Torque Loss, Survival, and Strain Distribution of Implant-Supported Prostheses with Zirconia and Cobalt–Chromium Hybrid Abutments
Abstract
1. Introduction
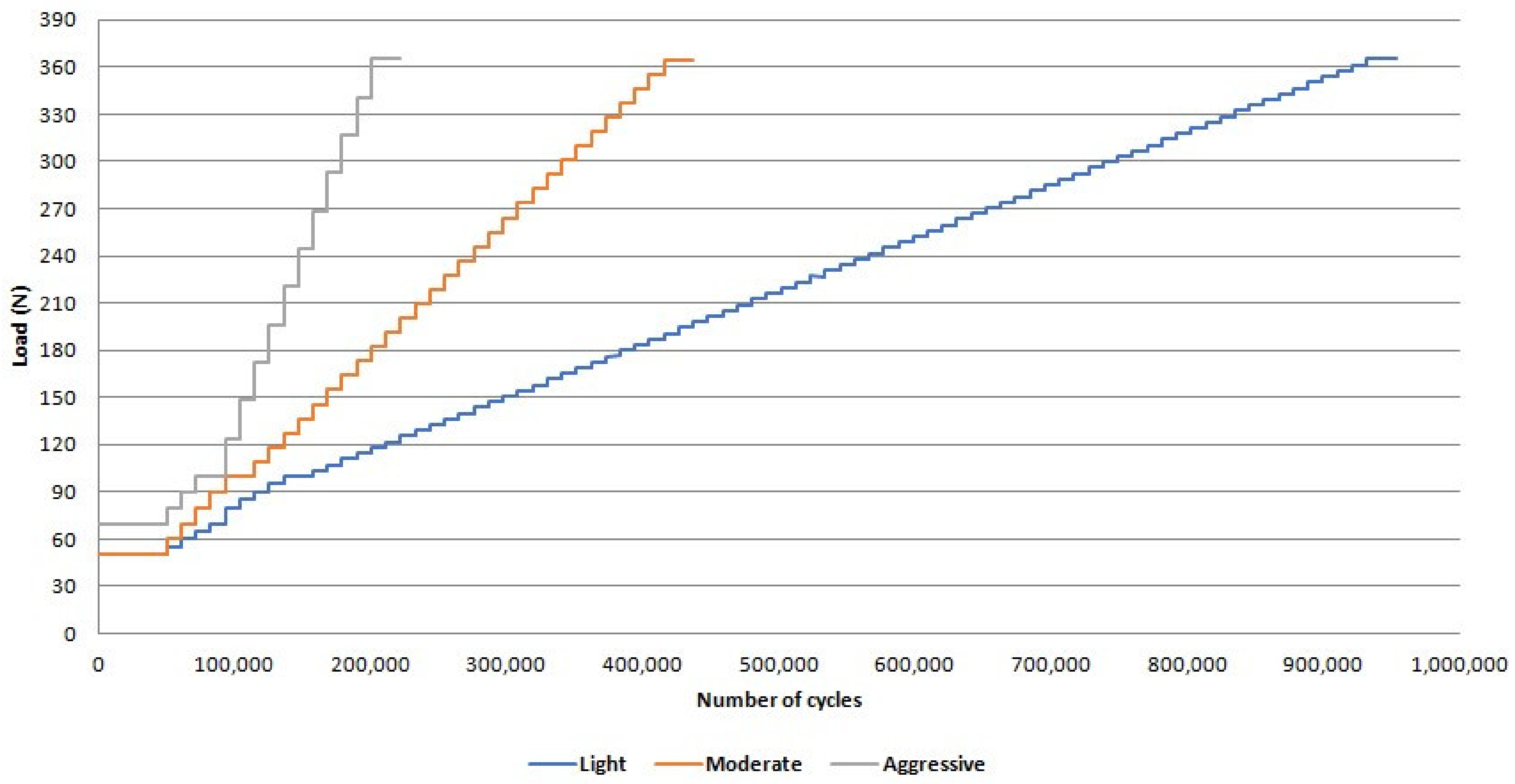
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- There was no difference between torque loss of zirconia and Co-Cr abutments after the thermomechanical cycling;
- There was no difference between survival and reliability of zirconia and Co-Cr abutments for the evaluated missions after the step-stress accelerated life testing;
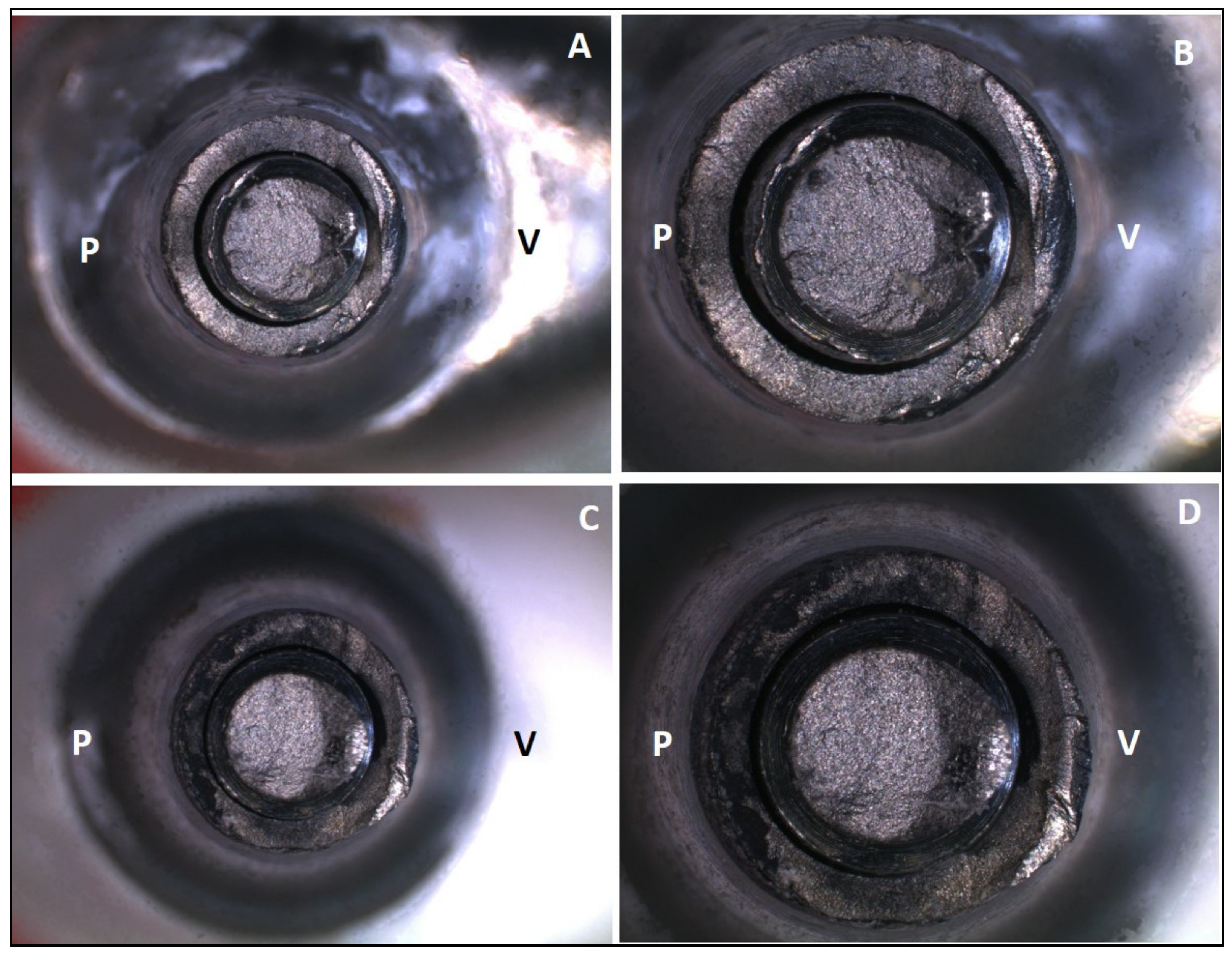
- Failure mode was similar for zirconia and Co-Cr hybrid abutments, and fracture occurred in the titanium base ;
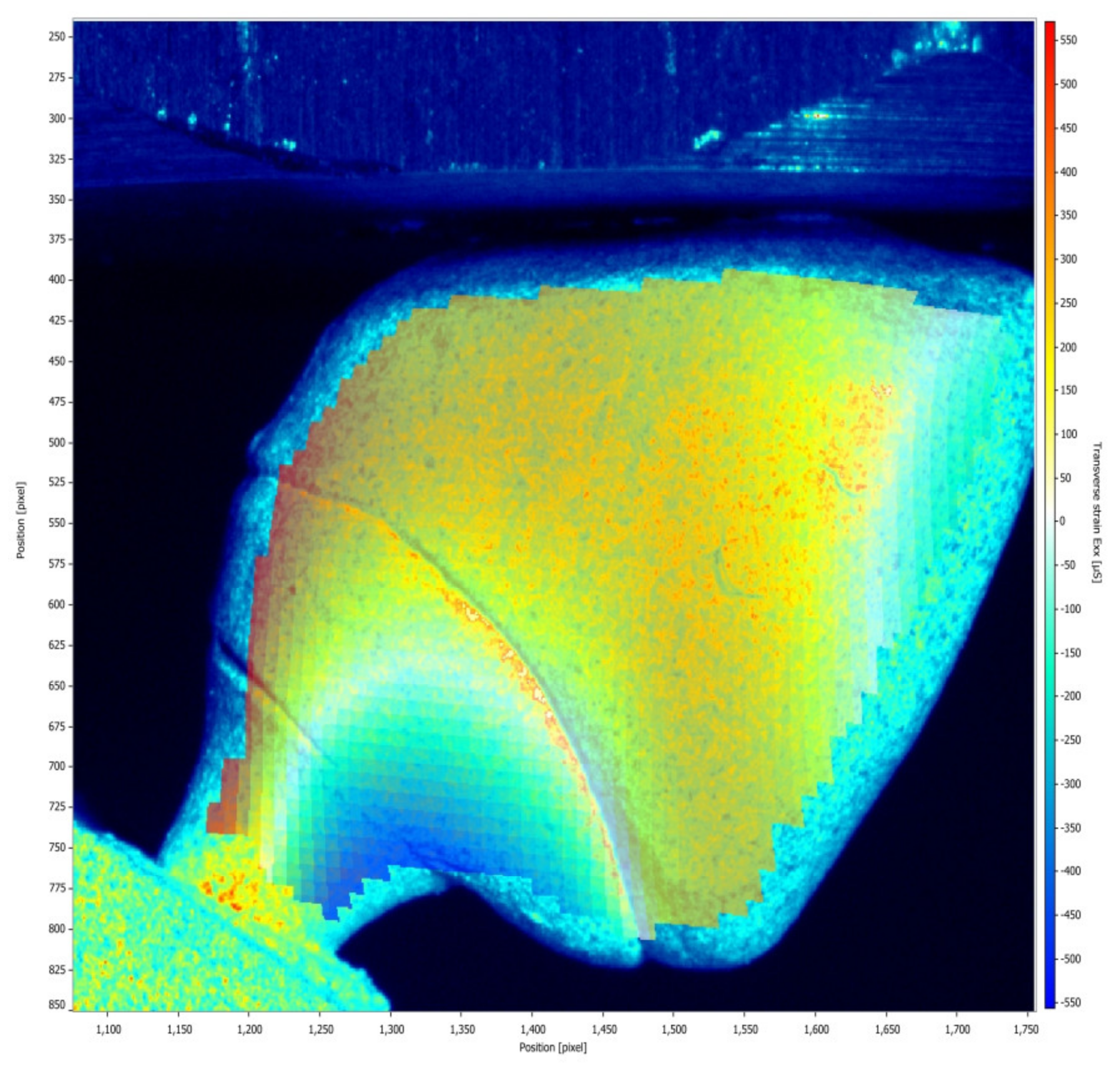
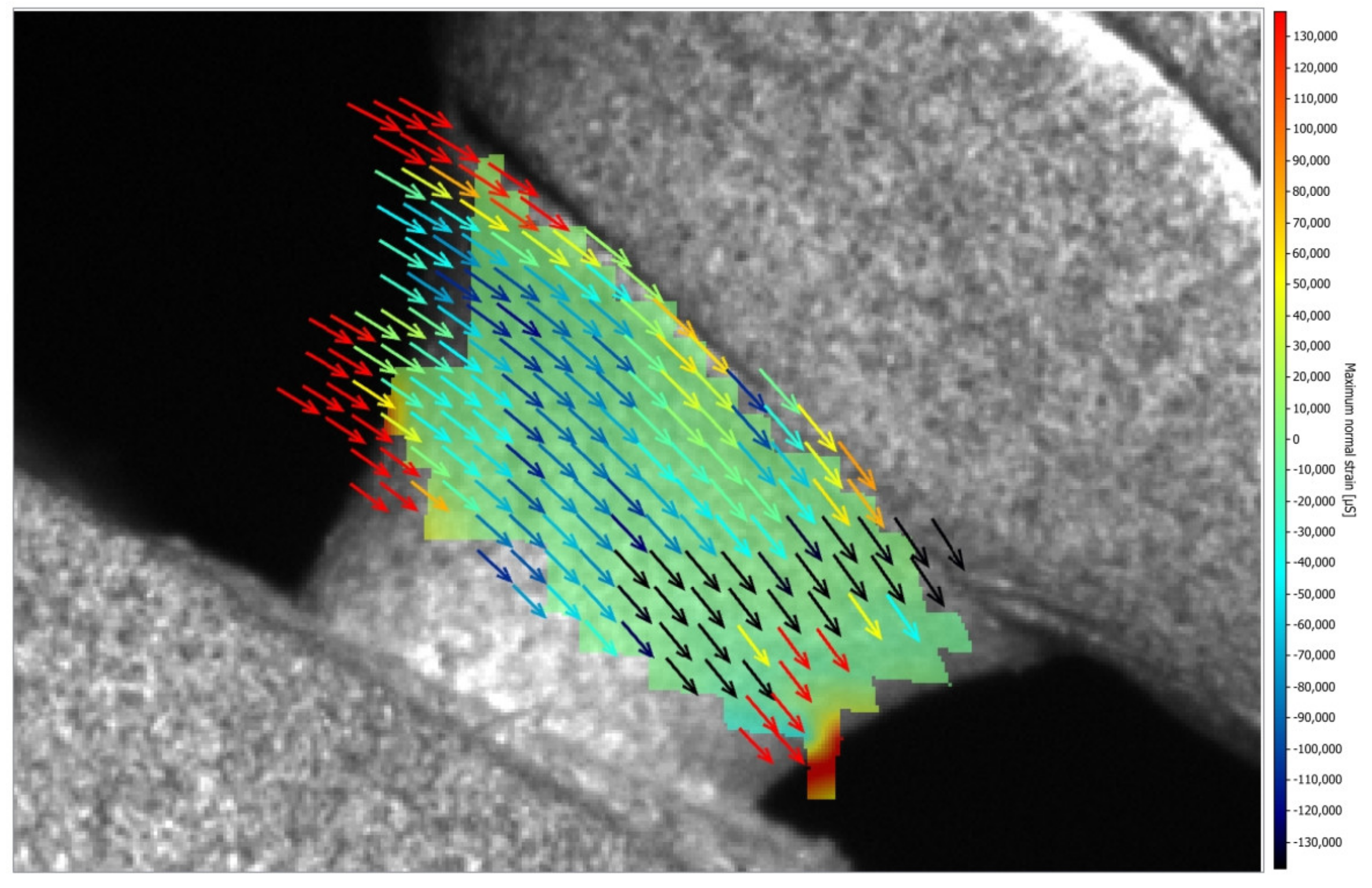
- The model of zirconia abutment presented only compression strain, while the model of Co-Cr abutment presented compression and tensile strains; however, all strains are within acceptable limits, indicating the safe use of both types of hybrid abutments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaleli, N.; Sarac, D.; Külünk, S.; Öztürk, O. Effect of different restorative crown and customized abutment materials on stress distribution in single implants and peripheral bone: A three-dimensional finite element analysis study. J. Prosthet. Dent. 2018, 119, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Farrag, G.; Chaar, M.S.; Abdelnabi, N.; Kern, M. Influence of different CAD/CAM crown materials on the fracture of custom-made titanium and zirconia implant abutments after artificial aging. Int. J. Prosthodont. 