High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine
Abstract
1. Introduction
2. Infections
3. Inflammatory Dermatoses
4. Metabolic and Genetic Disorders
5. Specific Cutaneous Structure and Sites of Skin Disorders
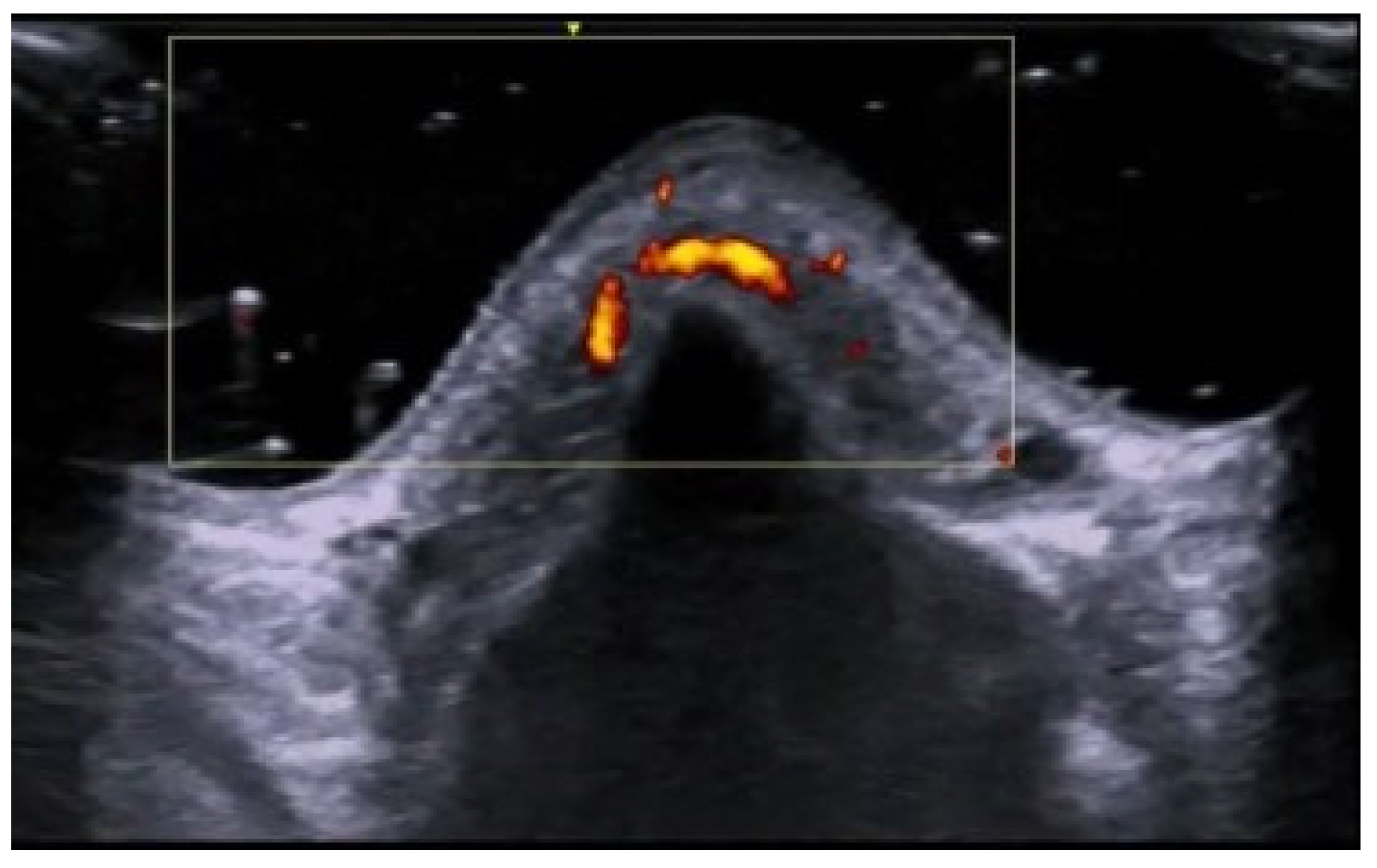
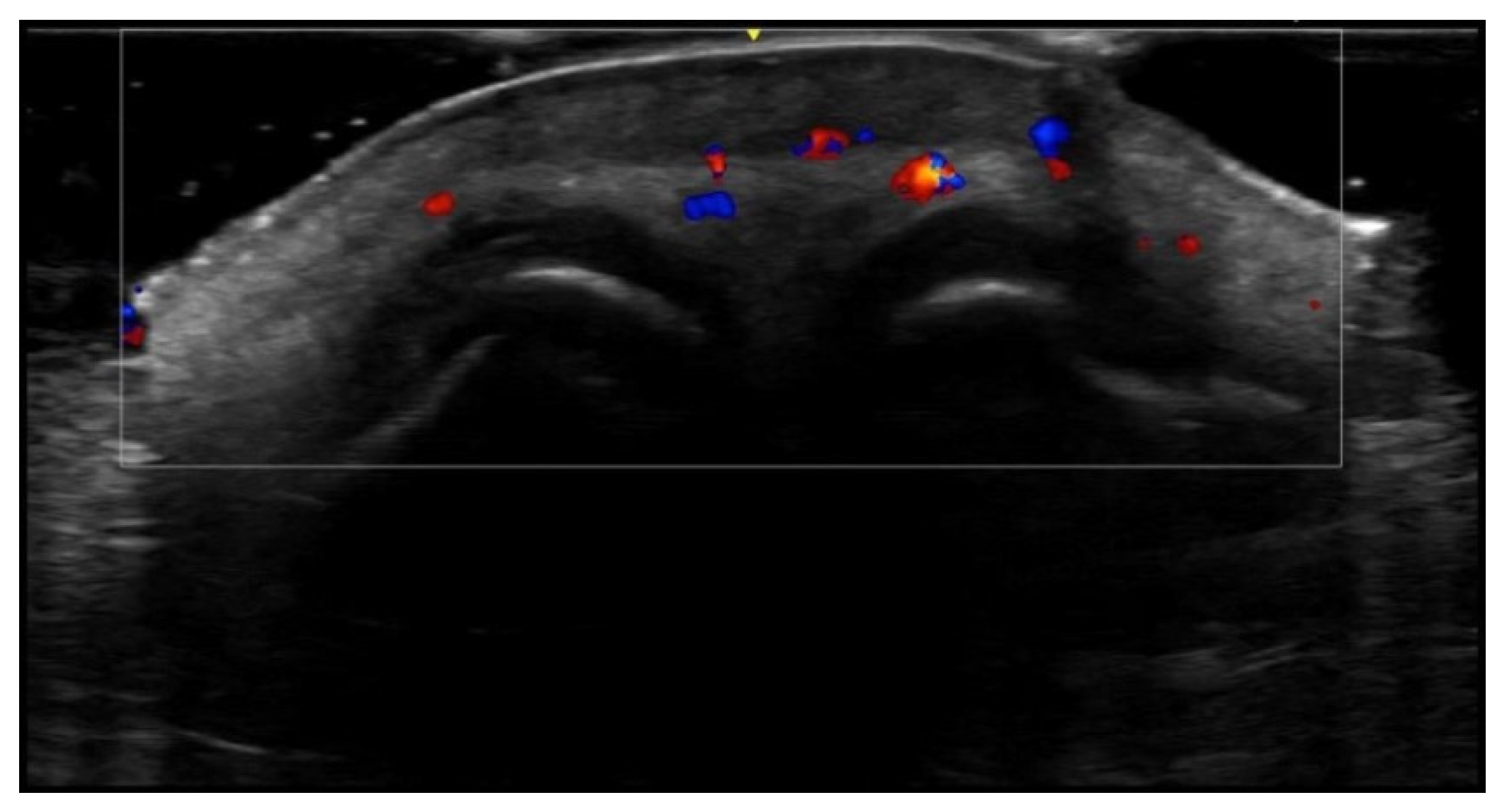
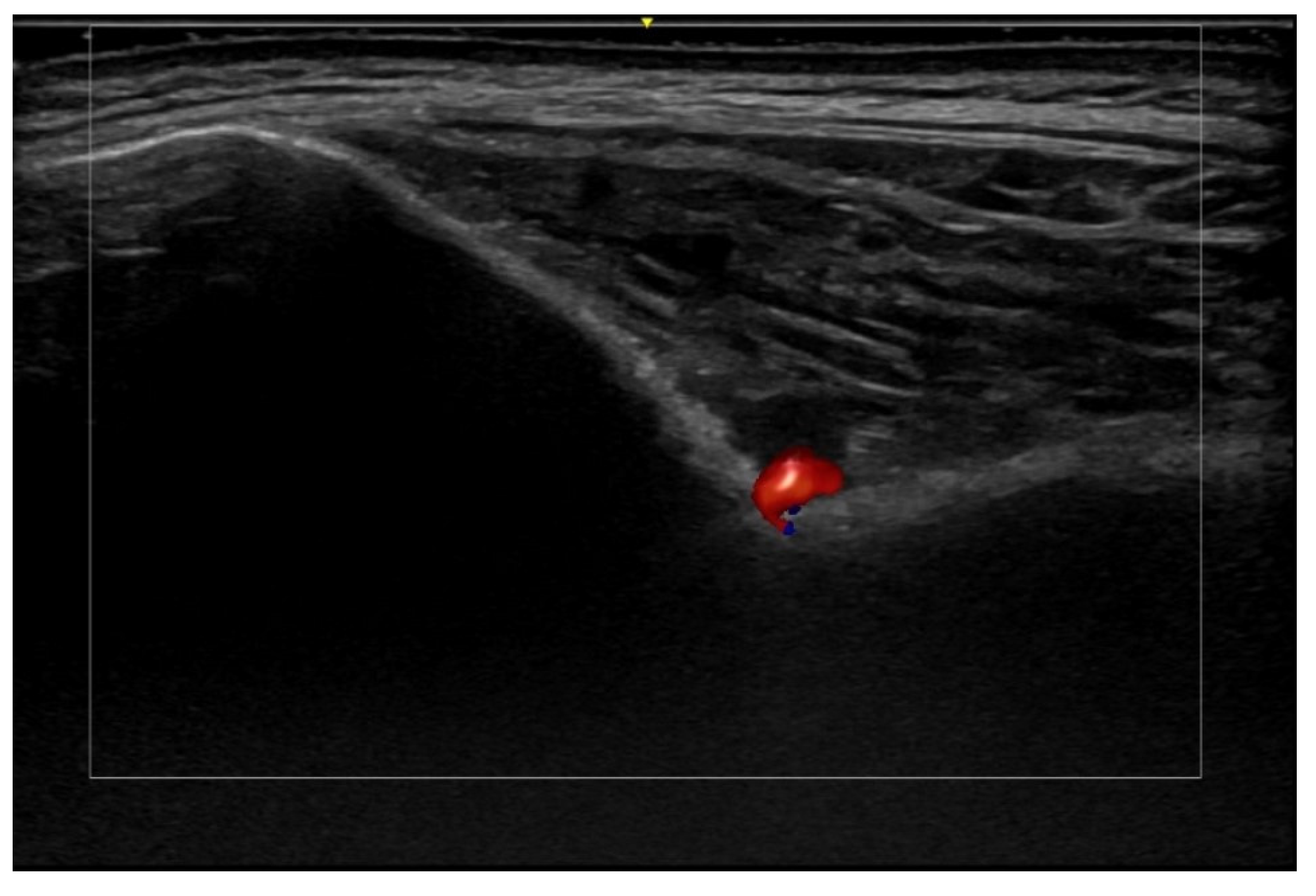
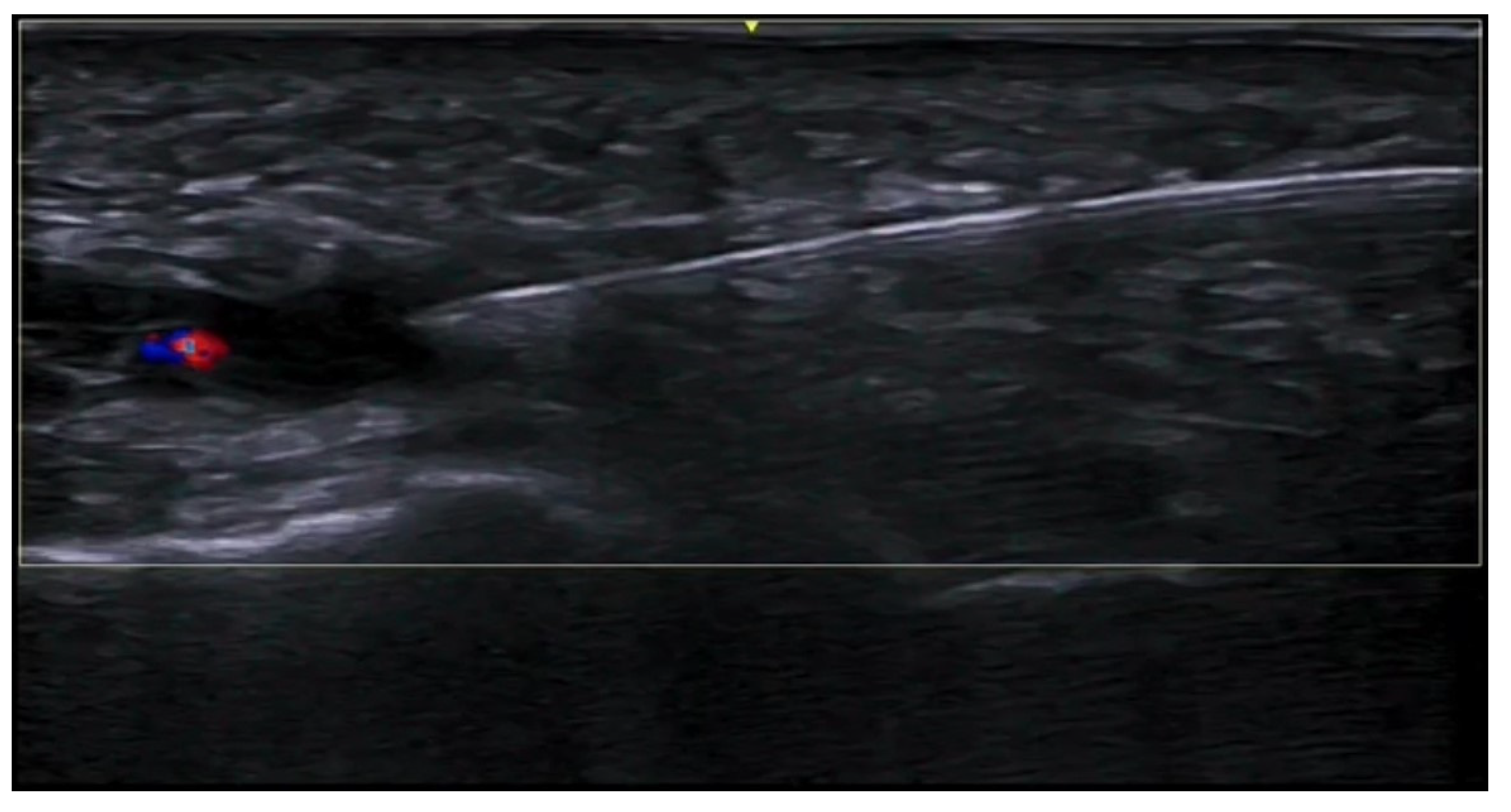
6. Vascular Disorders
7. External-Agent-Associated Disorders
8. Neoplastic Diseases
9. Aesthetic Medicine
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaurav, V.; Agrawal, S.; Najeeb, A.; Ahuja, R.; Saurabh, S.; Gupta, S. Advancements in Dermatological Imaging Modalities. Indian Dermatol. Online J. 2024, 15, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Alma, A.; Sticchi, A.; Chello, C.; Guida, S.; Farnetani, F.; Chester, J.; Bettoli, V.; Pellacani, G.; Manfredini, M. Dermoscopy, Reflectance Confocal Microscopy and Optical Coherence Tomography Features of Acne: A Systematic Review. J. Clin. Med. 2022, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Crisan, D.; Wortsman, X.; Alfageme, F.; Catalano, O.; Badea, A.; Scharffetter-Kochanek, K.; Sindrilaru, A.; Crisan, M. Ultraso-nography in dermatologic surgery: Revealing the unseen for improved surgical planning. J. Dtsch. Dermatol. Ges. 2022, 20, 913–926. [Google Scholar] [PubMed]
- Rahmani, E.; Fayyazishishavan, E.; Afzalian, A.; Varshochi, S.; Amani-Beni, R.; Ahadiat, S.A.; Moshtaghi, Z.; Shafagh, S.G.; Khorram, R.; Asadollahzade, E.; et al. Point-Of-Care Ultrasonography for Identification of Skin and Soft Tissue Abscess in Adult and Pediatric Patients; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2023, 11, e49. [Google Scholar]
- Barbic, D.; Chenkin, J.; Cho, D.D.; Jelic, T.; Scheuermeyer, F.X. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open 2017, 7, e013688. [Google Scholar]
- Mese, I. Expanding dermatologic ultrasonography applications: Further insights for enhanced patient management. Ultrasonography 2023, 42, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Santas, M.; Roustan-Gullon, G.; Alfageme-Roldan, F. Sonographic findings in scabies. J. Ultrasound 2023, 26, 549–551. [Google Scholar] [CrossRef]
- Wortsman, X.; Sazunic, I.; Jemec, G.B. Sonography of plantar warts: Role in diagnosis and treatment. J. Ultrasound Med. 2009, 28, 787–793. [Google Scholar] [CrossRef]
- Corvino, A.; Catalano, O.; Wortsman, X.; Roldán, F.A.; Cavallieri, F.; Gonzalez, C.; Tafuri, D.; Corvino, F.; Cocco, G.; Caruso, M. High-Resolution Ultrasound of Odontogenic Cutaneous Sinus Tract: An International Multicentric Experience and a Review of the Literature. J. Ultrasound Med. 2024, 43, 1489–1499. [Google Scholar] [CrossRef]
- Giraldelli, G.A.; Baka, J.L.C.S.; Orofino-Costa, R.; Piñeiro-Maceira, J.; Barcaui, E.; Barcaui, C.B. In vivo reflectance confocal microscopy, dermoscopy, high-frequency ultrasonography, and histopathology features in a case of chromoblastomycosis. PLoS Negl. Trop. Dis. 2022, 16, e0010226. [Google Scholar] [CrossRef]
- Vergara-de-la-Campa, L.; Cembrero-Saralegui, H.; Luna-Bastante, L.; Martínez-Lorenzo, E.R.; Alfageme-Roldán, F. Usefulness of ultrasound for treatment and follow-up of cutaneous leishmaniasis. J. Ultrasound 2021, 24, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Neri, I.; Patrizi, A.; Di Altobrando, A.; Clinca, R.; Caro, R.D.C.; Leuzzi, M.; Misciali, C.; Gaspari, V. Ultrasound patterns of localized cutaneous leishmaniasis and clinical correlations. J. Ultrasound 2022, 25, 343–348. [Google Scholar] [CrossRef]
- Frade, M.A.; Nogueira-Barbosa, M.H.; Lugão, H.B.; Furini, R.B.; Marques Júnior, W.; Foss, N.T. New sonographic measures of peripheral nerves: A tool for the diagnosis of peripheral nerve involvement in leprosy. Mem. Inst. Oswaldo Cruz 2013, 108, 257–262. [Google Scholar] [CrossRef]
- Rao, P.N.; Suneetha, S. Pure neuritic leprosy: Current status and relevance. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Voltan, G.; Marques-Júnior, W.; Santana, J.M.; Lincoln Silva, C.M.; Leite, M.N.; De Paula, N.A.; Bernardes Filho, F.; Barreto, J.G.; Da Silva, M.B.; Conde, G.; et al. Silent peripheral neuropathy determined by high-resolution ultrasound among contacts of patients with Hansen’s disease. Front. Med. 2023, 9, 1059448. [Google Scholar] [CrossRef] [PubMed]
- Bouer, M.; Rodriguez-Bandera, A.I.; Albizuri-Prado, F.; Lobos, A.; Gubeling, W.; Wortsman, X. Real-time high-frequency colour Doppler ultrasound detection of cutaneous Dermatobia hominis myiasis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e180–e181. [Google Scholar] [CrossRef] [PubMed]
- Ogueta, I.; Navajas-Galimany, L.; Concha-Rogazy, M.; Álvarez-Véliz, S.; Vera-Kellet, C.; Gonzalez-Bombardiere, S.; Wortsman, X. Very High- and High-Frequency Ultrasound Features of Cutaneous Larva Migrans. J. Ultrasound Med. 2019, 38, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Sonography of Dermatologic Emergencies. J. Ultrasound Med. 2017, 36, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, Q.; Ye, X. High-frequency ultrasound findings in gonococcal inflammation of the paraurethral glands in men. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, Q.; Ye, X.; Fan, Z. Dynamic Observation of the Morphological Changes in Paraurethral Ducts Infected with Gonococci in Men before and after Ceftriaxone Therapy Using High-Frequency Ultrasound. Urol. Int. 2018, 100, 240–244. [Google Scholar] [CrossRef]
