An Updated Review on the Use of Noninvasive Respiratory Supports in the Management of Severe Asthma Exacerbations
Abstract
1. Introduction
2. Asthma Pathophysiology
3. Respiratory System Mechanics, Gas Exchange and Heart–Lung Interactions in Acute Asthma Exacerbations
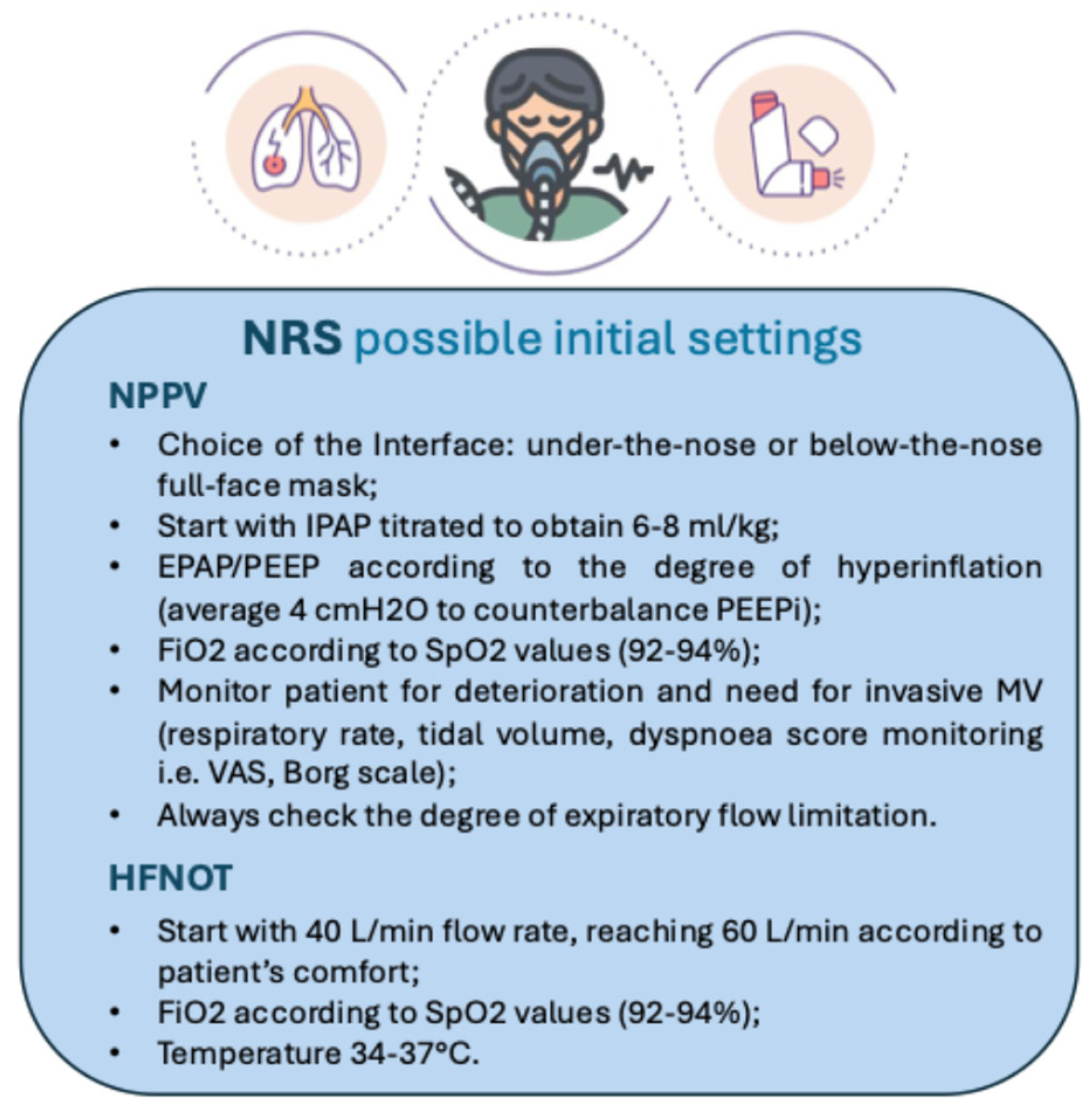
4. Noninvasive Positive Pressure Ventilation Use in Acute Asthma Exacerbations
| Study (First Author, Year) | Design | N. Patients Age (y) Sex | Intervention | Interfaces | Outcomes |
|---|---|---|---|---|---|
| Meduri GU et al., 1996 [34] | Prospective clinical study | 17 35 ± 11 41%M/59%F | IPAP 14 ± 5 cm H2I EPAP 4 ± 2 cm H2O | NPPV face mask | Baseline at 2 h: pH 7.25 ± 0.01; PaCO2 65 ± 2; PaO2 315 ± 41; From 2 h to 6 h: pH 7.32 ± 0.02; PaCO2 52 ± 3; PaO2 403 ± 47; From 12 h to 24 h: pH 7.36 ± 0.02; PaCO2 45 ± 3; PaO2 367 ± 47 At 12 h: pH 7.38 ± 0.02; PaCO2 45 ± 4; PaO2 472 ± 67 Two patients required intubation. All patients survived. Length of hospital stay was 5 ± 4 days |
| Fernandez MM et al., 2001 [33] | Retrospective observational study | 33 (22 NPPV vs. 11 ETI) NPPV 48 ± 21 ETI 53 ± 19 NPPV 27%M/73%F ETI 27%M/73%F | IPAP 10 cm H2O EPAP 5 cm H2O | NPPV face mask | NPPV PaCO2 89 ± 29 mmHg vs. ETI PaCO2 53 ± 13 mmHg; NPPV pH 7.05 ± 0.21 vs. ETI pH 7.28 ± 0.008; NPPV HCO3- level 22 ± 5 mmol/l vs. ETI HCO3-level 26 ± 6 mmol/l; No differences in the median length of ICU stay (NPPV 4.5 vs. ETI 3 days), median hospital stay (NPPV 15 vs. ETI 12 days) and mortality (NPPV 0 vs. ETI 4%) |
| Soroksky A et al., 2003 [8] | Prospective randomized placebo-controlled | 30 (NPPV 15 vs. Stm 15) NPPV 34 ± 8 Stm 32 ± 9 NPPV 47%M/53%F Stm 53%M/47%F | IPAP 15 cm H2O EPAP 5 cm H2O | NPPV nasal mask | Primary: Increase in FEV1 ≥ 50% Secondary: Need for hospitalization Need for MV Study over 4 h |
| Soma T et al., 2008 [41] | Prospective randomized trial | 44 (NPPV (HP) 14; NPPV (LP) 12; Stm 14) NPPV (High Pressure, HP) 37 ± 20 NPPV (Low Pressure, LP) 46 ± 14 Stm 44 ± 13 NPPV (HP) 57%M/43%F NPPV (LP) 33%M/67%F Stm 28%M/72%F | NPPV (HP) IPAP 8 cm H2O EPAP 6 cm H2O NPPV (LP) IPAP 6 cm H2O EPAP 4 cm H2O | NPPV nose or face mask | Primary: % Improvement in FEV1 Secondary: SpO2 Modified Borg dyspnea scale Adverse effects |
| Gupta D et al., 2010 [9] | Prospective randomized controlled trial | 53 (NPPV 28 vs. Stm 25) 44 ± 15 21%M/79%F | IPAP min 8 cm H2O EPAP 4 cm H2O; IPAP max 20 cm H2O EPAP 10 cm H2O | NPPV oro-nasal mask | Primary: Increase in FEV1 ≥ 50% ICU and hospital stay Secondary: RR Accessory muscle use ABG values at 1, 2 and 4 h Bronchodilator usage Failure of primary therapy |
