Immunohistochemical Expression of Differentiated Embryonic Chondrocyte 1 and Cluster of Differentiation 44 in Oral Potentially Malignant Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Immunohistochemical Analysis
2.3. Antibodies
2.4. Evaluation of Score
2.5. Statistical Analysis
3. Results
3.1. The Characteristics of the Study Specimens
3.2. Comparison of DEC1 and CD44 Immunoscores Among Patients with Oral Leukoplakia, Oral Lichen Planus, and Actinic Cheilitis
3.3. Comparison of DEC1 and CD44 Immunoscores Across Different Types of Oral Lesions and Levels of Dysplasia
3.4. Analysis of DEC1 and CD44 Immunoscores Between Normal Mucosa, Oral Premalignant Lesions, and Oral Squamous Cell Carcinoma
4. Discussion
5. Conclusions
- -
- The analysis of DEC1 and CD44 markers revealed important differences, demonstrating their complementary roles in diagnosing and managing premalignant and malignant oral lesions;
- -
- CD44 expression progressively increased from normal epithelium to dysplastic lesions and carcinoma, highlighting its relevance for the early diagnosis of dysplasia;
- -
- Analyzing DEC1, as a marker of early-stage lesions, and CD44, as an indicator of tumor progression, is a possible synergistic approach for optimal clinical management.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sreekumar, V.N. Global Scenario of Research in Oral Cancer. J. Maxillofac. Oral Surg. 2019, 18, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Ray, J.G. Oral potentially malignant disorders: Revisited. J. Oral Maxillofac. Pathol. 2017, 21, 326–327. [Google Scholar] [CrossRef]
- Onofrei, B.A.; Popa, C.; Sciuca, A.M.; Toader, M.P.; Condurache Hrițcu, O.M.; Baciu, E.R.; Ciobanu-Apostol, D.G.; Costan, V.V. Potentially malignant lesions in the oral cavity: A retrospective analysis. Rom. J. Oral Rehabil. 2024, 16, 327–333. [Google Scholar] [CrossRef]