- Negrutiu, M.; Danescu, S.; Popa, T.; Focsan, M.; Vesa, S.C.; Szasz, F.; Baican, A. Imaging Approach in the Diagnostics and Evaluation of the Psoriasis Plaque: A Preliminary Study and Literature Review. Diagnostics 2024, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Ianoși, S.L.; Forsea, A.M.; Lupu, M.; Ilie, M.A.; Zurac, S.; Boda, D.; Ianosi, G.; Neagoe, D.; Tutunaru, C.; Popa, C.M.; et al. Role of modern imaging techniques for the in vivo diagnosis of lichen planus. Exp. Ther. Med. 2019, 17, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, T.; Yazdani, K.; Humbert, P.; Khatami, A.; Ahmad Nasrollahi, S.; Zartab, H.; Izadi Firouzabadi, L.; Firooz, A. Biophysical and ultrasonographic changes in lichen planus compared with uninvolved skin. Int. J. Women’s Dermatol. 2018, 5, 100–104. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, Z.; Zhang, D.; Liu, J.; Zhu, Q. Improve the dupilumab therapy evaluation with dermoscopy and high-frequency ultrasound in moderate-to-severe atopic dermatitis. Ski. Res. Technol. 2023, 29, e13260. [Google Scholar] [CrossRef]
- Csány, G.; Gergely, L.H.; Kiss, N.; Szalai, K.; Lőrincz, K.; Strobel, L.; Csabai, D.; Hegedüs, I.; Marosán-Vilimszky, P.; Füzesi, K.; et al. Preliminary Clinical Experience with a Novel Optical-Ultrasound Imaging Device on Various Skin Lesions. Diagnostics 2022, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Bacchetti, T.; Ferretti, G.; Molinelli, E.; Rizzetto, G.; Bellachioma, L.; Offidani, A. Oxidative Stress and Alterations of Paraoxonases in Atopic Dermatitis. Antioxidants 2021, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, C.; Jin, H.; Liu, J.; Wang, H. Investigation of using very high-frequency ultrasound in the differential diagnosis of early-stage pemphigus vulgaris vs seborrheic dermatitis. Ski. Res. Technol. 2020, 26, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Mancini, G.; Brisigotti, V.; Sapigni, C.; Simonetti, O.; Olivieri, A.; Offidani, A. Performance of high-frequency ultrasound in the evaluation of skin involvement in cutaneous chronic graft-versus-host disease: A preliminary report. J. Am. Acad. Dermatol. 2022, 87, 1180–1181. [Google Scholar] [CrossRef]
- Elsaiey, A.; Mahmoud, H.S.; Jensen, C.T.; Klimkowski, S.; Taher, A.; Chaudhry, H.; Morani, A.C.; Wong, V.K.; Salem, U.I.; Palmquist, S.M.; et al. Mastocytosis-A Review of Disease Spectrum with Imaging Correlation. Cancers 2021, 13, 5102. [Google Scholar] [CrossRef] [PubMed]
- Białynicki-Birula, R.; Reszke, R.; Szepietowski, J.C. Two Ultrasonographic Patterns in Maculopapular Cutaneous Mastocytosis: A Preliminary Report. Acta Dermatovenerol. Croat. 2017, 25, 22–25. [Google Scholar] [PubMed]
- Granieri, G.; Michelucci, A.; Manzo Margiotta, F.; Cei, B.; Vitali, S.; Romanelli, M.; Dini, V. The Role of Ultra-High-Frequency Ultrasound in Pyoderma Gangrenosum: New Insights in Pathophysiology and Diagnosis. Diagnostics 2023, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, J.; Zhou, G.; Liu, Y.; Lu, X.; Wang, N.; Liu, H.; Zhang, F. High-Frequency Ultrasound in Blistering Skin Diseases: A Useful Method for Differentiating Blister Locations. J. Ultrasound Med. 2017, 36, 2367–2371. [Google Scholar] [CrossRef]
- Izzetti, R.; Nisi, M.; Aringhieri, G.; Vitali, S.; Oranges, T.; Romanelli, M.; Caramella, D.; Graziani, F.; Gabriele, M. Ultra-high frequency ultrasound in the differential diagnosis of oral pemphigus and pemphigoid: An explorative study. Ski. Res. Technol. 2021, 27, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.; Zhu, R.; Luo, F.; Shi, Y.; Liu, M.; Xiao, Y.; Xiao, R. Updated Role of High-frequency Ultrasound in Assessing Dermatological Manifestations in Autoimmune Skin Diseases. Acta Derm. Venereol. 2022, 102, adv00765. [Google Scholar] [CrossRef] [PubMed]
- Ewrew Pu, Y.; Zhang, L. Application of dermatoscopy, reflectance confocal microscopy, and high-frequency ultrasound for diagnosing neonatal lupus erythematosus: A case report. Ski. Res. Technol. 2023, 29, e13291. [Google Scholar]
- Giavedoni, P.; Podlipnik, S.; Fuertes de Vega, I.; Iranzo, P.; Mascaró, J.M., Jr. High-Frequency Ultrasound to Assess Activity in Connective Tissue Panniculitis. J. Clin. Med. 2021, 10, 4516. [Google Scholar] [CrossRef]
- Romaní, J.; Giavedoni, P.; Roé, E.; Vidal, D.; Luelmo, J.; Wortsman, X. Inter- and Intra-rater Agreement of Dermatologic Ultrasound for the Diagnosis of Lobular and Septal Panniculitis. J. Ultrasound Med. 2020, 39, 107–112. [Google Scholar] [CrossRef]
- Svensson, C.; Eriksson, P.; Zachrisson, H.; Sjöwall, C. High-Frequency Ultrasound of Multiple Arterial Areas Reveals Increased Intima Media Thickness, Vessel Wall Appearance, and Atherosclerotic Plaques in Systemic Lupus Erythematosus. Front. Med. 2020, 7, 581336. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.; Akhter, T.; Nordmark, G.; Rönnblom, L.; Naessen, T. Increased carotid intima thickness and decreased media thickness in premenopausal women with systemic lupus erythematosus: An investigation by non-invasive high-frequency ultrasound. Scand. J. Rheumatol. 2011, 40, 279–282. [Google Scholar] [CrossRef]