2019, 32, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Al-Zordk, W.; Elmisery, A.; Ghazy, M. Hybrid-abutment-restoration: Effect of material type on torque maintenance and fracture resistance after thermal aging. Int. J. Implants Dent. 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical evaluations of the long-term stability of dental implant using finite element modeling method: A systematic review. J. Adv. Prosthodont. 2022, 14, 182–202. [Google Scholar] [CrossRef]
- Sailer, I.; Makarov, N.A.; Thoma, D.S.; Zwahlen, M.; Pjetursson, B.E. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent. Mater. 2015, 31, 603–623. [Google Scholar] [CrossRef]
- Bidra, A.S.; Huynh-Ba, G. Implants in the pterygoid region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 773–781. [Google Scholar] [CrossRef]
- Wang, J.; Wu, P.; Liu, H.L.; Zhang, L.; Liu, L.P.; Ma, C.F.; Chen, J.H. Polyetheretherketone versus titanium CAD-CAM framework for implant-supported fixed complete dentures: A retrospective study with up to 5-year follow-up. J. Prosthodont. Res. 2022, 66, 279–287. [Google Scholar] [CrossRef]
- Pereira, A.K.H.C.; Limirio, J.P.J.O.; Vasconcelos, B.C.E.; Pellizzer, E.P.; de Moraes, S.L.D. Mechanical behavior of titanium and zirconia abutments at the implant-abutment interface: A systematic review. J. Prosthet. Dent. 2022, 131, 420–426. [Google Scholar] [CrossRef]
- Kaenploy, J.; Li, R.; Makowka, S.; Sadid-Zadeh, R. Fracture resistance of cement-retained lithium disilicate implant-supported crowns: Effect of material used for two-piece abutments. J. Prosthodont. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, B.J.; Kim, S.H.; Lee, K.B. Shear bond strength of porcelain to a new millable alloy and a conventional castable alloy. J. Prosthet. Dent. 2015, 113, 329–335. [Google Scholar] [CrossRef]