- Evren, I.; Brouns, E.R.; Wils, L.J.; Poell, J.B.; Peeters, C.F.W.; Brakenhoff, R.H.; Bloemena, E.; de Visscher, J.G.A.M. Annual malignant transformation rate of oral leukoplakia remains consistent: A long-term follow-up study. Oral Oncol. 2020, 110, 105014. [Google Scholar] [CrossRef] [PubMed]
- Carrard, V.C.; van der Waal, I. A clinical diagnosis of oral leukoplakia; A guide for dentists. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e59–e64. [Google Scholar] [CrossRef]
- Błochowiak, K.; Farynowska, J.; Sokalski, J.; Wyganowska-Świątkowska, M.; Witmanowski, H. Benign tumours and tumour-like lesions in the oral cavity: A retrospective analysis. Postępy Dermatol. Alergol. 2019, 36, 744–751. [Google Scholar] [CrossRef]
- Chiang, C.P.; Yu-Fong Chang, J.; Wang, Y.P.; Wu, Y.H.; Lu, S.Y.; Sun, A. Oral lichen planus—Differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2018, 117, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Poitevin, N.A.; Rodrigues, M.S.; Weigert, K.L.; Macedo, C.L.R.; Dos Santos, R.B. Actinic cheilitis: Proposition and reproducibility of a clinical criterion. BDJ Open 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Varela-Centelles, P.; Seoane-Romero, J.; García-Pola, M.J.; Leira-Feijoo, Y.; Seoane-Romero, J.M. Therapeutic approaches for actinic cheilitis: Therapeutic efficacy and malignant transformation after treatment. Int. J. Oral Maxillofac. Surg. 2020, 49, 1343–1350. [Google Scholar] [CrossRef]
- Bi, H.; Li, S.; Qu, X.; Wang, M.; Bai, X.; Xu, Z.; Ao, X.; Jia, Z.; Jiang, X.; Yang, Y.; et al. DEC1 regulates breast cancer cell proliferation by stabilizing cyclin E protein and delays the progression of cell cycle S phase. Cell Death Dis. 2015, 6, e1891. [Google Scholar] [CrossRef]
