and Exclusion Criteria of Studies and Data Extraction
2.3. Risk of Bias Assessment
2.4. Statistical Strategy for Data Synthesis
3. Results
3.1. Included Study Characteristics
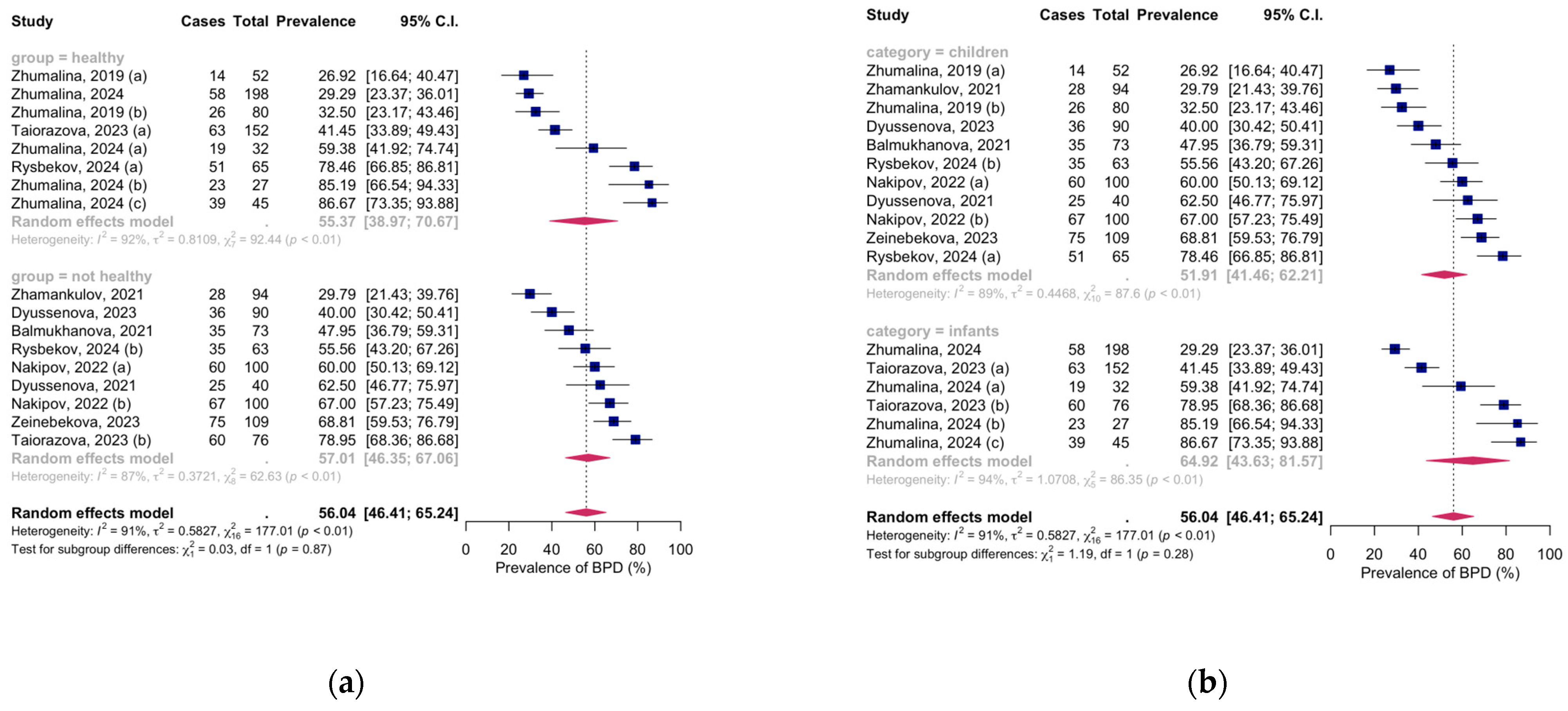
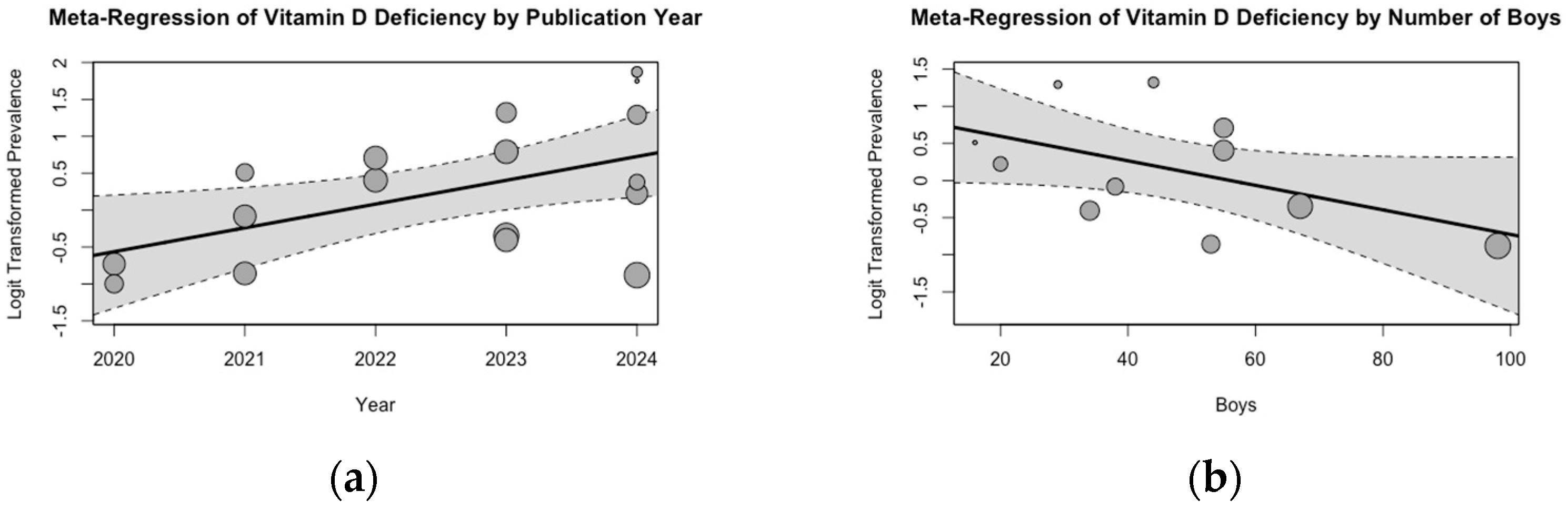
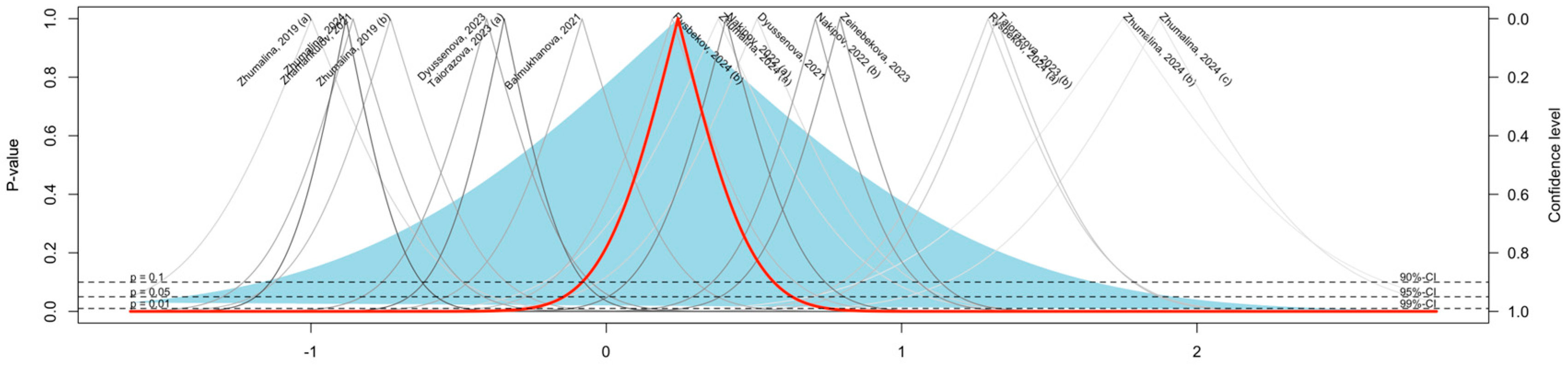