- França, D.G.B.; Morais, M.H.S.T.; Neves, F.D.; Barbosa, G.A.S. Influence of CAD/CAM on the fit accuracy of implant-supported zirconia and cobalt-chromium fixed dental prostheses. J. Prosthet. Dent. 2015, 113, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Barucca, G.; Santecchia, E.; Majni, G.; Girardin, E.; Bassoli, E.; Denti, L.; Gatto, A.; Iuliano, L.; Moskalewicz, T.; Mengucci, P. Structural characterization of biomedical Co–Cr–Mo components produced by direct metal laser sintering. Mater. Sci. Eng. C 2015, 48, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.M.; Rodrigues, R.C.; de Mattos, M.G.; Ribeiro, R.F. The effect of commercially pure titanium and alternative dental alloys on the marginal fit of one-piece cast implant frameworks. J. Dent. 2007, 35, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kocaağaoğlu, H.; Kılınç, H.İ.; Albayrak, H.; Kara, M. In vitro evaluation of marginal, axial, and occlusal discrepancies in metal ceramic restorations produced with new technologies. J. Prosthet. Dent. 2016, 116, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gungor, M.B.; Nemli, S.K.; Yilmaz, H.; Aydin, C. Fracture resistance of different implant supported ceramic abutment/crown systems. Eur. Oral Res. 2019, 53, 80–87. [Google Scholar]
- Silva, N.R.; Teixeira, H.S.; Silveira, L.M.; Bonfante, E.A.; Coelho, P.G.; Thompson, V.P. Reliability and failure modes of a hybrid ceramic abutment prototype. J. Prosthodont 2018, 27, 83–87. [Google Scholar] [CrossRef]
