Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
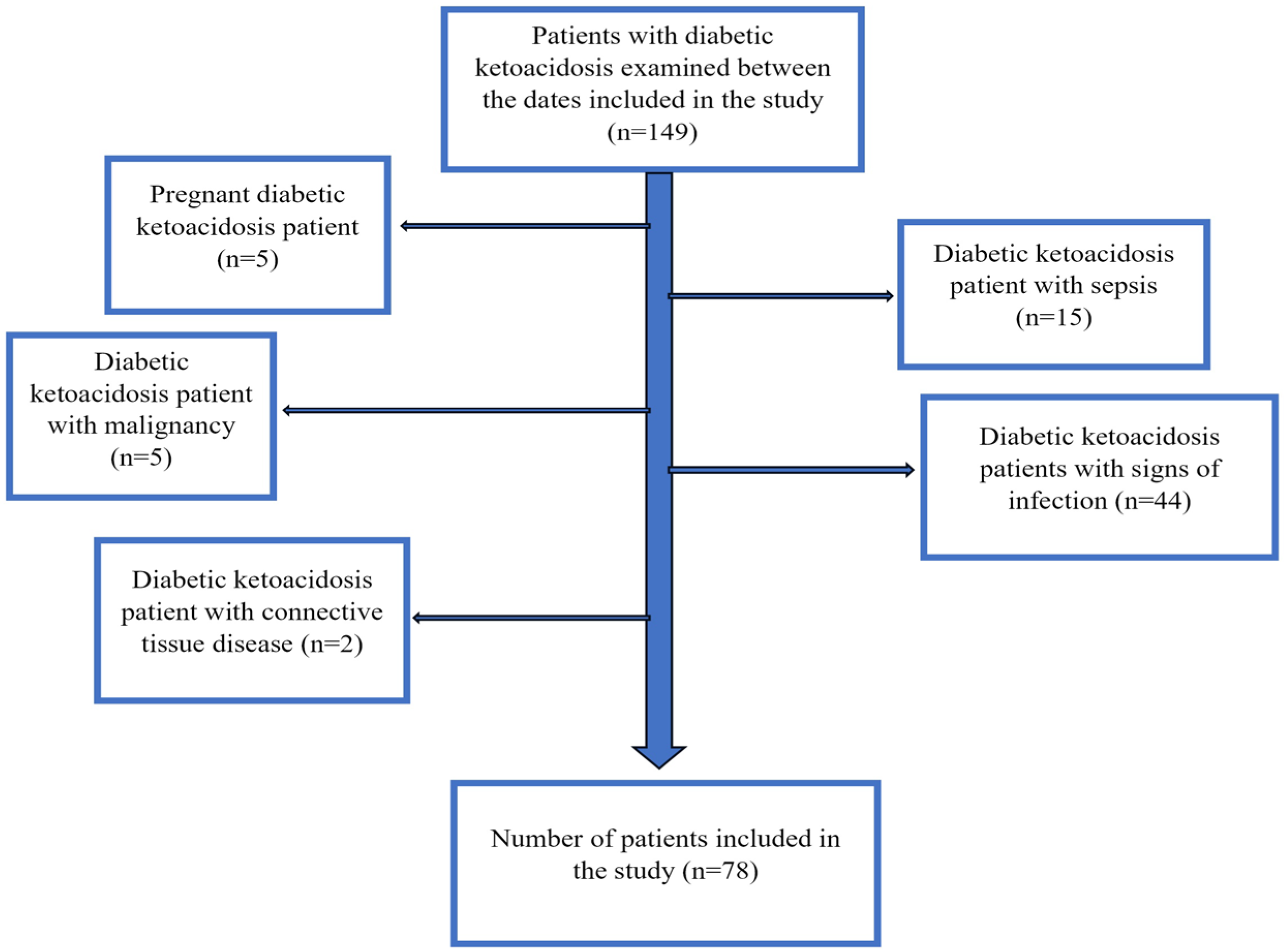
2.2. Study Population
2.3. Data Collection and Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DKA | Diabetic ketoacidosis |
| DM | Diabetes mellitus |
| ICU | Intensive care unit |
| NLR | Neutrophil-to-lymphocyte ratio |
| CAR | CRP-to-albumin ratio |
| IQR | Interquartile range |
| VIF | Variance Inflation Factor |
References
- Zhong, V.W.; Juhaeri, J.; Mayer-Davis, E.J. Trends in Hospital Admission for Diabetic Ketoacidosis in Adults with Type 1 and Type 2 Diabetes in England, 1998–2013: A Retrospective Cohort Study. Diabetes Care 2018, 41, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.G.; Sousa, L.P.; Sousa, M.O.; Pietrani, N.T.; Fernandes, A.P.; Gomes, K.B. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 99, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- von Herrath, M.; Bain, S.C.; Bode, B.; Clausen, J.O.; Coppieters, K.; Gaysina, L.; Gumprecht, J.; Hansen, T.K.; Mathieu, C.; Morales, C.; et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021, 9, 212–224. [Google Scholar] [CrossRef]
- Glaser, N.; Chu, S.; Weiner, J.; Zdepski, L.; Wulff, H.; Tancredi, D.; ODonnell, M.E. Effects of TRAM-34 and minocycline on neuroinflammation caused by diabetic ketoacidosis in a rat model. BMJ Open Diabetes Res. Care 2022, 10, e002777. [Google Scholar] [CrossRef]
- Gogos, C.A.; Giali, S.; Paliogianni, F.; Dimitracopoulos, G.; Bassaris, H.P.; Vagenakis, A.G. Interleukin-6 and C-reactive protein as early markers of sepsis in patients with diabetic ketoacidosis or hyperosmosis. Diabetologia 2001, 44, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Karavanaki, K.; Kakleas, K.; Georga, S.; Bartzeliotou, A.; Mavropoulos, G.; Tsouvalas, M.; Vogiatzi, A.; Papassotiriou, I.; Karayianni, C. Plasma high sensitivity C-reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clin. Biochem. 2012, 45, 1383–1388. [Google Scholar] [CrossRef]