| Murase K et al., 2010 [12] | Retrospective cohort study | 102 (pre-NPPV 48 vs. post-NPPV 54) Pre-NPPV 45 ± 20 Post-NPPV 52 ± 18 Pre-NPPV 46%M/64%F Post-NPPV 36%M/74%F | - | NPPV face mask | Pre-NPPV 9 were treated primarily by ETI; Post-NPPV 17 were treated primarily by NPPV The rate of ETI decreased in the post-NPPV period Post-NPPV: reduction in the duration of MV with ETI or NPPV (36.9 ± 38.4 h vs. 20.3 ± 35.8 h), and hospital stay was shortened (12.6 ± 4.2 vs. 8.4 ± 2.8 days) |
| Althoff MD et al., 2020 [42] | Retrospective cohort study | 53.654 (NPPV 13.540 vs. NO-NPPV 40.114) 51 NPPV 34%M/66%F NO-NPPV 31%M/61%F | - | - | NPPV 22.3% ETI and 136 died NPPV was associated with lower odds of receiving ETI and in-hospital mortality |
| Briones CKH et al., 2021 [43] | Prospective clinical study | 68 (2 asthma) 71 ± 19 66%M/34%F | IPAP 12 cm H2O EPAP 6–8 cm H2O | NPPV face mask | NPPV success rate was 69% and mortality rate was 20.6% |
5. Continuous Positive Airway Pressure (CPAP) Use in Acute Asthma Exacerbations
6. High-Flow Oxygen Therapy (HFNOT) Use in Acute Asthma Exacerbations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma—GINA. 2024 GINA Main Report. Available online: https://ginasthma.org/2024-report/ (accessed on 7 September 2024).
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.; Kotanidou, A.; Malagari, K.; Roussos, C. Clinical review: Severe asthma. Crit. Care Lond. Engl. 2001, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Corbridge, T.; Kazzi, A. Intubation and mechanical ventilation of the asthmatic patient in respiratory failure. J. Allergy Clin. Immunol. 2009, 124 (Suppl. S2), S19–S28. [Google Scholar] [CrossRef] [PubMed]
- Demoule, A.; Brochard, L.; Dres, M.; Heunks, L.; Jubran, A.; Laghi, F.; Mekontso-Dessap, A.; Nava, S.; Ouanes-Besbes, L.; Peñuelas, O.; et al. How to ventilate obstructive and asthmatic patients. Intensive Care Med. 2020, 46, 2436–2449. [Google Scholar] [CrossRef]
- Misseri, G.; Frassanito, L.; Simonte, R.; Rosà, T.; Grieco, D.L.; Piersanti, A.; De Robertis, E.; Gregoretti, C. Personalized Noninvasive Respiratory Support in the Perioperative Setting: State of the Art and Future Perspectives. J. Pers. Med. 2023, 14, 56. [Google Scholar] [CrossRef]
- Leatherman, J. Mechanical Ventilation for Severe Asthma. Chest 2015, 147, 1671–1680. [Google Scholar] [CrossRef]
- Soroksky, A.; Stav, D.; Shpirer, I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003, 123, 1018–1025. [Google Scholar] [CrossRef]
- Gupta, D.; Nath, A.; Agarwal, R.; Behera, D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir. Care 2010, 55, 536–543. [Google Scholar]
- Gregoretti, C.; Pisani, L.; Cortegiani, A.; Ranieri, V.M. Noninvasive ventilation in critically ill patients. Crit. Care Clin. 2015, 31, 435–457. [Google Scholar] [CrossRef]