3.2. Meta-Analysis of Vitamin D Deficiency Prevalence
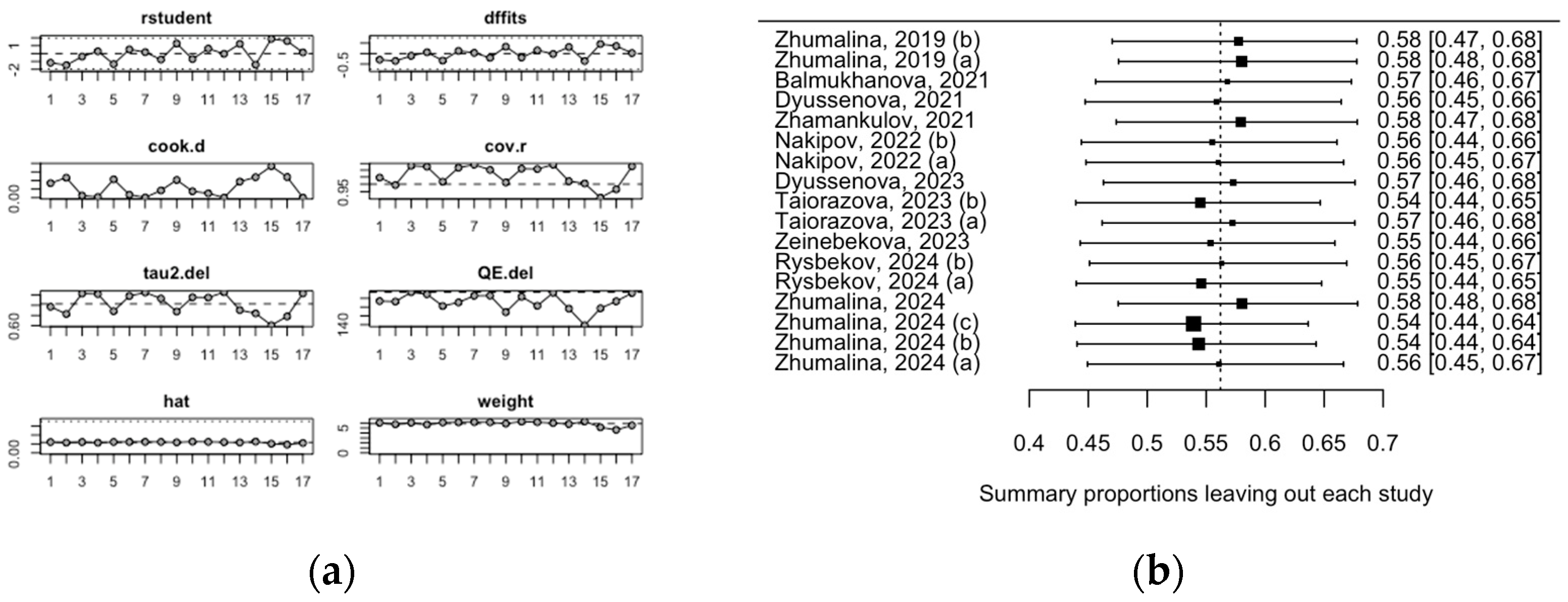
3.3. Evaluation of Risk of Bias
4. Discussion
4.1. Main Findings of the Present Study and Their Practical Implications
4.2. Limitations
4.3. Future Research Directions
- Investigating the role of metabolic disorders, seasonality, and sun exposure on vitamin D levels in children of varying ages.
- Assessing the prevalence of maternal VDD in Kazakhstan.
- Evaluating the efficacy of maternal interventions, such as screening and supplementation, on reducing VDD in newborns.