- Sudoł-Szopińska, I.; Żelnio, E.; Olesińska, M.; Gietka, P.; Ornowska, S.; Power, D.J.; Taljanovic, M.S. Update on Current Imaging of Systemic Lupus Erythematous in Adults and Juveniles. J. Clin. Med. 2022, 11, 5212. [Google Scholar] [CrossRef] [PubMed]
- Kotob, H.; Kamel, M. Identification and prevalence of rheumatoid nodules in the finger tendons using high frequency ultrasonography. J. Rheumatol. 1999, 26, 1264–1268. [Google Scholar] [PubMed]
- Di Battista, M.; Barsotti, S.; Vitali, S.; Palma, M.; Granieri, G.; Oranges, T.; Aringhieri, G.; Dini, V.; Della Rossa, A.; Neri, E.; et al. Multiparametric Skin Assessment in a Monocentric Cohort of Systemic Sclerosis Patients: Is There a Role for Ultra-High Frequency Ultrasound? Diagnostics 2023, 13, 1495. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; Kersten, B.E.; van den Ende, C.H.M.; van den Hoogen, F.H.J.; Vonk, M.C.; de Korte, C.L. Photoacoustic and high-frequency ultrasound imaging of systemic sclerosis patients. Arthritis Res. Ther. 2021, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, M.; Vitali, S.; Barsotti, S.; Granieri, G.; Aringhieri, G.; Morganti, R.; Dini, V.; Della Rossa, A.; Romanelli, M.; Neri, E.; et al. Ultra-high frequency ultrasound for digital arteries: Improving the characterization of vasculopathy in systemic sclerosis. Semin. Arthritis Rheum. 2022, 57, 152105. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, T.; Mohseni, A.; Dehghan, K.S.; Delavar, S.; Firooz, A. High-frequency ultrasound evaluation of morphea: Retrospective analytical study. Ski. Res. Technol. 2024, 30, e13818. [Google Scholar] [CrossRef] [PubMed]
- Ranosz-Janicka, I.; Lis-Święty, A.; Skrzypek-Salamon, A.; Brzezińska-Wcisło, L. An extended high-frequency ultrasound protocol for assessing and quantifying of inflammation and fibrosis in localized scleroderma. Ski. Res. Technol. 2019, 25, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Khorasanizadeh, F.; Kalantari, Y.; Etesami, I. Role of imaging in morphea assessment: A review of the literature. Ski. Res. Technol. 2023, 29, e13410. [Google Scholar] [CrossRef] [PubMed]
- Etesami, I.; Azizi, N.; Sabrinejad, R.; Montazeri, S.; Kamyab, K.; Nasimi, M.; Mahmoudi, H.; Khorasanizadeh, F.; Wortsman, X. Sonographic skin features and shear wave elastography in distinguishing active from inactive morphea lesions: A case-control study. J Am Acad Dermatol. 2025, 92, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cares, J.; Wortsman, X.; Alfaro-Sepúlveda, D.; Mellado-Francisco, G.; Ramírez-Cornejo, C.; Vera-Kellet, C. Color Doppler Ultrasound Assessment of Subclinical Activity with Scoring of Morphea. J. Cutan. Med. Surg. 2023, 27, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Vera-Kellet, C. Ultrasound Morphea Activity Scoring (US-MAS): Modified US-MAS. J. Ultrasound Med. 2023, 42, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Vera-Kellet, C.; Meza-Romero, R.; Moll-Manzur, C.; Ramírez-Cornejo, C.; Wortsman, X. Low effectiveness of methotrexate in the management of localised scleroderma (morphea) based on an ultrasound activity score. Eur. J. Dermatol. 2021, 31, 813–821. [Google Scholar] [CrossRef]
- Araneda-Ortega, P.; Poblete-Villacorta, M.J.; Muñoz-López, C.; Vera-Kellet, C.; Wortsman, X. Morphea After Liposuction Ultrasonography. J. Ultrasound Med. 2022, 41, 2629–2635. [Google Scholar] [CrossRef]
- Noe, M.H.; Rodriguez, O.; Taylor, L.; Sultan, L.; Sehgal, C.; Schultz, S.; Gelfand, J.M.; Judson, M.A.; Rosenbach, M. High frequency ultrasound: A novel instrument to quantify granuloma burden in cutaneous sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 136–141. [Google Scholar]
- Rodriguez, O.; Noe, M.H.; Sehgal, C.; Schultz, S.; Rosenbach, M. Quantification of granuloma volume and response to treatment in cutaneous sarcoidosis using 3-dimensional high-frequency ultrasound scan. JAAD Case Rep. 2017, 3, 522–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Planas-Ciudad, S.; Roé Crespo, E.; Mir-Bonafé, J.F.; Muñoz-Garza, F.Z.; Puig Sanz, L. Dystrophic Calcification in a Patient with Primary Localized Cutaneous Nodular Amyloidosis: An Uncommon Ultrasound Finding. Acta Derm. Venereol. 2018, 98, 144–145. [Google Scholar] [CrossRef]
- García-Arpal, M.; Bujalance-Cabrera, C.; Banegas-Illescas, M.E.; Sánchez-Caminero, M.P.; González-Ruiz, L.; Villasanti-Rivas, N. Scleredema diabeticorum in a patient: Un uncommon etiology of restrictive lung pattern Escleredema diabeticorum en un paciente: Una etiología infrecuente de patrón restrictivo pulmonar. Dermatol. Online J. 2021, 27, 7. [Google Scholar] [CrossRef]
- Shih, S.R.; Lin, M.S.; Li, H.Y.; Yang, H.Y.; Hsiao, Y.L.; Chang, M.T.; Chen, C.M.; Chang, T.C. Observing pretibial myxedema in patients with Graves’ disease using digital infrared thermal imaging and high-resolution ultrasonography: For better records, early detection, and further investigation. Eur. J. Endocrinol. 2011, 164, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Elahmar, H.; Feldman, B.M.; Johnson, S.R. Management of Calcinosis Cutis in Rheumatic Diseases. J. Rheumatol. 2022, 49, 980–989. [Google Scholar] [CrossRef]
- Tubau, C.; Cubiró, X.; Amat-Samaranch, V.; Garcia-Melendo, C.; Puig, L.; Roé-Crespo, E. Clinical and ultrasonography follow-up of five cases of calcinosis cutis successfully treated with intralesional sodium thiosulfate. J. Ultrasound 2022, 25, 995–1003. [Google Scholar] [CrossRef]