- Lim, W.J.; Mohammed Akram, R.; Carson, K.V.; Mysore, S.; Labiszewski, N.A.; Wedzicha, J.A.; Rowe, B.H.; Smith, B.J. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst. Rev. 2012, 12, CD004360. [Google Scholar] [CrossRef]
- Murase, K.; Tomii, K.; Chin, K.; Tsuboi, T.; Sakurai, A.; Tachikawa, R.; Harada, Y.; Takeshima, Y.; Hayashi, M.; Ishihara, K.; et al. The use of non-invasive ventilation for life-threatening asthma attacks: Changes in the need for intubation. Respirol. Carlton Vic. 2010, 15, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, P.; Terzi, N.; Mortamet, G.; Abroug, F.; Carteaux, G.; Charasse, C.; Chauvin, A.; Combes, X.; Dauger, S.; Demoule, A.; et al. Management of severe asthma exacerbation: Guidelines from the Société Française de Médecine d’Urgence, the Société de Réanimation de Langue Française and the French Group for Pediatric Intensive Care and Emergencies. Ann. Intensive Care 2019, 9, 115. [Google Scholar] [CrossRef]
- Pendergraft, T.B.; Stanford, R.H.; Beasley, R.; Stempel, D.A.; Roberts, C.; McLaughlin, T. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann. Allergy Asthma Immunol. Off Publ. Am. Coll. Allergy Asthma Immunol. 2004, 93, 29–35. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Cuttone, G.; Misseri, G.; Sorbello, M.; Pappalardo, F.; Maniaci, A.; Duarte-Medrano, G.; Nuño-Lámbarri, N.; Zanza, C.; Gregoretti, C. The use of noninvasive positive pressure ventilation for severe asthma: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Expert Rev. Respir. Med. 2025, 19, 1–9. [Google Scholar] [CrossRef]
- Peñuelas, O.; Muriel, A.; Abraira, V.; Frutos-Vivar, F.; Mancebo, J.; Raymondos, K.; Du, B.; Thille, A.W.; Ríos, F.; González, M.; et al. Inter-country variability over time in the mortality of mechanically ventilated patients. Intensive Care Med. 2020, 46, 444–453. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; William, O.C.M.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Mazzone, S.B.; Undem, B.J. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- Bergeron, C.; Tulic, M.K.; Hamid, Q. Airway remodelling in asthma: From benchside to clinical practice. Can. Respir. J. 2010, 17, e85–e93. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Wagner, C.; Wegmann, M. Airway remodeling in asthma: What really matters. Cell Tissue Res. 2017, 367, 551–569. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Davis, B.E. Mechanisms of airway hyperresponsiveness. J. Allergy Clin. Immunol. 2006, 118, 551–559; quiz 560–561. [Google Scholar] [CrossRef]
- Wagner, P.D. Ventilation-perfusion matching during exercise. Chest 1992, 101 (Suppl. S5), 192S–198S. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Toumpanakis, D.; Mancebo, J. What’s new about pulmonary hyperinflation in mechanically ventilated critical patients. Intensive Care Med. 2020, 46, 2381–2384. [Google Scholar] [CrossRef]