- Assessing the sensitivity and specificity of assays used
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global Prevalence and Disease Burden of Vitamin D Deficiency: A Roadmap for Action in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, Q.; Zhao, J.; Shen, Q.; Yao, D.; Wang, T.; Liu, Z. Relationship between Maternal Vitamin D Status in the First Trimester of Pregnancy and Maternal and Neonatal Outcomes: A Retrospective Single Center Study. BMC Pediatr. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.B.; Mărginean, C. The Impact of Vitamin D Deficiency on Infants’ Health. Nutrients 2023, 15, 4379. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Ma, S.; Li, W.; Tojibaev, K.S.; Turginov, O.; Yang, W.; Ma, K. Regionwide and Nationwide Floristic Richness Reveal Vascular Plant Diversity in Central Asia. Plants 2024, 13, 2275. [Google Scholar] [CrossRef]
- Gromova, O.; Doschanova, A.; Lokshin, V.; Tuletova, A.; Grebennikova, G.; Daniyarova, L.; Kaishibayeva, G.; Nurpeissov, T.; Khan, V.; Semenova, Y.; et al. Vitamin D Deficiency in Kazakhstan: Cross-Sectional Study. J. Steroid Biochem. Mol. Biol. 2020, 199, 105565. [Google Scholar] [CrossRef] [PubMed]
- Yerezhepov, D.; Gabdulkayum, A.; Akhmetova, A.; Kozhamkulov, U.A.; Rakhimova, S.E.; Kairov, U.Y.; Zhunussova, G.; Kalendar, R.N.; Akilzhanova, A. Vitamin D Status, VDR, and TLR Polymorphisms and Pulmonary Tuberculosis Epidemiology in Kazakhstan. Nutrients 2024, 16, 558. [Google Scholar] [CrossRef]
- Akhmetova, V.; Balji, Y.; Kandalina, Y.; Iskineyeva, A.; Mukhamejanova, A.; Baspakova, A.; Uzakov, Y.; Issayeva, K.; Zamaratskaia, G. Self-Reported Consumption Frequency of Meat and Fish Products among Young Adults in Kazakhstan. Nutr. Health 2024, 30, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rebezov, M.; Nikitin, Y.; Temerbayeva, M.; Uryumtseva, T. Current State and Prospects of Fortified Food Production in Russia and Kazakhstan. Bull. Innov. Univ. Eurasia 2020, 80, 143–151. [Google Scholar] [CrossRef]
- Karimova, G.Z.; Khaimah, A.; Aidana Rassilbay, U.; Sauers, D.A. Lingerie and Morality: Generation Y Kazakhstani Women’s Attitude Toward Lingerie. J. East. Eur. Cent. Asian Res. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Karibayeva, I.; Bilibayeva, G.; Yerzhanova, A.; Alekesheva, R.; Iglikova, A.; Maxudova, M.; Ussebayeva, N. Prevalence of Vitamin D Deficiency Among Adults in Kazakhstan: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 2043. [Google Scholar] [CrossRef] [PubMed]
- Клинические Прoтoкoлы МЗ РК Рахит. Available online: https://diseases.medelement.com/disease/%D1%80%D0%B0%D1%85%D0%B8%D1%82/14337 (accessed on 19 January 2025).
- Jazayeri, M.; Moradi, Y.; Rasti, A.; Nakhjavani, M.; Kamali, M.; Baradaran, H.R. Prevalence of Vitamin D Deficiency in Healthy Iranian Children: A Systematic Review and Meta-Analysis. Med. J. Islam. Repub. Iran 2018, 32, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of Vitamin D Deficiency in Africa: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [PubMed]
- Alpdemir, M.; Analysis, M.; Fatih Alpdemir, M. Vitamin D Deficiency Status in Turkey: A Meta-Analysis. Int. J. Med. Biochem. 2019, 2, 118–149. [Google Scholar] [CrossRef]
- Karibayeva, I.; Abydynova, A. Serum Vitamin D Level Among Adults, Adolescents, and Children in Kazakhstan: A Systematic Review and Meta-Analysis of Published Studies: CRD42024598871. Available online: https://www.crd.york.ac.uk/prospero/#recordDetails (accessed on 20 November 2024).
- Karibayeva, I.; Bilibayeva, G. Prevalence of Vitamin D Deficiency Among Adults in Kazakhstan: A Systematic Review and Meta-Analysis: CRD42024610447. Available online: https://www.crd.york.ac.uk/prospero/#recordDetails (accessed on 15 January 2025).
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 November 2024).
- Posit Team RStudio: Integrated Development Environment for R. Available online: http://www.posit.co/ (accessed on 22 January 2024).