- Nouh, I.; Kern, M.; Sabet, A.E.; Aboelfadl, A.K.; Hamdy, A.M.; Chaar, M.S. Mechanical behavior of posterior all-ceramic hybrid-abutment-crowns versus hybrid-abutments with separate crowns-A laboratory study. Clin. Oral Implants Res. 2019, 30, 90–98. [Google Scholar] [CrossRef]
- Pitta, J.; Hicklin, S.P.; Fehmer, V.; Boldt, J.; Gierthmuehlen, P.C.; Sailer, I. Mechanical stability of zirconia meso-abutments bonded to titanium bases restored with different monolithic all-ceramic crowns. Int. J. Oral Maxillofac. Implants 2019, 34, 1091–1097. [Google Scholar] [CrossRef]
- Pitta, J.; Hicklin, S.P.; Fehmer, V.; Boldt, J.; Gierthmuehlen, P.C.; Sailer, I. Mechanical stability and technical outcomes of monolithic CAD/CAM fabricated abutment-crowns supported by titanium bases: An in vitro study. Clin. Oral Implants Res. 2021, 32, 222–232. [Google Scholar] [CrossRef]
- Izadi, A.; Vafaee, F.; Shishehian, A.; Roshanaei, G.; Afkari, B.F. Evaluation of dimensional accuracy of dental bridges manufactured with conventional casting technique and CAD/CAM system with ceramill sintron blocks using CMM. J. Dent. Res. Dent. Clin. Dent. Prospect 2018, 12, 264–271. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Del Río Highsmith, J.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of CAD/CAM Cobalt-Chrome and Zirconia Abutments on a Conical Connection Implant: An In Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Mourelle, P.; Roccuzzo, A.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Highsmith, J.D.R.; Gómez-Polo, M. Microleakage assessment of CAD-CAM Cobalt-Chrome and Zirconia abutments on a conical connection dental implant: A comparative in vitro study. Clin. Oral Implants Res. 2022, 33, 945–952. [Google Scholar] [CrossRef]