- Smith, T.C.; Marini, J.J. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J. Appl. Physiol. 1988, 65, 1488–1499. [Google Scholar] [CrossRef]
- Tobin, M.J. Asthma, airway biology, and nasal disorders in AJRCCM 2003. Am. J. Respir. Crit. Care Med. 2004, 169, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.M.; Villagrá, A.; Blanch, L.; Fernández, R. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Med. 2001, 27, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Cook, T.R.; Turner, R.E.; Cohen, M.; Leeper, K.V. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996, 110, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Young, I.H.; Bye, P.T.P. Gas exchange in disease: Asthma, chronic obstructive pulmonary disease, cystic fibrosis, and interstitial lung disease. Compr. Physiol. 2011, 1, 663–697. [Google Scholar]
- Cheyne, W.S.; Gelinas, J.C.; Eves, N.D. Hemodynamic effects of incremental lung hyperinflation. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H474–H481. [Google Scholar] [CrossRef]
- Corbridge, T.C.; Hall, J.B. The assessment and management of adults with status asthmaticus. Am. J. Respir. Crit. Care Med. 1995, 151, 1296–1316. [Google Scholar] [CrossRef]
- Manglani, R.; Landaeta, M.; Maldonado, M.; Hoge, G.; Basir, R.; Menon, V. The use of non-invasive ventilation in asthma exacerbation—A two year retrospective analysis of outcomes. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 727–732. [Google Scholar] [CrossRef]
- Nava, S.; Hill, N. Non-invasive ventilation in acute respiratory failure. Lancet Lond. Engl. 2009, 374, 250–259. [Google Scholar] [CrossRef]
- Ari, A. How to optimize aerosol drug delivery during noninvasive ventilation: What to use, how to use it, and why? Eurasian J. Pulmonol. 2019, 21, 1. [Google Scholar] [CrossRef]
- Soma, T.; Hino, M.; Kida, K.; Kudoh, S. A prospective and randomized study for improvement of acute asthma by non-invasive positive pressure ventilation (NPPV). Intern. Med. 2008, 47, 493–501. [Google Scholar] [CrossRef]
- Althoff, M.D.; Holguin, F.; Yang, F.; Grunwald, G.K.; Moss, M.; Vandivier, R.W.; Ho, P.M.; Kiser, T.H.; Burnham, E.L. Noninvasive Ventilation Use in Critically Ill Patients with Acute Asthma Exacerbations. Am. J. Respir. Crit. Care Med. 2020, 202, 1520. [Google Scholar] [CrossRef] [PubMed]
- Briones Claudett, K.H.; Rodriguez, A.E.; Briones Claudett, M.H.; Tejada, M.P.; del Pilar Cabrera Baños, M.; Jorge, D.N.; Bermeo, B.; Grunauer, M. Non-invasive mechanical ventilation with average volume-assured pressure support. Results according to the aetiology of acute respiratory failure. Anaesthesiol. Intensive Ther. 2021, 53, 403–410. [Google Scholar] [CrossRef]