- You, J.; Lin, L.; Liu, Q.; Zhu, T.; Xia, K.; Su, T. The correlation between the expression of differentiated embryo-chondrocyte expressed gene l and oral squamous cell carcinoma. Eur. J. Med. Res. 2014, 19, 21. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.; Wu, K. The role of CD44 in epithelial–mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792. [Google Scholar]
- Zhang, H.; Cao, H.; Luo, H.; Zhang, N.; Wang, Z.; Dai, Z.; Wu, W.; Liu, G.; Xie, Z.; Cheng, Q.; et al. RUNX1/CD44 axis regulates the proliferation, migration, and immunotherapy of gliomas: A single-cell sequencing analysis. Front. Immunol. 2023, 14, 1086280. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Ranganathan, K.; Kavitha, L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J. Oral Maxillofac. Pathol. 2019, 23, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.; Saghravanian, N.; Taghi Shakeri, M.; Jamali, M. Evaluation of CD44 and TGF-B Expression in Oral Carcinogenesis. J. Dent. 2021, 22, 33–40. [Google Scholar]
- Han, Z.; Chen, Z.; Zheng, R.; Cheng, Z.; Gong, X.; Wang, D. Clinicopathological significance of CD133 and CD44 expression in infiltrating ductal carcinoma and their relationship to angiogenesis. World J. Surg. Oncol. 2015, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Thankappan, P.; Augustine, P.I.; Shaga, I.B.; Nirmal, R.M.; Joseph, T.I.; Girish, K.L. Immunohistochemical expression of putative cancer stem cell markers aldehyde dehydrogenase 1, SOX2, CD44 and OCT4 in different grades of oral epithelial dysplasia. J. Oral Maxillofac. Pathol. 2022, 26, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, M.; Ghazi, N.; Saghravanian, N.; Taghipour, A.; Mohajertehran, F. Evaluation of CD24 and CD44 as cancer stem cell markers in squamous cell carcinoma and epithelial dysplasia of the oral cavity by q-RT-PCR. Dent. Res. J. 2020, 17, 208–212. [Google Scholar]