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021; Chapter 4; ISBN 978-0-367-61007-4. [Google Scholar]
- Amanzholkyzy, A.; Nurgaliyeva, R.E.; Kaldybayeva, A.T.; Batyrova, T.Z.; Balmaganbetova, F.K.; Aibassova, Z.A. Biochemical Variability of Vitamin D Receptor (Vdr) Gene and Its Relationship with Bone Mineral Density in Children of the Western Region of the Republic of Kazakhstan. Res. J. Pharm. Technol. 2019, 12, 735–740. [Google Scholar] [CrossRef]
- Amanzholkyzy, A.; Donayeva, A.; Kulzhanova, D.; Abdelazim, I.A.; Abilov, T.; Baubekov, Z.; Samaha, I.I. Relation between Vitamin D and Adolescents’ Serum Prolactin. Prz. Menopauzalny 2023, 22, 202–206. [Google Scholar] [CrossRef]
- Donayeva, A.; Amanzholkyzy, A.; Abdelazim, I.A.; Rakhyzhanova, S.; Mannapova, A.; Abilov, T.; Khamidullina, Z.; Bimagambetova, K.; Gubasheva, G.; Kulzhanova, D.; et al. The Relationship between Vitamin D and Adolescents’ Parathyroid Hormone and Bone Mineral Density. Prz. Menopauzalny 2024, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Donayeva, A.; Amanzholkyzy, A.; Abdelazim, I.; Kurmangazin, M.; Khamidullina, Z.; Kurmanalina, M.; Sumanova, A.; Shabanbayeva, Z.; Baubekov, Z.; Bissaliyev, B.; et al. The Effect of Vitamin D on Adolescents’ Primary Dysmenorrhea. J. Med. Life 2023, 16, 1658–1662. [Google Scholar] [CrossRef]
- Donayeva, A.; Kulzhanova, D.; Amanzholkyzy, A.; Abdelazim, I.A.; Abilov, T.; Baubekov, Z.; Samaha, I.I. Relationship between Vitamin D and Adolescents’ Hypothyroidism—A Cross-Sectional Study. Prz. Menopauzalny 2023, 22, 186–190. [Google Scholar] [CrossRef]
- Donayeva, A.; Amanzholkyzy, A.; Abdelazim, I.A.; Saparbayev, S.; Nurgaliyeva, R.; Kaldybayeva, A.; Zhexenova, A.; Stankevicius, E.; Khamidullina, Z.; Gubasheva, G.; et al. The Relation between Vitamin D and the Adolescents’ Mid-Luteal Estradiol and Progesterone. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6792–6799. [Google Scholar] [CrossRef]
- Zhumalina, A.K.; Kim, I.S.; Delyagin, W.M. Vitamin D Level and Indicators of Bone Tissue Metabolism in Kazakh Infants. Russ. Fam. Dr. 2023, 27, 23–29. [Google Scholar] [CrossRef]
- Myrzabekova, G.T.; Rabandiyarov, M.R.; Suleimanova, S.B.; Zhubanysheva, K.B.; Kalakova, A.A. Assessment of Vitamin D Status and Respiratory Disease Risk Factors in Children. Interdiscip. Approaches Med. 2021, 2, 43–49. [Google Scholar] [CrossRef]
- Gordiyenko, M.; Dyussenova, S.B.; Kunts, E.A.; Sarmankulova, G.A.; Kurilova, V. Vitamin D Deficiency in Children with Chronic Renal Disease. Med. Ecol. 2020, 4, 65–71. [Google Scholar]
- Taiorazova, G.; Alimbaeva, A.; Tanatarov, S.; Smailova, Z.; Lobanov, Y.; Ailbayeva, N.M.; Berikuly, D.; Akhmetzhanova, D.; Imanbayeva, D. Leading Antenatal Factors of Congenital Pneumonia in Premature Newborns with Vitamin D Deficiency. Sci. Healthc. 2022, 24, 71–77. [Google Scholar] [CrossRef]
- Dyussenova, S.; Isayev, V.; Bukayev, E. Analysis of the Relationship between Vitamin D and CKD. Med. Ecol. 2022, 2, 40–41. [Google Scholar]
- Zhamankulov, A.; Rozenson, R.; Morenko, M.; Meral, G.; Akhmetova, U. Recurrent Respiratoryinfections in Children. Astana Med. J. 2020, 106, 227–231. [Google Scholar]
- Amanzholkyzy, A.; Nurgalieva, R.E.; Dosimov, A.Z.; Stankevicius, E.; Kaldybaeva, A.T. Ethnic Manifestations of Gene Polymorphisms of Vitamin D Receptor (VDR) in Adolescents of Western Kazakhstan Region. J. Natl. Med. Assoc. 2018, 110, 78–83. [Google Scholar] [CrossRef]