- Mohafez, H.; Ahmad, S.A.; Hadizadeh, M.; Moghimi, S.; Roohi, S.A.; Marhaban, M.H.; Saripan, M.I.; Rampal, S. Quantitative assessment of wound healing using high-frequency ultrasound image analysis. Ski. Res. Technol. 2018, 24, 45–53. [Google Scholar] [CrossRef]
- Chao, C.Y.; Zheng, Y.P.; Cheing, G.L. The association between skin blood flow and edema on epidermal thickness in the diabetic foot. Diabetes Technol. Ther. 2012, 14, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Zheng, Y.P.; Cheing, G.L. Epidermal thickness and biomechanical properties of plantar tissues in diabetic foot. Ultrasound Med. Biol. 2011, 37, 1029–1038. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Dong, Q.; Xu, C.; Zhou, X.; Ouyang, Y.; Liu, Y.; Lee, J.J.; Hu, N.; Wang, K.; et al. X-linked dominant protoporphyria in a Chinese pedigree reveals a four-based deletion of ALAS2. Ann. Transl. Med. 2020, 8, 344. [Google Scholar] [CrossRef]
- Goldberg, I.; Sprecher, E.; Schwartz, M.E.; Gaitini, D. Comparative study of high-resolution multifrequency ultrasound of the plantar skin in patients with various types of hereditary palmoplantar keratoderma. Dermatology 2013, 226, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Moreau, M.; Leftheriotis, G.; Le Corre, Y.; Etienne, M.; Amode, R.; Hamel, J.F.; Croué, A.; Le Saux, O.; Machet, L.; Martin, L. High-frequency (20–50 MHz) ultrasonography of pseudoxanthoma elasticum skin lesions. Br. J. Dermatol. 2013, 169, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Mikiel, D.; Polańska, A.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. Suitability of high-frequency ultrasonography (20 MHz) in evaluation of various forms of primary cicatricial alopecia in relation to trichoscopy—Pilot study. Ski. Res. Technol. 2021, 27, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Mikiel, D.; Polańska, A.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. Usefulness of high-frequency ultrasonography in the assessment of alopecia areata—Comparison of ultrasound images with trichoscopic images. Postep. Dermatol. Alergol. 2022, 39, 132–140. [Google Scholar] [CrossRef]
- Paun, M.; Tiplica, G.S. Non-Invasive Techniques for Evaluating Alopecia Areata. Maedica 2023, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita-Ise, M.; Ohyama, M.; Ramjist, J.M.; Foster, F.S.; Yang, V.X.D.; Sachdeva, M.; Sade, S.; Shear, N.H. Ultra high-frequency ultrasound with seventy-MHz transducer in hair disorders: Development of a novel noninvasive diagnostic methodology. J Dermatol. Sci. 2021, 102, 167–176. [Google Scholar] [CrossRef]
- Cataldo-Cerda, K.; Wortsman, X. Dissecting Cellulitis of the Scalp Early Diagnosed by Color Doppler Ultrasound. Int. J. Trichology 2017, 9, 147–148. [Google Scholar] [CrossRef]
- Zattar, L.; Wortsman, X. Ultrasound of Benign Cutaneous Tumors and Pseudotumors: The Key Lesions. Semin. Ultrasound CT MRI 2024, 45, 192–215. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Wortsman, J.; Matsuoka, L.; Saavedra, T.; Mardones, F.; Saavedra, D.; Guerrero, R.; Corredoira, Y. Sonography in pathologies of scalp and hair. Br. J. Radiol. 2012, 85, 647–655. [Google Scholar] [CrossRef]
- Cedirian, S.; Rapparini, L.; Sechi, A.; Piraccini, B.M.; Starace, M. Diagnosis and Management of Scalp Metastases: A Review. Diagnostics 2024, 14, 1638. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Orellana, G.; Ferreira-Wortsman, C.; Wortsman, X. Ultrasound Pattern of Cutis Verticis Gyrata. J. Ultrasound Med. 2024, 43, 405–409. [Google Scholar] [CrossRef]
- Wortsman, X.; Araya, I.; Maass, M.; Valdes, P.; Zemelman, V. Ultrasound Patterns of Vitiligo at High Frequency and Ultra-High Frequency. J. Ultrasound Med. 2024, 43, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bai, Y.; Duan, Z.; Yuan, R.; Liu, X.; Liu, Y.; Liang, X.; Wu, H.; Zhuo, F. Efficacy and Safety of Long-Pulsed 755-nm Alexandrite Laser for Keratosis Pilaris: A Split-Body Randomized Clinical Trial. Dermatol. Ther. 2022, 12, 1897–1906. [Google Scholar] [CrossRef]
- Tao, Y.; Wei, C.; Su, Y.; Hu, B.; Sun, D. Emerging High-Frequency Ultrasound Imaging in Medical Cosmetology. Front. Physiol. 2022, 13, 885922. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, S.; Jaguś, D.; Woźniak, W.; Mlosek, R.K. Usefulness of high-frequency ultrasound in the monitoring of laser treatment of acne scars. J. Ultrason. 2021, 20, e279–e283. [Google Scholar] [CrossRef] [PubMed]
- Naouri, M.; Atlan, M.; Perrodeau, E.; Georgesco, G.; Khallouf, R.; Martin, L.; Machet, L. High-resolution ultrasound imaging to demonstrate and predict efficacy of carbon dioxide fractional resurfacing laser treatment. Dermatol. Surg. 2011, 37, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Ñanco-Meléndez, C.; Yagnam-Díaz, M.; Muñoz-Cáceres, M.; Contador-González, J.; Gubelin-Harcha, W.; Chicao-Carmona, F.; Tan, J.; Wortsman, X. Evaluation of Ultrasound Changes with the Use of Microneedling Versus Fractional CO2 Laser in Atrophic Acne Scars. Dermatol. Pract. Concept. 2024, 14, e2024168. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Update on Ultrasound Diagnostic Criteria and New Ultrasound Severity and Activity Scorings of Hidradenitis Suppurativa: Modified SOS-HS and US-HSA. J. Ultrasound Med. 2024, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Bastos, P.; Martorell, A.; Bettoli, V.; Matos, A.P.; Muscianisi, E.; Wortsman, X. The use of ultrasound and magnetic resonance imaging in the management of hidradenitis suppurativa: A narrative review. Br. J. Dermatol. 2023, 188, 591–600. [Google Scholar] [CrossRef]