- Zargaran, M.; Baghaei, F.; Moghimbeigi, A. Comparative study of β-catenin and CD44 immunoexpression in oral lichen planus and squamous cell carcinoma. Int. J. Dermatol. 2018, 57, 794–798. [Google Scholar] [CrossRef]
- Abdal, K.; Mostafazadeh, S.; Ghorbani, N. Comparison of E-cadherin and CD44 markers expression in oral lichen planus, oral leukoplakia and oral squamous cell carcinoma. J. Dent. 2021, 13, 6–10. [Google Scholar] [CrossRef]
- Mao, T.; Xiong, H.; Hu, X.; Hu, Y.; Wang, C.; Yang, L.; Huang, D.; Xia, K.; Su, T. DEC1: A potential biomarker of malignant transformation in oral leukoplakia. Braz. Oral Res. 2020, 34, e052. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Sharaf, K.; Schirmer, M.; Leu, M.; Küffer, S.; Bertlich, M.; Ihler, F.; Haubner, F.; Canis, M.; Kitz, J. Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC. Strahlenther. Onkol. 2021, 197, 231–245. [Google Scholar] [CrossRef]
- Thankappan, P.; Ramadoss, M.N.; Joseph, T.I.; Augustine, P.I.; Shaga, I.B.; Thilak, J. Human Papilloma Virus and Cancer Stem Cell markers in Oral Epithelial Dysplasia-An Immunohistochemical Study. Rambam Maimonides Med. J. 2021, 12, e0028. [Google Scholar] [CrossRef]
- Saghravanian, N.; Anvari, K.; Ghazi, N.; Memar, B.; Shahsavari, M.; Aghaeef, M.A. Expression of p63 and CD44 in oral squamous cell carcinoma and correlation with clinicopathological parameters. Arch. Oral. Biol. 2017, 82, 160–165. [Google Scholar] [CrossRef]
- Singh, B.; Aggarwal, S.; Das, P.; Srivastava, S.K.; Sharma, S.C.; Das, S.N. Over Expression of Cancer Stem Cell Marker CD44 and Its Clinical Significance in Patients with Oral Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 109–114. [Google Scholar] [CrossRef]