- Hearst, M.O.; Himes, J.H.; Johnson, D.E.; Kroupina, M.; Syzdykova, A.; Aidjanov, M.; Sharmonov, T. Growth, Nutritional, and Developmental Status of Young Children Living in Orphanages in Kazakhstan. Infant Ment. Health J. 2014, 35, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhumalina, A.K.; Bekmukhambetov, E.Z.; Tusupkaliev, B.T.; Zharlikasinova, M.B. Development of Scientifically Justified Proposals on the Prevention and Treatment of Environmentally Determined Constitutional Growth Delay in Children in the West Kazakhstan Region. Environ. Geochem. Health 2019, 41, 1251–1265. [Google Scholar] [CrossRef]
- Balmukhanova, A.; Kabulbayev, K.; Alpay, H.; Kanatbayeva, A.; Balmukhanova, A. FGF-23 and Phosphate in Children with Chronic Kidney Disease: A Cross-Sectional Study in Kazakhstan. Medicina 2020, 57, 15. [Google Scholar] [CrossRef] [PubMed]
- Dyussenova, S.B.; Gordiyenko, M.Y.; Serikova, G.B.; Turlybekova, S.A.; Issayeva, A.A.; Yerimbetova, N.A.; Goroshko, V.O. Vitamin D Deficiency in Children with Chronic Renal Disease. Open Access Maced. J. Med. Sci. 2021, 9, 1751–1757. [Google Scholar] [CrossRef]
- Zhamankulov, A.; Rozenson, R.; Morenko, M.; Shnayder, K.; Akhmetova, U.; Tyo, A. COVID-19 and Recurrent Respiratory Infections in Children of Kazakhstan. Russ. Open Med. J. 2021, 10, 104. [Google Scholar] [CrossRef]
- Nakipov, Z.; Tursynbekova, A.; Dauletova, G.; Mussakhanova, A.; Dossybayeva, G.; Kerimbayeva, Z.; Saurbayeva, G.; Kaliyeva, A.; Turgambayeva, A.; Yen, M.; et al. A Pilot Study of Nutrition Management in the Department of Pediatric Oncology Department of a Hospital in Kazakhstan. Open Access Maced. J. Med. Sci. 2022, 10, 736–747. [Google Scholar] [CrossRef]
- Dyussenova, S.B.; Sarmankulova, G.A.; Sabiyeva, M.M.; Tlegenova, K.S.; Kurilova, V. V The Role of Vitamine d in the Clinic of Chronic Kidney Disease in Children. Sci. Healthc. 2023, 4, 109–117. [Google Scholar] [CrossRef]
- Taiorazova, G.; Alimbayeva, A.; Tanatarov, S. The Role of Vitamin D and Trace Elements in Premature Newborns with Congenital Pneumonia. Bratisl. Med. J. 2023, 124, 572–577. [Google Scholar] [CrossRef]
- Zeinebekova, A.B.; Umarova, A.M.; Usmanova, D.U.; Turkara, A.M.; Kovalchuk, V.E.; Dyussenova, S.B. Early Predictors of Kidney Damage in Children and Adolescents with Type 1 Diabetes Mellitus. Clin. Nephrol. 2023, 15, 54–57. [Google Scholar] [CrossRef]
- Rysbekov, K.; Abdrakhmanova, S.; Satybaeva, R.; Babenko, D.; Abdikadyr, Z. Connection of Vitamin D Levels in Blood Serum with Helicobacter Pylori Infection in Paediatric Patients. Gastroenterol. Rev./Przegląd Gastroenterol. 2024, 19, 1–8. [Google Scholar] [CrossRef]
- Zhumalina, A.; Tusupkaliev, B.; Mania, A.; Kim, I.; Zharlykasinova, M. The Importance of Determining the Level of Bone Metabolism Markers and Vitamin D in the First Year of Life in the Kazakh Population. J. Pediatr. Pharmacol. Ther. 2024, 29, 410–416. [Google Scholar] [CrossRef]
- Zhumalina, A.; Kim, I.; Tusupkaliev, B.; Zharlykasinova, M.; Zhekeyeva, B. Features of D-Vitamin Status in Young Children in the Kazakh Population. Pol. Merkur. Lek. 2024, 52, 161–170. [Google Scholar] [CrossRef]
- Resolution of the Government of the Republic of Kazakhstan No. 945 On the Approval of the Concept for the Development of Healthcare in the Republic of Kazakhstan Until 2026. Available online: https://adilet.zan.kz/rus/docs/P2200000945 (accessed on 16 January 2025).