- Soroksky, A.; Klinowski, E.; Ilgyev, E.; Mizrachi, A.; Miller, A.; Yehuda, T.M.B.; Shpirer, I.; Leonov, Y. Noninvasive positive pressure ventilation in acute asthmatic attack. Eur. Respir. Rev. 2010, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2014, 69 (Suppl. S1), 1–192. [Google Scholar]
- Martin, J.G.; Shore, S.; Engel, L.A. Effect of continuous positive airway pressure on respiratory mechanics and pattern of breathing in induced asthma. Am. Rev. Respir. Dis. 1982, 126, 812–817. [Google Scholar]
- Wilson, B.A.; Jackson, P.J.; Evans, J. Effects of positive end-expiratory pressure breathing on exercise-induced asthma. Int. J. Sports Med. 1981, 2, 27–30. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, C.H.; Yang, C.T.; Huang, T.J.; Yu, C.T.; Shieh, W.B.; Kuo, H.P. Effect of nasal continuous positive airway pressure on methacholine-induced bronchoconstriction. Respir. Med. 1995, 89, 121–128. [Google Scholar] [CrossRef]
- Shivaram, U.; Miro, A.M.; Cash, M.E.; Finch, P.J.; Heurich, A.E.; Kamholz, S.L. Cardiopulmonary responses to continuous positive airway pressure in acute asthma. J. Crit. Care 1993, 8, 87–92. [Google Scholar] [CrossRef]
- Renda, T.; Corrado, A.; Iskandar, G.; Pelaia, G.; Abdalla, K.; Navalesi, P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br. J. Anaesth. 2018, 120, 18–27. [Google Scholar] [CrossRef]
- Roca, O.; Riera, J.; Torres, F.; Masclans, J.R. High-flow oxygen therapy in acute respiratory failure. Respir. Care 2010, 55, 408–413. [Google Scholar]
- Grieco, D.L.; Maggiore, S.M.; Roca, O.; Spinelli, E.; Patel, B.K.; Thille, A.W.; Barbas, C.S.V.; de Acilu, M.G.; Cutuli, S.L.; Bongiovanni, P.; et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021, 47, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, S.M.; Idone, F.A.; Vaschetto, R.; Festa, R.; Cataldo, A.; Antonicelli, F.; Montini, L.; De Gaetano, A.; Navalesi, P.; Antonelli, M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am. J. Respir. Crit. Care Med. 2014, 190, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Corley, A.; Caruana, L.R.; Barnett, A.G.; Tronstad, O.; Fraser, J.F. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br. J. Anaesth. 2011, 107, 998–1004. [Google Scholar] [CrossRef]
- Ricard, J.D.; Roca, O.; Lemiale, V.; Corley, A.; Braunlich, J.; Jones, P.; Kang, B.J.; Lellouche, F.; Nava, S.; Rittayamai, N.; et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020, 46, 2238–2247. [Google Scholar] [CrossRef]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef]
- Genecand, L.; Agoritsas, T.; Ehrensperger, C.; Kharat, A.; Marti, C. High-flow nasal oxygen in acute hypoxemic respiratory failure: A narrative review of the evidence before and after the COVID-19 pandemic. Front. Med. 2022, 9, 1068327. [Google Scholar] [CrossRef]