- Dhumal, S.N.; Choudhari, S.K.; Patankar, S.; Ghule, S.S.; Jadhav, Y.B.; Masne, S. Cancer Stem Cell Markers, CD44 and ALDH1, for Assessment of Cancer Risk in OPMDs and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Head Neck Pathol. 2022, 16, 453–465. [Google Scholar] [CrossRef] [PubMed]
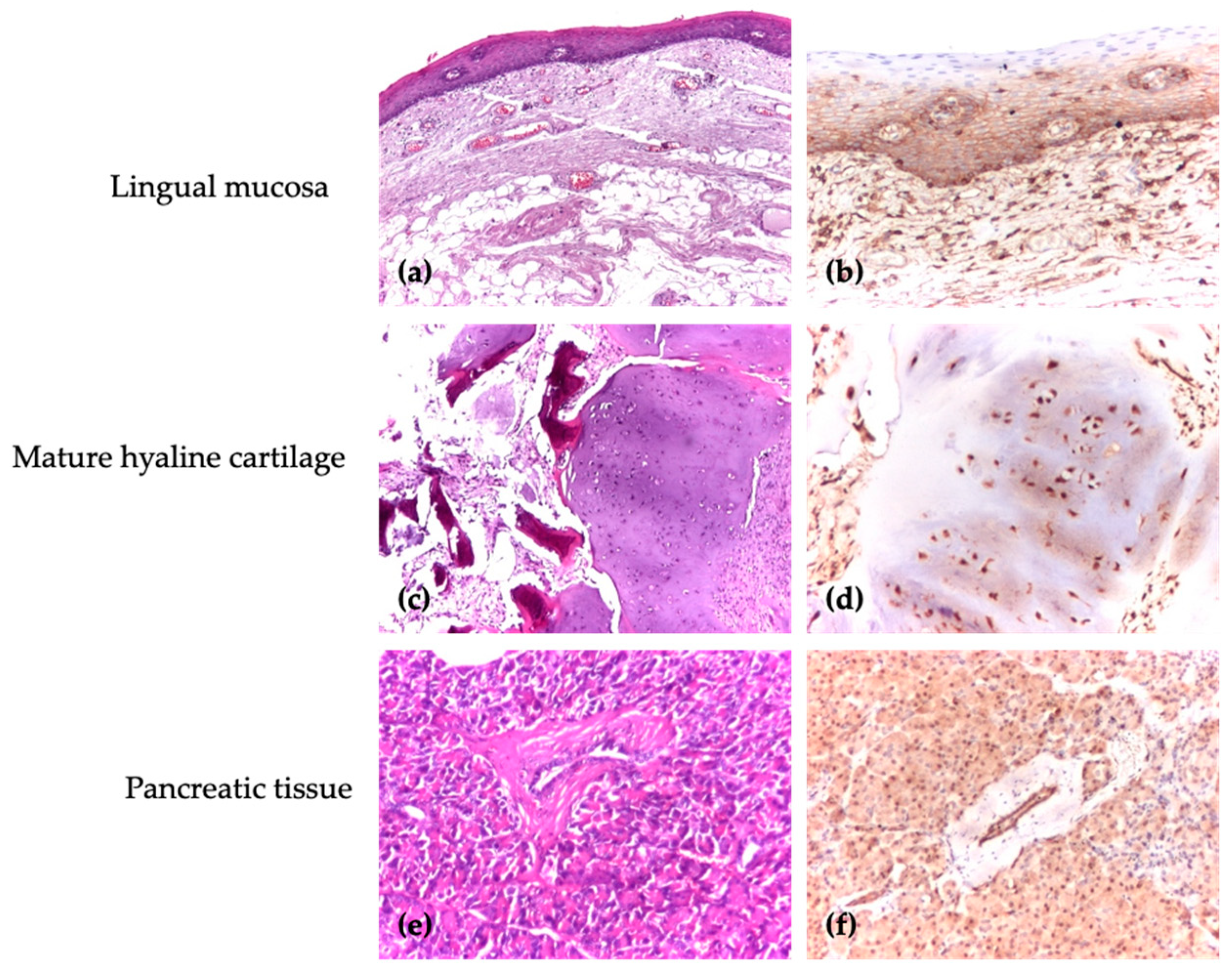

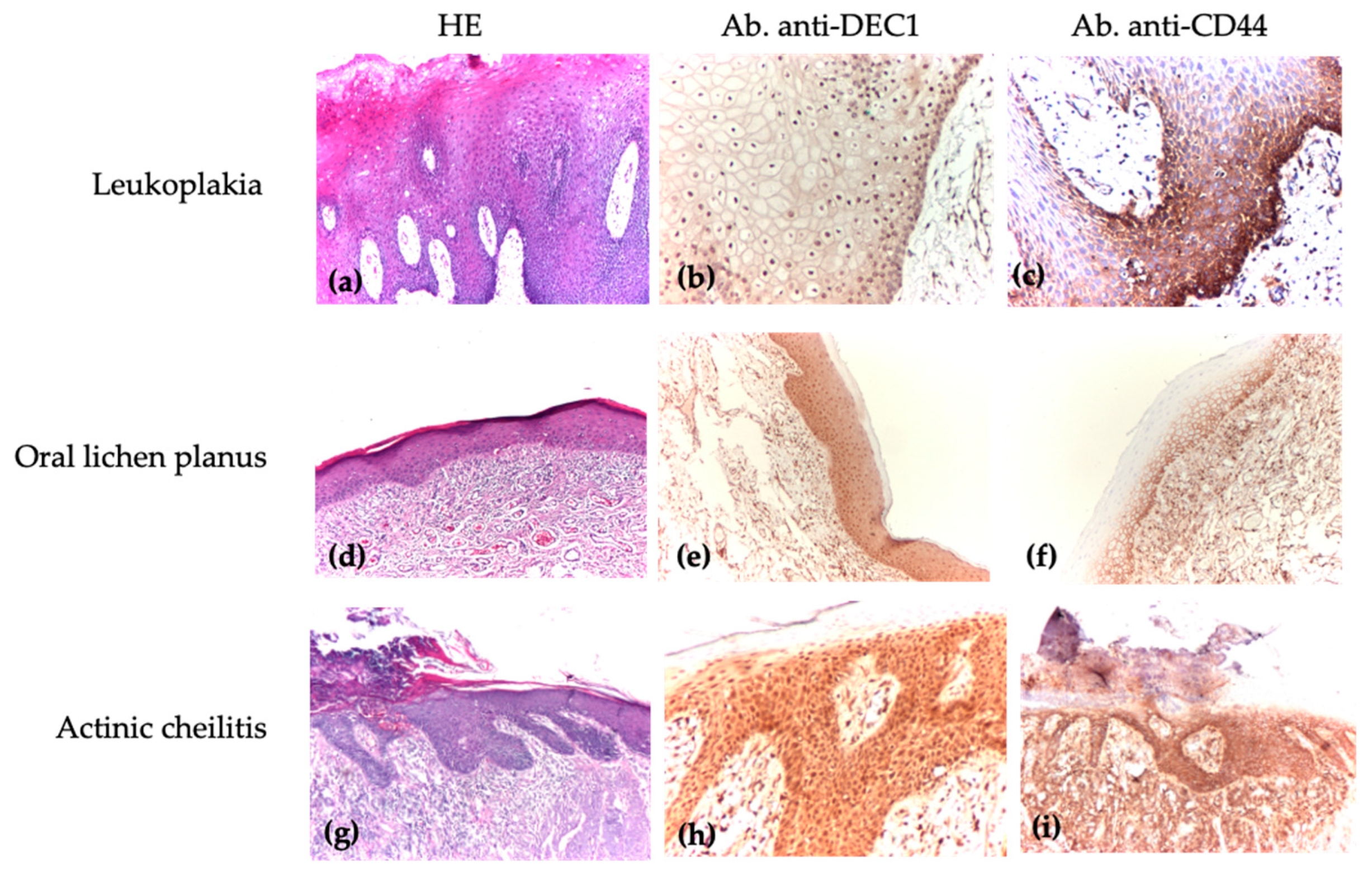
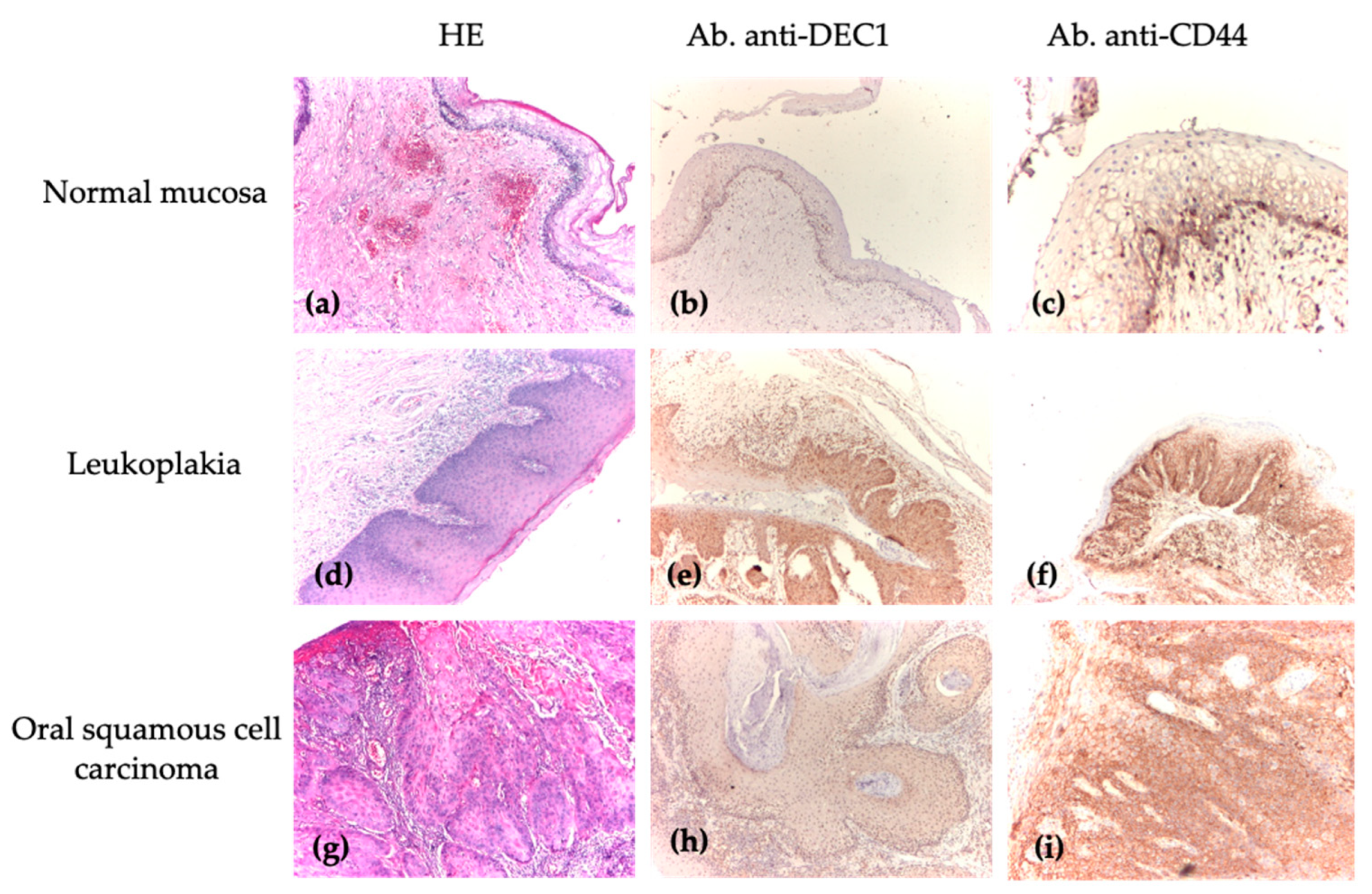
| Group | Diagnosis | Number |
|---|---|---|
| Group 0—control group | Normal mucosa (NM) | 63 |
| Group 1 | Oral leukoplakia (OLK) | 24 |
| Group 2 | Oral lichen planus (OLP) | 13 |
| Group 3 | Actinic cheilitis (AC) | 27 |
| Group 4 | Oral squamous cell carcinoma (OSCC) | 18 |
| Total = 145 |
| Antibody | Clone | pH | Class | Dilution | Expression |
|---|---|---|---|---|---|
| DEC1 (Antibodies Online, Aachen, Germany) | BHLHE40 | 9 | rabbit, polyclonal | 1:100 | nuclear |
| CD44 (ABCAM, Cambridge, UK) | C44Mab-5 | 9 | mouse, monoclonal | 1:500 | membrane |
| Immunohistochemical Staining | Score | |
|---|---|---|
| Extent of Positivity | 1 | 5–24% |
| 2 | 25–49% | |
| 3 | 50–74% | |
| 4 | 75–100% | |
| Intensity of Positivity | 0 | no staining |
| 1 | weak staining | |
| 2 | moderate staining | |
| 3 | strong staining | |
| Final Immunoscores = Extent of Positivity × Intensity of Positivity (resulting in a range from 0 to 12) | ||
| Location | Histopathological Diagnosis | Pearson Chi-Squared Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | ||||||
| N | % | N | % | N | % | N | % | ||
| Lower labial mucosa | 7 | 29.2% | - | - | 27 | 100.0% | 11 | 61.1% | Chi2 = 79.826 p < 0.001 * |
| Buccal mucosa | 3 | 12.5% | 11 | 84.6% | - | - | - | - | |
| Lingual mucosa | 8 | 33.3% | 1 | 7.7% | - | - | 4 | 22.2% | |