- Wortsman, X.; Reyes-Baraona, F.; Ramirez-Cornejo, C.; Ferreira-Wortsman, C.; Caposiena Caro, R.D.; Molina-Leyva, A.; Arias-Santiago, S.; Giavedoni, P.; Martorell, A.; Romani, J.; et al. Can Ultrasound Examinations Generate Pain in Hidradenitis Suppurativa Patients? Results from a Multicentric Cross-Sectional Study. Dermatology 2023, 239, 277–282. [Google Scholar] [CrossRef]
- Iannone, M.; Janowska, A.; Oranges, T.; Balderi, L.; Benincasa, B.B.; Vitali, S.; Tonini, G.; Morganti, R.; Romanelli, M.; Dini, V. Ultrasound-guided injection of intralesional steroids in acute hidradenitis suppurativa lesions: A prospective study. Dermatol. Ther. 2021, 34, e15068. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Patrizi, A.; Raone, B. Intralesional steroid injections to target sinus tract fibrosis in hidradenitis suppurativa: Results from an ultrasound-based retrospective study. Dermatol. Ther. 2022, 35, e15710. [Google Scholar] [CrossRef]
- Dini, V.; Michelucci, A.; Granieri, G.; Zerbinati, N.; Margiotta, F.M.; Romanelli, M. Evaluation of post-surgical complications of hidradenitis suppurativa lesions explored with presurgical ultra-high frequency ultrasound mapping. J. Wound Care 2024, 33, S10–S16. [Google Scholar] [CrossRef]
- Michelucci, A.; Fidanzi, C.; Manzo Margiotta, F.; Granieri, G.; Salvia, G.; Janowska, A.; Romanelli, M.; Dini, V. Presurgical Mapping with Ultra-high Frequency Ultrasound of Hidradenitis Suppurativa Lesions Treated with Wide Local Excision and Secondary Intention Healing. Dermatol. Surg. 2025, 51, 36–39. [Google Scholar] [CrossRef]
- Wortsman, X.; Ortiz-Orellana, G.; Valderrama, Y.; Ferreira-Wortsman, C.; Reyes, F.; Herane, M.I. Ultrasonography of Facial and Submandibular Hidradenitis Suppurativa and Concomitance with Acne Vulgaris. J. Ultrasound Med. 2024, 43, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, P.; Kopeć, J.; Przewratil, P. Sequential, ultrasound-guided, minimally invasive pit-picking procedure with Nd:YAG laser epilation treatment for pilonidal disease. Pol. Prz. Chir. 2023, 96, 13–16. [Google Scholar] [CrossRef]
- Kaiyasah, H.; Abufool, L.; Al Ozaibi, L. The Role of Point-of-Care Ultrasound in Pilonidal Sinus Disease. POCUS J. 2022, 7, 205–207. [Google Scholar] [CrossRef]
- Yilmaz, T.U.; Yavuz, O.; Yirmibesoglu, A.O.; Sarisoy, H.T.; Vural, C.; Kiraz, U.; Utkan, N.Z. Radiological, Clinical, and Histological Findings in the Treatment of Pilonidal Sinus with Phenol Injection. Medeni. Med. J. 2022, 37, 29–35. [Google Scholar] [CrossRef]
- Aksoy, H.M.; Aksoy, B.; Ozkur, E.; Calikoglu, E. Topical polyphenol treatment of sacrococcygeal pilonidal sinus disease: Use of ultrasonography to evaluate response to treatment—Clinical case series study. Postep. Dermatol. Alergol. 2019, 36, 431–443. [Google Scholar] [CrossRef]
- Puranik, C.I.; Wadhwani, V.J.; Vora, D.M. Role of transperineal ultrasound in infective and inflammatory disorders. Indian J. Radiol. Imaging 2017, 27, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Berritto, D.; Iacobellis, F.; Rossi, C.; Reginelli, A.; Cappabianca, S.; Grassi, R. Ultra high-frequency ultrasound: New capabilities for nail anatomy exploration. J. Dermatol. 2017, 44, 43–46. [Google Scholar] [CrossRef]
- Szymoniak-Lipska, M.; Polańska, A.; Jenerowicz, D.; Lipski, A.; Żaba, R.; Adamski, Z.; Dańczak-Pazdrowska, A. High-Frequency Ultrasonography and Evaporimetry in Non-invasive Evaluation of the Nail Unit. Front. Med. 2021, 8, 686470. [Google Scholar] [CrossRef]
- Ortner, V.K.; Mandel, V.D.; Bertugno, S.; Philipsen, P.A.; Haedersdal, M. Imaging of the nail unit in psoriatic patients:A sys-tematic scoping review of techniques and terminology. Exp. Dermatol. 2022, 31, 828–840. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A. Usefulness of Ultrasound Examination in the Assessment of the Nail Apparatus in Psoriasis. Int. J. Environ. Res. Public Health 2022, 19, 5611. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Żuber, Z.; Owczarczyk-Saczonek, A. Ultrasound Evaluation of the Effectiveness of the Use of Acitretin in the Treatment of Nail Psoriasis. J. Clin. Med. 2021, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Wortsman, X.; Tosti, A.; Iorizzo, M. Advances in image-based diagnosis of nail disorders. J. Eur. Acad. Dermatol. Venereol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wiktorowicz, A.; Wojtkiewicz, J. Distal interphalangeal joint extensor tendon enthesopathy in patients with nail psoriasis. Sci. Rep. 2019, 9, 3628. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Dini, V.; Salvia, G.; Granieri, G.; Manzo Margiotta, F.; Panduri, S.; Morganti, R.; Romanelli, M. Assessment and Monitoring of Nail Psoriasis with Ultra-High Frequency Ultrasound: Preliminary Results. Diagnostics 2023, 13, 2716. [Google Scholar] [CrossRef]