- Kethireddy, R.; Gandhi, D.; Kichloo, A.; Patel, L. Challenges in hyperglycemia management in critically ill patients with COVID-19. World J. Crit. Care Med. 2022, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Ning, B. Evaluating IL-6 and IL-10 as rapid diagnostic tools for Gram-negative bacteria and as disease severity predictors in pediatric sepsis patients in the intensive care unit. Front. Immunol. 2022, 13, 1043968. [Google Scholar] [CrossRef]
- Kumarasamy, C.; Sabarimurugan, S.; Madurantakam, R.M.; Lakhotiya, K.; Samiappan, S.; Baxi, S.; Nachimuthu, R.; Gothandam, K.M.; Jayaraj, R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine 2019, 98, e14834. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, A.; Dymicka-Piekarska, V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023, 16, 69–81. [Google Scholar] [CrossRef]
- Kang, P.; Kang, W.; Li, Y.; Li, T. C-Reactive Protein-to-Albumin Ratio as an Early Biomarker to Identify Sepsis in Neonates with Pneumonia. Mediat. Inflamm. 2022, 2022, 4711018. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Chung, K.S.; Song, J.H.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; et al. The C-Reactive Protein/Albumin Ratio as a Predictor of Mortality in Critically Ill Patients. J. Clin. Med. 2018, 7, 333. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycaemic crises in adults with diabetes: A consensus report. Diabetologia 2024, 67, 1455–1479. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell. Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Mashaba, R.G.; Phoswa, W.N.; Lebelo, S.L. Curcumin Attenuates Hyperglycemia and Inflammation in Type 2 Diabetes Mellitus: Quantitative Analysis of Randomized Controlled Trial. Nutrients 2024, 16, 4177. [Google Scholar] [CrossRef]
- Pawluk, H.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Woźniak, A.; Grzechowiak, E.; Szumny, M.; Sobolewski, P.; Bieniaszewski, L.; Kozera, G. Effect of IL-6 and hsCRP Serum Levels on Functional Prognosis in Stroke Patients Undergoing IV-Thrombolysis: Retrospective Analysis. Clin. Interv. Aging 2020, 15, 1295–1303. [Google Scholar] [CrossRef]