- Vohra, F.; Alsaif, R.; Khan, R.; Bukhari, I.A. Comparison of De-Torque and Failure Load Evaluation of Selective-Laser-Sintered CoCr, CAD-CAM ZrO, and Machined Implant Abutment/Restoration. Bioengineering 2024, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Coelho, P.G.; Guess, P.C.; Thompson, V.P.; Silva, N.R. Fatigue and damage accumulation of veneer porcelain pressed on Y-TZP. J. Dent. 2010, 38, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Coelho, P.G. A critical perspective on mechanical testing of implants and prostheses. Adv. Dent. Res. 2016, 28, 18–27. [Google Scholar] [CrossRef]
- ISO 14801:2016; Dentistry—Implants—Dynamic Loading Test for Endosseous Dental Implants. International Organization for Standardization: Geneva, Switzerland, 2016.
- Moris, I.C.M.; Faria, A.C.L.; Ribeiro, R.F.; Rodrigues, R.C.S. Torque loss of differente abutment sizes before and after cyclic loading. Int. J. Oral Maxillofac. Implants 2015, 30, 1256–1261. [Google Scholar] [CrossRef]
- Dittmer, M.; Dittmer, S.; Borchers, L.; Kohorst, P.; Stiesch, M. Influence of the interface design on the yield force of the implant—Abutment complex before and after cyclic mechanical loading. J. Prosthodont. Res. 2012, 56, 19–24. [Google Scholar] [CrossRef]
- Yilmaz, B.; Gouveia, D.; Seghi, R.; Johnston, W.; Lang, L.A. Effect of crown height on the screw joint stability of zirconia screw-retained crowns. J. Prosthet. Dent. 2021, 128, 1328–1334. [Google Scholar] [CrossRef]
- Barbosa-Júnior, S.A.; Pereira, G.K.R.; Dapieve, K.S.; Machado, P.S.; Valandro, L.F.; Schuh, C.; Consani, R.L.X.; Bacchi, A. Mechanical Fatigue Analysis of PEEK as Alternative to Zirconia for Definitive Hybrid Abutments Supporting All-Ceramic Crowns. Int. J. Oral Maxillofac. Implants 2020, 35, 1209–1217. [Google Scholar] [CrossRef]