| Other locations | 6 | 25.0% | 1 | 7.7% | - | - | 3 | 16.7% | |
| Histopathological Diagnosis | Pearson Chi-Squared Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||||||
| N | % | N | % | N | % | ||||
| Extent of positivity | DEC1 | 0 | 5 | 20.8% | 6 | 46.2% | 2 | 7.4% | Chi2 = 13.725 p = 0.089 |
| 1 | 4 | 16.7% | 1 | 7.7% | 3 | 11.1% | |||
| 2 | 7 | 29.2% | 3 | 23.1% | 7 | 25.9% | |||
| 3 | 5 | 20.8% | 2 | 15.4% | 14 | 51.9% | |||
| 4 | 3 | 12.5% | 1 | 7.7% | 1 | 3.7% | |||
| CD44 | 0 | 1 | 4.2% | 2 | 15.4% | - | - | Chi2 = 26.756 p < 0.001 * | |
| 1 | 4 | 16.7% | 7 | 53.8% | 1 | 3.7% | |||
| 2 | 9 | 37.5% | 4 | 30.8% | 9 | 33.3% | |||
| 3 | 9 | 37.5% | - | - | 17 | 63.0% | |||
| 4 | 1 | 4.2% | - | - | - | - | |||
| Intensity of positivity | DEC1 | 0 | 5 | 20.8% | 6 | 46.2% | 2 | 7.4% | Chi2 = 10.238 p = 0.115 |
| 1 | 9 | 37.5% | 3 | 23.1% | 16 | 59.3% | |||
| 2 | 9 | 37.5% | 4 | 30.8% | 8 | 29.6% | |||
| 3 | 1 | 4.2% | - | - | 1 | 3.7% | |||
| CD44 | 0 | 1 | 4.2% | 2 | 15.4% | - | - | Chi2 = 11.658 p = 0.070 | |
| 1 | 6 | 25.0% | 5 | 38.5% | 2 | 7.4% | |||
| 2 | 8 | 33.3% | 3 | 23.1% | 12 | 44.4% | |||
| 3 | 9 | 37.5% | 3 | 23.1% | 13 | 48.1% | |||
| Immunoscore | DEC1 (mean ± SD; min ÷ max; mediana) | 3.21 ± 2.919 0 ÷ 9 2.50 | 2.00 ± 2.415 0 ÷ 8 3.00 | 3.48 ± 2.592 0 ÷ 12 3.00 | Kruskal–Wallis H = 3.278 p = 0.194 | ||||
| CD44 (m ± SD; min ÷ max; mediana) | 4.87 ± 2.894 0 ÷ 12 6.00 | 2.38 ± 2.293 0 ÷ 6 1.00 | 6.22 ± 2.154 2 ÷ 9 6.00 | Kruskal–Wallis H = 16.127 p < 0.001 * | |||||
| Types of Dysplasia | Pearson Chi-Squared Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||
| N | % | N | % | N | % | N | % | ||||
| Extent of positivity | DEC1 | 0 | 6 | 31.6% | 2 | 10.5% | 3 | 15.8% | 2 | 28.6% | Chi2 = 14.083 p = 0.295 |
| 1 | 2 | 10.5% | 4 | 21.1% | 2 | 10.5% | - | - | |||