- Yoon, J.A.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Ahn, H.J.; Jeon, S.Y.; Kim, D.; Kang, C. Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest. Brain Sci. 2024, 14, 779. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Simon, D.W.; Wagner, A.K. Targeting interleukin-6 after cardiac arrest-Let us not forget the brain. Resuscitation 2023, 184, 109715. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.R.; Hoffman, W.H.; Passmore, G.G.; Martin, S.L.A. Plasma C-reactive protein levels in severe diabetic ketoacidosis. Ann. Clin. Lab. Sci. 2003, 33, 435–442. [Google Scholar]
- Aslan Sirakaya, H. Evaluating Monocyte-to-high-density Lipoprotein Ratio Across Age and Gender in Healthy Individuals. Bağcılar Tıp Bülteni 2024, 9, 38–43. [Google Scholar] [CrossRef]
- Aslan Sirakaya, H.; Sirakaya, E. Association of triglyceride-glucose index in branch retinal vein occlusion. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2024, 262, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Sirakaya, E.; Duru, Z.; Kuçuk, B.; Duru, N. Monocyte to high-density lipoprotein and neutrophil-to-lymphocyte ratios in patients with acute central serous chorioretinopathy. Indian J. Ophthalmol. 2020, 68, 854–858. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n:78) | Moderate DKA (n:32) | Severe DKA (n:46) | p-Value | |
|---|---|---|---|---|
| Sex (male/female) | 29/49 | 11/21 (34.4/65.6) | 18/28 (39.1/60.9) | 0.923 |
| Age (years) | 46.01 ± 19.82 | 44.56 ± 20.58 | 45.02 ± 19.55 | 0.673 |
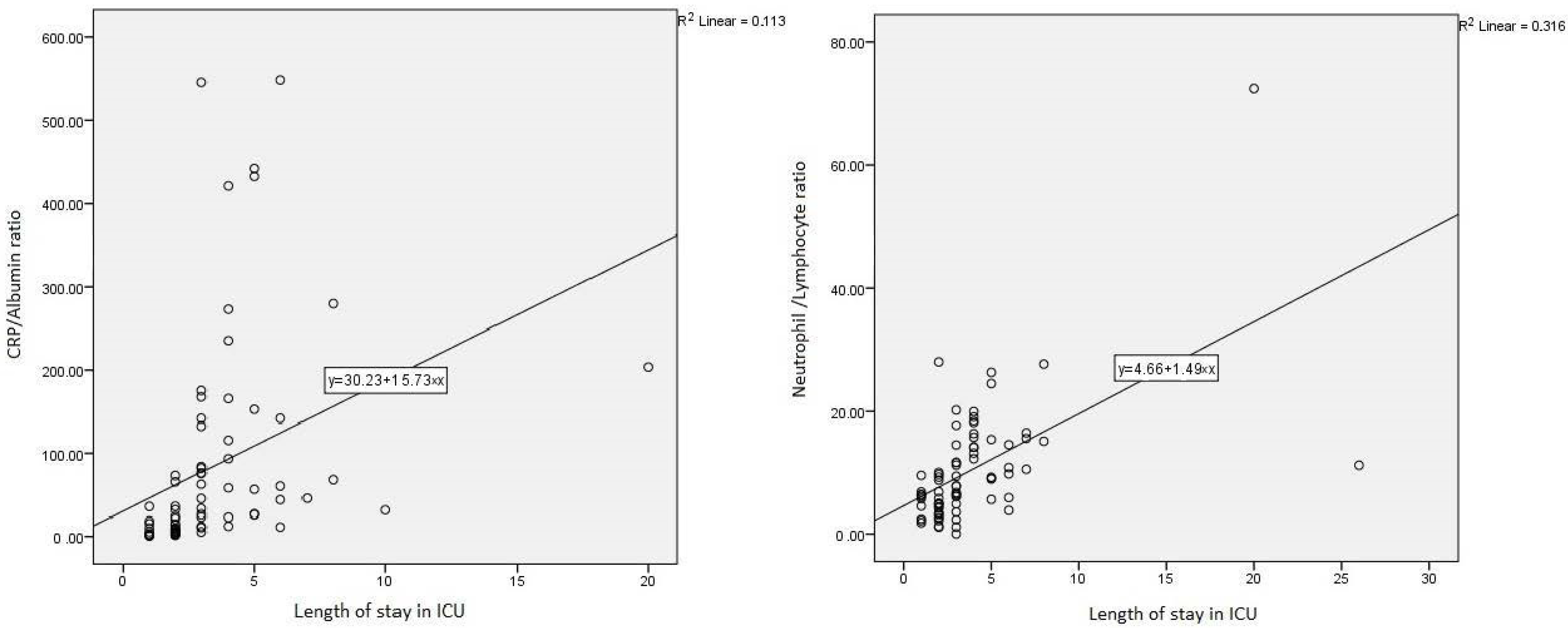
| Intensive Care Hospitalization Duration (days) | 3.78 (1–26) | 2.00 (1–6) | 3.00 (1–26) | 0.001 |
| GCS score | 13.92 ± 1.06 | 14.03 ± 1.06 | 13.84 ± 1.07 | 0.458 |
| APACHE II score | 22.85 ± 5.83 | 21.78 ± 6.00 | 23.60 ± 5.65 | 0.175 |
| NUTRIC score | 3.78 ± 1.69 | 3.56 ± 1.72 | 3.93 ± 1.67 | 0.109 |
| BMI (kg/m2) | 24.12 ± 2.28 | 24.42 ± 2.25 | 23.84 ± 2.33 | 0.889 |
| Glukoz (mg/dL) | 573.55 ± 198.29 | 567.89 ± 236.228 | 596.76 ± 182.63 | 0.698 |
| HbA1C (%) | 11.68 ± 2.43 | 11.88 ± 2.51 | 11.88 ± 2.25 | 0.995 |
| CRP (mg/L) | 17.05 (0.3–386) | 8.90 (0.6–386) | 19.05 (0.3–292.5) | 0.541 |
| Albumin (mg/dL) | 38.50 ± 7.67 | 37.19 ± 7.89 | 38.93 ± 7.54 | 0.656 |