- Nishigawa, K.; Bando, E.; Nakano, M. Quantitative study of biteforce during sleep associated bruxism. J. Oral Rehabil. 2001, 28, 485–491. [Google Scholar] [CrossRef]
- Moris, I.C.M.; Chen, Y.C.; Faria, A.C.L.; Ribeiro, R.F.; Fok, A.S.; Rodrigues, R.C.S. Fracture loads and failure modes of customized and non-customized zirconia abutments. Dent. Mater. 2018, 34, e197–e204. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, D.; Muftu, S.; Muftu, A. Evaluation of load transfer characteristics of five different implants in compact bone at different load levels by finite elements analysis. J. Prosthet. Dent. 2004, 92, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–10101. [Google Scholar] [CrossRef] [PubMed]
- Duyck, J.; Ronold, H.J.; Van Oosterwyck, H.; Naert, I.; Vander Sloten, J.; Ellingsen, J.E. The influence of static and dynamic loading on marginal bone reactions around osseointegrated implants: An animal experimental study. Clin. Oral Implants Res. 2001, 12, 207–218. [Google Scholar] [CrossRef]







| Groups | Thermomechanical Cycling | Mean | Standard Deviation | Lower Limit | Upper Limit |
|---|---|---|---|---|---|
| Co-Cr | Before | 22.18 Aa | 7.43 | 11.30 | 35.00 |
| After | 42.75 Ab | 24.57 | −17.30 | 66.90 | |
| Zirconia | Before | 18.75 Aa | 9.70 | 6.20 | 32.20 |
| After | 40.71 Ab | 28.20 | −6.80 | 78.20 |
| Groups | ||
|---|---|---|
| Co-Cr | Zirconia | |
| Upper limit | 5.56 | 3.54 |
| β | 3.51 | 1.91 |
| Lower limit | 2.21 | 1.03 |