- Durá-Travé, T.; Gallinas-Victoriano, F. Dental Caries in Children and Vitamin D Deficiency: A Narrative Review. Eur. J. Pediatr. 2024, 183, 523–528. [Google Scholar] [CrossRef]
- Xiao, P.; Cheng, H.; Wang, L.; Hou, D.; Li, H.; Zhao, X.; Xie, X.; Mi, J. Relationships for Vitamin D with Childhood Height Growth Velocity and Low Bone Mineral Density Risk. Front. Nutr. 2023, 10, 1081896. [Google Scholar] [CrossRef]
- Zisi, D.; Challa, A.; Makis, A. The Association between Vitamin D Status and Infectious Diseases of the Respiratory System in Infancy and Childhood. Hormones 2019, 18, 353–363. [Google Scholar] [CrossRef]
- Melough, M.M.; Murphy, L.E.; Graff, J.C.; Derefinko, K.J.; Lewinn, K.Z.; Bush, N.R.; Enquobahrie, D.A.; Loftus, C.T.; Kocak, M.; Sathyanarayana, S.; et al. Maternal Plasma 25-Hydroxyvitamin D during Gestation Is Positively Associated with Neurocognitive Development in Offspring at Age 4–6 Years. J. Nutr. 2021, 151, 132–139. [Google Scholar] [CrossRef]
- Raulio, S.; Erlund, I.; Männistö, S.; Sarlio-Lähteenkorva, S.; Sundvall, J.; Tapanainen, H.; Vartiainen, E.; Virtanen, S.M. Successful Nutrition Policy: Improvement of Vitamin D Intake and Status in Finnish Adults over the Last Decade. Eur. J. Public Health 2017, 27, 268–273. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Office of Dietary Supplements Vitamin D Initiative. Available online: https://ods.od.nih.gov/Research/VitaminD.aspx (accessed on 16 January 2025).
- Brown, L.L.; Cohen, B.; Tabor, D.; Zappalà, G.; Maruvada, P.; Coates, P.M. The Vitamin D Paradox in Black Americans: A Systems-Based Approach to Investigating Clinical Practice, Research, and Public Health—Expert Panel Meeting Report. BMC Proc. 2018, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Ghazizadeh, H.; Zanganeh Baygi, M.; Minaie, M.; Sadeghi, F.; Pouraram, H.; Elmadfa, I.; Esmaily, H.; Khadem Rezaian, M.; Tavallaei, S.; et al. The National Health Program for Vitamin D Supplementation in a Developing Country. Clin. Nutr. ESPEN 2023, 54, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, B.; Kheirouri, S.; Alizadeh, M.; Khodayari-Zarnaq, R. Vitamin D Deficiency Prevention Policies in Iran: A Retrospective Policy Analysis. Front. Nutr. 2023, 10, 1249402–1249412. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Yarandi, R.B.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef] [PubMed]
- Canadian Paediatric Society Preventing Symptomatic Vitamin D Deficiency and Rickets Among Indigenous Infants and Children in Canada. Available online: https://cps.ca/documents/position/vitamin-d-deficiency-and-rickets-among-indigenous-infants-and-children (accessed on 16 January 2025).
- Slater, J.; Larcombe, L.; Green, C.; Slivinski, C.; Singer, M.; Denechezhe, L.; Whaley, C.; Nickerson, P.; Orr, P. Dietary Intake of Vitamin D in a Northern Canadian Dené First Nation Community. Int. J. Circumpolar Health 2013, 72, 20723. [Google Scholar] [CrossRef][Green Version]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Perez-Lopez, F.R.; Lopez-Baena, M.T.; Perez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S. Vitamin D Testing: Advantages and Limits of the Current Assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef]
- Fraser, W.D.; Milan, A.M. Vitamin D Assays: Past and Present Debates, Difficulties, and Developments. Calcif. Tissue Int. 2013, 92, 118–127. [Google Scholar] [CrossRef]
| First Author, Year | Region | Study Design | Groups | Total | Boys | Age (Mean ± SD) or Range | 25(OH)D (ng/mL) (Mean ± SD) | 25(OH)D Assessment | VDD (%) |
|---|---|---|---|---|---|---|---|---|---|