| 2 | 5 | 26.3% | 3 | 15.8% | 6 | 31.6% | 3 | 42.9% | |||
| 3 | 3 | 15.8% | 10 | 52.6% | 6 | 31.6% | 2 | 28.6% | |||
| 4 | 3 | 15.8% | - | - | 2 | 10.5% | - | - | |||
| CD44 | 0 | 2 | 10.5% | 1 | 5.3% | - | - | - | - | Chi2 = 13.340 p = 0.345 | |
| 1 | 7 | 36.8% | 3 | 15.8% | 2 | 10.5% | - | - | |||
| 2 | 6 | 31.6% | 6 | 31.6% | 7 | 36.8% | 3 | 42.9% | |||
| 3 | 4 | 21.1% | 9 | 47.4% | 9 | 47.4% | 4 | 57.1% | |||
| 4 | - | - | - | - | 1 | 5.3% | - | - | |||
| Intensity of positivity | DEC1 | 0 | 6 | 31.6% | 2 | 10.5% | 3 | 15.8% | 2 | 28.6% | Chi2 = 8.715 p = 0.464 |
| 1 | 6 | 31.6% | 8 | 42.1% | 9 | 47.4% | 5 | 71.4% | |||
| 2 | 7 | 36.8% | 8 | 42.1% | 6 | 31.6% | - | - | |||
| 3 | - | - | 1 | 5.3% | 1 | 5.3% | - | - | |||
| CD44 | 0 | 2 | 10.5% | 1 | 5.3% | - | - | - | - | Chi2 = 12.083 p = 0.209 | |
| 1 | 6 | 31.6% | 3 | 15.8% | 4 | 21.1% | - | - | |||
| 2 | 6 | 31.6% | 4 | 21.1% | 8 | 42.1% | 5 | 71.4% | |||
| 3 | 5 | 26.3% | 11 | 57.9% | 7 | 36.8% | 2 | 28.6% | |||
| Immunoscore | DEC1 (mean ± SD; min ÷ max; mediana) | 2.74 ± 2.766 0 ÷ 8 2.00 | 3.63 ± 2.671 0 ÷ 9 3.00 | 3.37 ± 3.004 0 ÷ 12 3.00 | 1.71 ± 1.254 0 ÷ 3 2.00 | Kruskal–Wallis H = 3.100 p = 0.376 | |||||
| CD44 (m ± SD; min ÷ max; mediana) | 3.42 ± 2.673 0 ÷ 9 3.00 | 5.47 ± 2.894 0 ÷ 9 6.00 | 5.58 ± 2.854 1 ÷ 12 6.00 | 5.86 ± 1.676 4 ÷ 9 6.00 | Kruskal–Wallis H = 7.655 p = 0.054 | ||||||
| Groups | DEC1 Immunoscores | CD44 Immunoscores | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min ÷ Max | Median | Mann–Whitney U Test | Mean ± SD | Min ÷ Max | Median | Mann–Whitney U Test | |
| Group 0 | 1.32 ± 1.73 | 0 ÷ 8 | 1.00 | p < 0.001 * | 2.21 ± 1.94 | 0 ÷ 9 | 2.00 | p < 0.001 * |
| Groups 1 + 2 + 3 | 3.08 ± 2.70 | 0 ÷ 12 | 3.00 | 4.94 ± 2.83 | 0 ÷ 12 | 6.00 | ||