- Ballestero, Y.; De Pedro, J.; Portillo, N.; Martinez-Mugica, O.; Arana-Arri, E.; Benito, J. Pilot Clinical Trial of High-Flow Oxygen Therapy in Children with Asthma in the Emergency Service. J. Pediatr. 2018, 194, 204–210.e3. [Google Scholar] [CrossRef]
- Martínez, F.G.; Sánchez, M.I.G.; Del Castillo, B.T.; Moreno, J.P.; Muñoz, M.M.; Jiménez, C.R.; Fernández, R.R. Treatment with high-flow oxygen therapy in asthma exacerbations in a paediatric hospital ward: Experience from 2012 to 2016. An. Pediatr. 2019, 90, 72–78. [Google Scholar]
- Ruangsomboon, O.; Limsuwat, C.; Praphruetkit, N.; Monsomboon, A.; Chakorn, T. Nasal High-flow Oxygen Versus Conventional Oxygen Therapy for Acute Severe Asthma Patients: A Pilot Randomized Controlled Trial. Acad. Emerg. Med. 2021, 28, 530–541. [Google Scholar] [CrossRef]
- Pilar, J.; Modesto I Alapont, V.; Lopez-Fernandez, Y.M.; Lopez-Macias, O.; Garcia-Urabayen, D.; Amores-Hernandez, I. High-flow nasal cannula therapy versus non-invasive ventilation in children with severe acute asthma exacerbation: An observational cohort study. Med. Intensiva. 2017, 41, 418–424. [Google Scholar] [CrossRef]
- Chao, K.Y.; Chien, Y.H.; Mu, S.C. High-flow nasal cannula in children with asthma exacerbation: A review of current evidence. Paediatr. Respir. Rev. 2021, 40, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.; Atalay, O.; Harwood, R.; Sheard, M.; Aljamhan, E.; Fink, J. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir. Care 2010, 55, 845–851. [Google Scholar] [PubMed]
- Li, J.; Tu, M.; Yang, L.; Jing, G.; Fink, J.; Burtin, C.; de Andrade, A.D.; Gong, L.; Xie, L.; Ehrmann, S. Worldwide clinical practice of high-flow nasal cannula and concomitant aerosol therapy in the adult ICU setting. Respir. Care 2021, 66, 1416–1424. [Google Scholar] [CrossRef]
- Li, J.; Gong, L.; Fink, J. The ratio of nasal cannula gas flow to patient inspiratory flow on trans-nasal pulmonary aerosol delivery for adults: An in vitro study. Pharmaceutics 2019, 11, 225. [Google Scholar] [CrossRef]
- Colaianni-Alfonso, N.; Toledo, A.; Montiel, G.; Castro-Sayat, M.; Crimi, C.; Vetrugno, L. High-Flow Nasal Cannula and in-line aerosolised bronchodilator delivery during severe exacerbation of asthma in adults: A feasibility Observational Study. Anaesth. Crit. Care Pain Med. 2024, 43, 101414. [Google Scholar] [CrossRef]
- Gates, R.M.; Haynes, K.E.; Rehder, K.J.; Zimmerman, K.O.; Rotta, A.T.; Miller, A.G. High-Flow Nasal Cannula in Pediatric Critical Asthma. Respir. Care 2021, 66, 1240–1246. [Google Scholar] [CrossRef]
- Gross, J.L.; Park, G.R. Humidification of inspired gases during mechanical ventilation. Minerva. Anestesiol. 2012, 78, 496–502. [Google Scholar]