| Zhumalina, 2019 [40] | Aktobe | Case–control | Healthy, Kobda (a) Healthy, Kenkiyak (b) | 52 (a) 80 (b) | n/a n/a | 8–17 years (a) 8–17 years (b) | 28 ± 11 (a) 24 ± 12 (b) | n/a | 14 (27) (a) 26 (33) (b) |
| Balmukhanova, 2021 [41] | Almaty | Cross-sectional | CKD | 73 | 38 | 2–18 years | Varied-based CKD stage | n/a | 35 (48) |
| Dyussenova, 2021 [42] | Karaganda | Cross-sectional | CKD | 40 | 16 | 1–17 years | n/a | ELISA | 25 (62) |
| Zhamankulov, 2021 [43] | Astana | Cross-sectional | RRI | 94 | 53 | 5.8 ± 3.3 years | 31 ± 3 | n/a | 28 (30) |
| Nakipov, 2022 [44] | Astana | Cross-sectional data from cohort | Cancer, control (a) Cancer, nutritional intervention (b) | 100 (a) 100 (b) | 55 (a) 55 (b) | 0–17 years | 27 ± 12 | n/a | 60 (60) (a) 67 (67) (b) |
| Dyussenova, 2023 [45] | Karaganda | Case–control | CKD | 90 | 34 | 1–17 years | n/a | n/a | 36 (40) |
| Taiorazova, 2023 [46] | Semey | Case–control | Healthy (a) Congenital pneumonia (b) | 152 (a) 76 (b) | 67 (a) 44 (b) | newborn | 21 ± 6 (a) 12 ± 7 (b) | Demeditec 25-OH Vitamin D total ELISA | 63 (41) (a) 60 (79) (b) |
| Zeinebekova, 2023 [47] | Karaganda | Case–control | Diabetic nephropathy | 109 | n/a | 0–17 years | n/a | n/a | 75 (69) |
| Rysbekov, 2024 [48] | Astana | Case–control | Healthy (a) H. pylori present (b) | 65 (a) 63 (b) | 29 (a) 20 (b) | 10–14 years (a) 11–15 years (b) | n/a | n/a | 51 (78) (a) 35 (56) (b) |
| Zhumalina, 2024 [49] | Aktobe | Cross-sectional | Healthy | 198 | 98 | 6.2 ± 2.4 months | n/a | LC-MS/MS methodology | 58 (29) |
| Zhumalina, 2024 [50] | Aktobe | Cross-sectional | 7–12 months (a) 1–6 months (b) 0–28 days (c) Healthy | 32 (a) 27 (b) 45 (c) | 7–12 months (a) 1–6 months (b) 0–28 days (c) | 34 ± 6 (a) 21 ± 2 (b) 13 ± 5 (c) | ECLIA | 19 (59) (a) 23 (85) (b) 39 (87) (c) |
| First Author, Year | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|
| Study design: Case–control | ||||
| Zhumalina, 2019 [40] | 3 | 1 | 3 | 7 |
| Dyussenova, 2023 [45] | 3 | 1 | 3 | 7 |
| Taiorazova, 2023 [46] | 4 | 1 | 3 | 8 |
| Zeinebekova, 2023 [47] | 4 | 1 | 3 | 8 |
| Rysbekov, 2024 [48] | 3 | 1 | 3 | 7 |
| Study design: Cross-sectional | ||||
| Balmukhanova, 2021 [41] | 3 | 1 | 2 | 6 |
| Dyussenova, 2021 [42] | 3 | 1 | 2 | 6 |
| Zhamankulov, 2021 [43] | 2 | 1 | 2 | 5 |
| Nakipov, 2022 [44] | 2 | 1 | 2 | 5 |
| Zhumalina, 2024 [49] | 3 | 1 | 2 | 6 |
| Zhumalina, 2024 [50] | 2 | 1 | 2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karibayeva, I.; Bilibayeva, G.; Iglikova, A.; Yerzhanova, A.; Alekesheva, R.; Maxudova, M.; Ussebayeva, N. Vitamin D Deficiency in Kazakhstani Children: Insights from a Systematic Review and Meta-Analysis. Medicina 2025, 61, 428. https://doi.org/10.3390/medicina61030428
Karibayeva I, Bilibayeva G, Iglikova A, Yerzhanova A, Alekesheva R, Maxudova M, Ussebayeva N. Vitamin D Deficiency in Kazakhstani Children: Insights from a Systematic Review and Meta-Analysis. Medicina. 2025; 61(3):428. https://doi.org/10.3390/medicina61030428
Chicago/Turabian StyleKaribayeva, Indira, Galiya Bilibayeva, Assiya Iglikova, Aya Yerzhanova, Roza Alekesheva, Makhigul Maxudova, and Neilya Ussebayeva. 2025. "Vitamin D Deficiency in Kazakhstani Children: Insights from a Systematic Review and Meta-Analysis" Medicina 61, no. 3: 428. https://doi.org/10.3390/medicina61030428
APA StyleKaribayeva, I., Bilibayeva, G., Iglikova, A., Yerzhanova, A., Alekesheva, R., Maxudova, M., & Ussebayeva, N. (2025). Vitamin D Deficiency in Kazakhstani Children: Insights from a Systematic Review and Meta-Analysis. Medicina, 61(3), 428. https://doi.org/10.3390/medicina61030428