| Groups | DEC1 Immunoscores | CD44 Immunoscores | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min ÷ Max | Median | Mann–Whitney U Test | Mean ± SD | Min ÷ Max | Median | Mann–Whitney U Test | |
| Group 0 | 1.32 ± 1.73 | 0 ÷ 8 | 1.00 | p < 0.001 * | 2.21 ± 1.94 | 0 ÷ 9 | 2.00 | p < 0.001 * |
| Group 4 | 3.83 ± 2.99 | 0 ÷ 12 | 3.50 | 7.06 ± 3.24 | 0 ÷ 12 | 8.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofrei, B.-A.; Ciobanu Apostol, D.G.; Tanasă, M.-G.; Baciu, E.-R.; Popa, C.; Sciuca, A.M.; Toader, M.P.; Costan, V.-V. Immunohistochemical Expression of Differentiated Embryonic Chondrocyte 1 and Cluster of Differentiation 44 in Oral Potentially Malignant Disorders. Medicina 2025, 61, 251. https://doi.org/10.3390/medicina61020251
Onofrei B-A, Ciobanu Apostol DG, Tanasă M-G, Baciu E-R, Popa C, Sciuca AM, Toader MP, Costan V-V. Immunohistochemical Expression of Differentiated Embryonic Chondrocyte 1 and Cluster of Differentiation 44 in Oral Potentially Malignant Disorders. Medicina. 2025; 61(2):251. https://doi.org/10.3390/medicina61020251
Chicago/Turabian StyleOnofrei, Bianca-Andreea, Delia Gabriela Ciobanu Apostol, Mădălina-Gabriela Tanasă, Elena-Raluca Baciu, Cristina Popa, Ana Maria Sciuca, Mihaela Paula Toader, and Victor-Vlad Costan. 2025. "Immunohistochemical Expression of Differentiated Embryonic Chondrocyte 1 and Cluster of Differentiation 44 in Oral Potentially Malignant Disorders" Medicina 61, no. 2: 251. https://doi.org/10.3390/medicina61020251
APA StyleOnofrei, B.-A., Ciobanu Apostol, D. G., Tanasă, M.-G., Baciu, E.-R., Popa, C., Sciuca, A. M., Toader, M. P., & Costan, V.-V. (2025). Immunohistochemical Expression of Differentiated Embryonic Chondrocyte 1 and Cluster of Differentiation 44 in Oral Potentially Malignant Disorders. Medicina, 61(2), 251. https://doi.org/10.3390/medicina61020251