| Missions | Groups | Upper Limit | Reliability 200 N | Lower Limit |
|---|---|---|---|---|
| 200,000 cycles | Co-Cr | 99 | 96 | 81 |
| Zirconia | 97 | 91 | 71 | |
| 300,000 cycles | Co-Cr | 93 | 83 | 62 |
| Zirconia | 91 | 81 | 61 |
| Missions | Groups | Upper Limit | Reliability 300 N | Lower Limit |
|---|---|---|---|---|
| 200,000 cycles | Co-Cr | 98 | 85 | 31 |
| Zirconia | 95 | 76 | 19 | |
| 300,000 cycles | Co-Cr | 87 | 52 | 5 |
| Zirconia | 86 | 54 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.C.S.; Fiorin, L.; Faria, A.C.L.; Bonfante, E.A.; Ribeiro, R.F. Torque Loss, Survival, and Strain Distribution of Implant-Supported Prostheses with Zirconia and Cobalt–Chromium Hybrid Abutments. Medicina 2025, 61, 274. https://doi.org/10.3390/medicina61020274
Rodrigues RCS, Fiorin L, Faria ACL, Bonfante EA, Ribeiro RF. Torque Loss, Survival, and Strain Distribution of Implant-Supported Prostheses with Zirconia and Cobalt–Chromium Hybrid Abutments. Medicina. 2025; 61(2):274. https://doi.org/10.3390/medicina61020274
Chicago/Turabian StyleRodrigues, Renata Cristina Silveira, Lívia Fiorin, Adriana Cláudia Lapria Faria, Estevam Augusto Bonfante, and Ricardo Faria Ribeiro. 2025. "Torque Loss, Survival, and Strain Distribution of Implant-Supported Prostheses with Zirconia and Cobalt–Chromium Hybrid Abutments" Medicina 61, no. 2: 274. https://doi.org/10.3390/medicina61020274
APA StyleRodrigues, R. C. S., Fiorin, L., Faria, A. C. L., Bonfante, E. A., & Ribeiro, R. F. (2025). Torque Loss, Survival, and Strain Distribution of Implant-Supported Prostheses with Zirconia and Cobalt–Chromium Hybrid Abutments. Medicina, 61(2), 274. https://doi.org/10.3390/medicina61020274