- Aluja Jaramillo, F.; Quiasúa Mejía, D.C.; Martínez Ordúz, H.M.; González Ardila, C. Nail unit ultrasound: A complete guide of the nail diseases. J. Ultrasound 2017, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Starace, M.; Piraccini, B.M.; Wortsman, X. Ultrasound Features of Onychopapilloma at High-Frequency and Ultra-High Frequency. J. Ultrasound Med. 2024, 43, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Lipner, S.R. Optimal diagnosis and management of common nail disorders. Ann. Med. 2022, 54, 694–712. [Google Scholar] [CrossRef]
- Turner, V.L.; Wortsman, X. Ultrasound Features of Nail Lichen Planus. J. Ultrasound Med. 2024, 43, 781–788. [Google Scholar] [CrossRef]
- Sechi, A.; Alessandrini, A.; Patrizi, A.; Starace, M.; Caposiena Caro, R.D.; Vara, G.; Brandi, N.; Golfieri, R.; Piraccini, B.M. Ultrasound features of the subungual glomus tumor and squamous cell carcinomas. Ski. Res. Technol. 2020, 26, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Pinto, C.; Amorim-Alves, L. Digital Mucous Cyst: An Unusual Presentation of Osteoarthritis. Acta Medica Port. 2022, 35, 591–592. [Google Scholar] [CrossRef]
- Patel, R.A.; Ariza-Hutchinson, A.; Emil, N.S.; Muruganandam, M.; Nunez, S.E.; McElwee, M.K.; O’Sullivan, F.X.; Hayward, W.A.; Haseler, L.J.; Sibbitt, W.L., Jr. Intraarticular injection of the interphalangeal joint for therapy of digital mucoid cysts. Rheumatol. Int. 2022, 42, 861–868. [Google Scholar] [CrossRef]
- Sechi, A.; Starace, M.; Alessandrini, A.; Caposiena Caro, R.D.; Piraccini, B.M. Digital Myxoid Cysts: Correlation of Initial and Long-Term Response to Steroid Injections. Dermatol. Surg. 2021, 47, e146–e152. [Google Scholar] [CrossRef]
- Vargas, E.A.T.; Finato, V.M.L.; Azulay-Abulafia, L.; Leverone, A.; Nakamura, R.; Wortsman, X. Ultrasound of Nails: Why, How, When. Semin. Ultrasound CT MRI 2024, 45, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Crisan, D.; Wortsman, X.; Catalano, O.; Badea, R.; Kastler, S.; Badea, A.; Manea, A.; Scharffetter-Kochanek, K.; Strilciuc, S.; Crisan, M.; et al. Pre-operative high-frequency ultrasound: A reliable management tool in auricular and nasal non-melanoma skin cancer. J. Dtsch. Dermatol. Ges. 2024, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Patanè, V.; Fusco, L.; Faggioni, L.; Boschetti, C.E.; Santagata, M.; Neri, E.; Cappabianca, S.; Reginelli, A. Reliability of Ultrasonographic Assessment of Depth of Invasion and Tumor Thickness in Intraoral Mucosa Lesions: A Preliminary Expe-rience. J. Clin. Med. 2024, 13, 2595. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Rubini, C.; Lazzarini, R.; DI Primio, R.; Offidani, A. Clinical and prognostic significance of survivin, AKT and VEGF in primary mucosal oral melanoma. Anticancer Res. 2015, 35, 2113–2120. [Google Scholar]
- Del Puerto, C.; Wortsman, X.; Downey, C. Lingual Epidermal Choristoma: Ultrasonography as a Diagnostic Tool. Dermatol. Pract. Concept. 2024, 14, e2024014. [Google Scholar] [CrossRef] [PubMed]
- Buch, J.; Karagaiah, P.; Raviprakash, P.; Patil, A.; Kroumpouzos, G.; Kassir, M.; Goldust, M. Noninvasive diagnostic techniques of port wine stain. J. Cosmet. Dermatol. 2021, 20, 2006–2014. [Google Scholar] [CrossRef]
- Rodríguez Bandera, A.I.; Feito Rodríguez, M.; Chiloeches Fernández, C.; Stewart, N.; Valdivielso-Ramos, M. Role of colour-Doppler high-frequency ultrasonography in capillary malformation-arteriovenous malformation syndrome: A case series. Australas. J. Dermatol. 2020, 61, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Qi, H.; Wang, T. The diagnostic value of ultrasonography in evaluation of the intraneural vascular anomalies of peripheral nerves. Acta Radiol. 2024, 65, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Pragasam, S.; Kumari, R.; Munisamy, M.; Mohan Thappa, D. Utility of high-frequency ultrasound in assessing cutaneous edema in venous ulcer patients. Ski. Res. Technol. 2021, 27, 904–908. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, J.; Wang, W.; Chen, X.; Xu, Y.; Huang, H.; Mi, J. A prospective study of super-thin anterolateral thigh flap harvesting assisted by high-frequency color Doppler ultrasound in detecting perforators in deep adipose layers. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2024, 38, 62–68. [Google Scholar] [PubMed]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.; Mann, G.; Salibian, A.A. Ultrasound in Microsurgery: Current Applications and New Frontiers. J. Clin. Med. 2024, 13, 3412. [Google Scholar] [CrossRef]
- Visconti, G.; Hayashi, A.; Bianchi, A.; Tartaglione, G.; Bartoletti, R.; Salgarello, M. Lymphaticovenular Anastomosis for Advanced-Stage Peripheral Lymphedema: Expanding Indication and Introducing the Hand/Foot Sign. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2153–2163. [Google Scholar] [CrossRef]
- Pazdrowski, J.; Dańczak-Pazdrowska, A.; Polańska, A.; Kaźmierska, J.; Barczak, W.; Szewczyk, M.; Golusiński, P.; Adamski, Z.; Żaba, R.; Golusiński, W. An ultrasonographic monitoring of skin condition in patients receiving radiotherapy for head and neck cancers. Ski. Res. Technol. 2019, 25, 857–861. [Google Scholar] [CrossRef]