| pH | 7.09 ± 0.13 | 7.20 ± 0.07 | 7.01 ± 0.11 | <0.001 |
| HCO3 (mEq/L) | 9.71 ± 3.70 | 13.58 ± 2.11 | 7.11 ± 1.91 | <0.001 |
| PCO2 (mmHg) | 24.46 ± 7.09 | 29.45 ± 6.27 | 20.35 ± 4.91 | <0.001 |
| Lactate (mmol/L) | 3.05 (1.20–12.10) | 2.80 (1.20–12.10) | 3.25 (1.20–9.40) | 0.615 |
| White blood cell (103/μL) | 15.89 ± 8.90 | 14.49 ± 7.82 | 17.56 ± 9.84 | 0.995 |
| Absolute neutrophil count (103/μL) | 13.37 ± 7.96 | 11.77 ± 7.26 | 15.10 ± 8.55 | 0.106 |
| Absolute lymphocyte count (103/μL) | 1.76 ± 1.02 | 1.75 ± 1.17 | 1.81 ± 0.94 | 0.941 |
| CRP/albumin ratio | 35.82 (0.57–1289.67) | 23.12 (1.94–1289.67) | 51.00 (0.57–1063.33) | 0.600 |
| Neutrophil/lymphocyte ratio | 8.89 (0.03–72.43) | 9.28 (1.11–72.43) | 9.03 (0.03–27.64) | 0.473 |
| Interleukin 6 (pg/mL) | 40.40 (5.04–460) | 19.70 (5.60–73.10) | 50.35 (5.04–460) | 0.001 |
| r-Value | p-Value | |
|---|---|---|
| IL-6 | 0.813 | <0.001 |
| Degree of ketoacidosis | 0.475 | <0.001 |
| CRP/albumin ratio | 0.336 | <0.001 |
| Neutrophil/lymphocyte ratio | 0.562 | <0.001 |
| IRR | z | p | 95% CI | |
|---|---|---|---|---|
| IL-6 | 1.04 | 7.77 | <0.001 | 1.02–1.06 |
| HCO3 | −0.44 | −2.47 | 0.014 | −0.80–−0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan Sirakaya, H.; Sipahioglu, H.; Cetinkaya, A.; Aydin, K. Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes. Medicina 2025, 61, 321. https://doi.org/10.3390/medicina61020321
Aslan Sirakaya H, Sipahioglu H, Cetinkaya A, Aydin K. Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes. Medicina. 2025; 61(2):321. https://doi.org/10.3390/medicina61020321
Chicago/Turabian StyleAslan Sirakaya, Hatice, Hilal Sipahioglu, Ali Cetinkaya, and Kaniye Aydin. 2025. "Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes" Medicina 61, no. 2: 321. https://doi.org/10.3390/medicina61020321
APA StyleAslan Sirakaya, H., Sipahioglu, H., Cetinkaya, A., & Aydin, K. (2025). Relationship Between Inflammatory Markers (IL-6, Neutrophil–Lymphocyte Ratio, and C-Reactive Protein-Albumin Ratio) and Diabetic Ketoacidosis Severity: Correlation with Clinical Outcomes. Medicina, 61(2), 321. https://doi.org/10.3390/medicina61020321