- Geng, W.; Batu, W.; You, S.; Tong, Z.; He, H. High-Flow Nasal Cannula: A Promising Oxygen Therapy for Patients with Severe Bronchial Asthma Complicated with Respiratory Failure. Can. Respir. J. 2020, 2020, 2301712. [Google Scholar] [CrossRef]
- Vaschetto, R.; Gregoretti, C.; Scotti, L.; De Vita, N.; Carlucci, A.; Cortegiani, A.; Crimi, C.; Mattei, A.; Scala, R.; Rocca, E.; et al. A pragmatic, open-label, multi-center, randomized controlled clinical trial on the rotational use of interfaces vs standard of care in patients treated with noninvasive positive pressure ventilation for acute hypercapnic respiratory failure: The ROTAtional-USE of interface STUDY (ROTA-USE STUDY). Trials 2023, 24, 527. [Google Scholar]
- Pierucci, P.; Portacci, A.; Carpagnano, G.E.; Banfi, P.; Crimi, C.; Misseri, G.; Gregoretti, C. The right interface for the right patient in noninvasive ventilation: A systematic review. Expert Rev. Respir. Med. 2022, 16, 931–944. [Google Scholar] [CrossRef]
- Vitale, F.; Misseri, G.; Ingoglia, G.; Bonanno, G.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Fake news and patient-family-physician interaction in critical care: Concepts, beliefs and potential countermeasures. Anaesthesiol. Intensive Ther. 2020, 52, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Misseri, G.; Piattoli, M.; Cuttone, G.; Gregoretti, C.; Bignami, E.G. Artificial Intelligence for Mechanical Ventilation: A Transformative Shift in Critical Care. Ther. Adv. Pulm. Crit. Care Med. 2024, 19, 29768675241298918. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuttone, G.; La Via, L.; Pappalardo, F.; Sorbello, M.; Paternò, D.S.; Piattoli, M.; Gregoretti, C.; Misseri, G. An Updated Review on the Use of Noninvasive Respiratory Supports in the Management of Severe Asthma Exacerbations. Medicina 2025, 61, 328. https://doi.org/10.3390/medicina61020328
Cuttone G, La Via L, Pappalardo F, Sorbello M, Paternò DS, Piattoli M, Gregoretti C, Misseri G. An Updated Review on the Use of Noninvasive Respiratory Supports in the Management of Severe Asthma Exacerbations. Medicina. 2025; 61(2):328. https://doi.org/10.3390/medicina61020328
Chicago/Turabian StyleCuttone, Giuseppe, Luigi La Via, Federico Pappalardo, Massimiliano Sorbello, Daniele Salvatore Paternò, Matteo Piattoli, Cesare Gregoretti, and Giovanni Misseri. 2025. "An Updated Review on the Use of Noninvasive Respiratory Supports in the Management of Severe Asthma Exacerbations" Medicina 61, no. 2: 328. https://doi.org/10.3390/medicina61020328
APA StyleCuttone, G., La Via, L., Pappalardo, F., Sorbello, M., Paternò, D. S., Piattoli, M., Gregoretti, C., & Misseri, G. (2025). An Updated Review on the Use of Noninvasive Respiratory Supports in the Management of Severe Asthma Exacerbations. Medicina, 61(2), 328. https://doi.org/10.3390/medicina61020328