- Garnier, M.; Champeaux, E.; Laurent, E.; Boehm, A.; Briard, O.; Wachter, T.; Vaillant, L.; Patat, F.; Bens, G.; Machet, L. High-frequency ultrasound quantification of acute radiation dermatitis: Pilot study of patients undergoing radiotherapy for breast cancer. Ski. Res. Technol. 2017, 23, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Meikle, B.; Simons, M.; Mahoney, T.; Reddan, T.; Dai, B.; Kimble, R.M.; Tyack, Z. Ultrasound measurement of traumatic scar and skin thickness: A scoping review of evidence across the translational pipeline of research-to-practice. BMJ Open 2024, 14, e078361. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.D.; Celestin, A.; Fish, E.; Glass, C.C.; Eskander, M.F.; Murillo, R.; Gospodinov, G.; Gupta, A.; Hauser, C.J. Surgical wound assessment by sonography in the prediction of surgical wound infections. J. Trauma Acute Care Surg. 2016, 80, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D.; Caffarini, M.; Orciani, M.; Giovanetti, E.; et al. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med. Microbiol. Immunol. 2019, 208, 877–883. [Google Scholar] [CrossRef]
- Krauze, A.; Woźniak, W.; Mlosek, R.K. Usefulness of high-frequency ultrasound to assess the healing progress of shin ulcers. J. Ultrason. 2021, 20, e254–e260. [Google Scholar] [CrossRef] [PubMed]
- Tantray, M.D.; Rather, A.; Manaan, Q.; Andleeb, I.; Mohammad, M.; Gull, Y. Role of ultrasound in detection of radiolucent foreign bodies in extremities. Strateg. Trauma Limb Reconstr. 2018, 13, 81–85. [Google Scholar] [CrossRef]
- Viviano, S.L.; Chandler, L.K.; Keith, J.D. Ultrahigh Frequency Ultrasound Imaging of the Hand: A New Diagnostic Tool for Hand Surgery. Hand 2018, 13, 720–725. [Google Scholar] [CrossRef]
- Crisan, D.; Tarnowietzki, E.; Bernhard, L.; Möller, M.; Scharffetter-Kochanek, K.; Crisan, M.; Schneider, L.A. Rationale for Using High-Frequency Ultrasound as a Routine Examination in Skin Cancer Surgery: A Practical Approach. J. Clin. Med. 2024, 13, 2152. [Google Scholar] [CrossRef]
- Moscarella, E.; Brancaccio, G.; Briatico, G.; Ronchi, A.; Verolino, P.; Argenziano, G.; Alfano, R. Management of advanced basal cell carcinoma: Real-life data with sonidegib. Dermatol. Ther. 2021, 34, e14948. [Google Scholar] [CrossRef] [PubMed]
- Laverde-Saad, A.; Simard, A.; Nassim, D.; Jfri, A.; Alajmi, A.; O’Brien, E.; Wortsman, X. Performance of Ultrasound for Identifying Morphological Characteristics and Thickness of Cutaneous Basal Cell Carcinoma: A Systematic Review. Dermatology 2022, 238, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Crisan, D.; Díaz, C.P.G.; Cavallieri, F.; Varelli, C.; Wortsman, X. Ultrasound Assessment of Skin Tumors Local Recurrence. J. Ultrasound Med. 2023, 42, 2439–2446. [Google Scholar] [CrossRef]
- Basra, M.; Shapiro, L.; Patel, H.; Payne, C.; Brazen, B.; Biglione, A. Exploring the Utilization of Imaging Modalities in the Diagnosis of Basal Cell Carcinoma: A Scoping Review. Cureus 2024, 16, e56047. [Google Scholar] [CrossRef]
- Bozsányi, S.; Boostani, M.; Farkas, K.; Hamilton-Meikle, P.; Varga, N.N.; Szabó, B.; Vasanits, F.; Kuroli, E.; Meznerics, F.A.; Lőrincz, K.; et al. Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study. J. Clin. Med. 2023, 12, 6910. [Google Scholar] [CrossRef]
- Russo, G.M.; Russo, A.; Urraro, F.; Cioce, F.; Gallo, L.; Belfiore, M.P.; Sangiovanni, A.; Napolitano, S.; Troiani, T.; Verolino, P.; et al. Management of Non-Melanoma Skin Cancer: Radiologists Challenging and Risk Assessment. Diagnostics 2023, 13, 793. [Google Scholar] [CrossRef]
- Wortsman, X.; Vergara, P.; Castro, A.; Saavedra, D.; Bobadilla, F.; Sazunic, I.; Zemelman, V.; Wortsman, J. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Licata, G.; Scharf, C.; Ronchi, A.; Pellerone, S.; Argenziano, G.; Verolino, P.; Moscarella, E. Diagnosis and Management of Melanoma of the Scalp: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1435–1447. [Google Scholar] [CrossRef]
- Varga, N.N.; Boostani, M.; Farkas, K.; Bánvölgyi, A.; Lőrincz, K.; Posta, M.; Lihacova, I.; Lihachev, A.; Medvecz, M.; Holló, P.; et al. Optically Guided High-Frequency Ultrasound Shows Superior Efficacy for Preoperative Estimation of Breslow Thickness in Comparison with Multispectral Imaging: A Single-Center Prospective Validation Study. Cancers 2023, 16, 157. [Google Scholar] [CrossRef]
- Reginelli, A.; Russo, A.; Berritto, D.; Patane, V.; Cantisani, C.; Grassi, R. Ultra-High-Frequency Ultrasound: A Modern Diagnostic Technique for Studying Melanoma. Ultraschall Med. 2023, 44, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of High-Frequency Ultrasonography in the Diagnosis of Melanoma: Mini Review. Front. Oncol. 2021, 11, 673026. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Sica, A.; et al. A Preliminary Study for Quantitative Assessment with HFUS (High- Frequency Ultrasound) of Nodular Skin Melanoma Breslow Thickness in Adults Before Surgery: Interdisciplinary Team Experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar]
- Płocka, M.; Czajkowski, R. High-frequency ultrasound in the diagnosis and treatment of skin neoplasms. Postep. Dermatol. Alergol. 2023, 40, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aragüés, I.; Vázquez-Osorio, I.; Alfageme, F.; Ciudad-Blanco, C.; Casas-Férnandez, L.; Rodríguez-Blanco, M.I.; Suárez-Fernández, R. Skin ultrasound features of Merkel cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e315–e318. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Vitiello, P.; Caccavale, S.; Sagnelli, C.; Calogero, A.; Doraro, C.A.; Pastore, F.; Ciardiello, F.; Argenziano, G.; Reginelli, A.; et al. Primary Cutaneous DLBCL Non-GCB Type: Challenges of a Rare Case. Open Med. 2020, 15, 119–125. [Google Scholar] [CrossRef]
- Russo, A.; Patanè, V.; Gagliardi, F.; Urraro, F.; Ronchi, A.; Vitiello, P.; Sica, A.; Argenziano, G.; Nardone, V.; Reginelli, A. Preliminary Experience in Ultra-High Frequency Ultrasound Assessment of Cutaneous Primary Lymphomas: An Innovative Classification. Cancers 2024, 16, 2456. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth-Wieser, I.; Ramjist, J.M.; Shear, N.; Alhusayen, R. Morphologic Features of Cutaneous T-Cell Lymphomas Using Dermoscopy and High Frequency Ultrasound. J. Clin. Med. 2020, 10, 17. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J. Dermoscopy and high-frequency ultrasound provide diagnostic clues in a gastric adenocarcinoma with cutaneous metastasis as the initial presentation: A case report. Ski. Res. Technol. 2023, 29, e13380. [Google Scholar] [CrossRef] [PubMed]
- Solivetti, F.M.; Elia, F.; Latini, A.; Cota, C.; Cordiali-Fei, P.; Di Carlo, A. AIDS-Kaposi Sarcoma and Classic Kaposi Sarcoma: Are different ultrasound patterns related to different variants? J. Exp. Clin. Cancer Res. 2011, 30, 40. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Wang, S.; Yang, J. Ultra-High-Frequency Ultrasound in the Evaluation of Paediatric Pilomatricoma Based on the Histopathologic Classification. Front. Med. 2021, 8, 673861. [Google Scholar] [CrossRef]
- Rodríguez Bandera, A.I.; Moreno Bonilla, G.; Feito Rodríguez, M.; Beato Merino, M.J.; de Lucas Laguna, R. Jellyfish-like sonographic pattern can help recognition of dermatofibrosarcoma protuberans. Report of 3 new cases and review of the literature. Australas. J. Dermatol. 2019, 60, e148–e150. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Huang, Q.; Yang, T.; Jiang, Y.; Zhang, L.J.; Xie, Y.; Zheng, R.Q. Role of ultrasound in the diagnosis of primary and recurrent dermatofibrosarcoma protuberans. BMC Cancer 2021, 21, 909. [Google Scholar] [CrossRef] [PubMed]
- Helbig, D.; Ziemer, M.; Dippel, E.; Erdmann, M.; Hillen, U.; Leiter, U.; Mentzel, T.; Osterhoff, G.; Ugurel, S.; Utikal, J.; et al. S1-guideline atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS). J. Dtsch. Dermatol. Ges. 2022, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.; Nguyen, D.A.; Malouf, P.; Ingersoll, Z.; Hosler, G.A.; Weis, S.E. Pleomorphic Dermal Sarcoma: A Clinical and Histopathologic Emulator of Atypical Fibroxanthoma, but Different Biologic Behavior. HCA Healthc. J. Med. 2022, 3, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Taleb, E.; Saldías, C.; Gonzalez, S.; Misad, C.; Wortsman, X. Sonographic Characteristics of Leiomyomatous Tumors of Skin and Nail: A Case Series. Dermatol. Pract. Concept. 2022, 12, e2022082. [Google Scholar] [CrossRef] [PubMed]
- Hobayan, C.G.P.; Gray, A.N.; Waters, M.F.; Mager, L.A.; Kobayashi, S.; Essien, E.W.; Ulman, C.A.; Kaffenberger, B.H. Diagnostic accuracy of high-frequency ultrasound for cutaneous neoplasms: A narrative review of the literature. Arch. Dermatol. Res. 2024, 316, 419. [Google Scholar] [CrossRef]
- Li, D.; Yang, F.; Zhao, Y.; Wang, Q.; Ren, W.; Sun, L.; Shan, D.; Qin, C. High-Frequency Ultrasound Imaging to Distinguish High-Risk and Low-Risk Dermatofibromas. Diagnostics 2023, 13, 3305. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Malinowska, S.P. High-Frequency Ultrasound in the Assessment of Cellulite-Correlation between Ultrasound-Derived Measurements, Clinical Assessment, and Nürnberger-Müller Scale Scores. Diagnostics 2024, 14, 1878. [Google Scholar] [CrossRef]
- Whipple, L.A.; Fournier, C.T.; Heiman, A.J.; Awad, A.A.; Roth, M.Z.; Cotofana, S.; Ricci, J.A. The Anatomical Basis of Cellulite Dimple Formation: An Ultrasound-Based Examination. Plast. Reconstr. Surg. 2021, 148, 375e–381e. [Google Scholar] [CrossRef]
- Sandby-Møller, J.; Wulf, H.C. Ultrasonographic subepidermal low-echogenic band, dependence of age and body site. Ski. Res. Technol. 2004, 10, 57–63. [Google Scholar] [CrossRef]
- Crisan, D.; Roman, I.; Crisan, M.; Scharffetter-Kochanek, K.; Badea, R. The role of vitamin C in pushing back the boundaries of skin aging: An ultrasonographic approach. Clin. Cosmet. Investig. Dermatol. 2015, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Pequeno, A.L.V.; Bagatin, E. Dermatological ultrasound in assessing skin aging. Front Med. 2024, 11, 1353605. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, J.; Juszczyk, J.; Bugdol, M.N.; Glenc-Ambroży, M.; Polak, A.; Piejko, L.; Pietka, E. High-frequency ultrasound in anti-aging skin therapy monitoring. Sci. Rep. 2023, 13, 17799. [Google Scholar] [CrossRef]
- Salvia, G.; Zerbinati, N.; Manzo Margiotta, F.; Michelucci, A.; Granieri, G.; Fidanzi, C.; Morganti, R.; Romanelli, M.; Dini, V. Ultra-High-Frequency Ultrasound as an Innovative Imaging Evaluation of Hyaluronic Acid Filler in Nasolabial Folds. Diagnostics 2023, 13, 2761. [Google Scholar] [CrossRef]
- Cavallieri, F.A.; Balassiano, L.K.A.; Munhoz, G.; Tembra, M.F.; Wortsman, X. Ultrasound in Aesthetics: Filler and Non-Filler Applications. Semin. Ultrasound CT MRI 2024, 45, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Top applications of dermatologic ultrasonography that can modify management. Ultrasonography 2023, 42, 183–202. [Google Scholar] [CrossRef]
- Sigrist, R.; Fassina, M.; Wortsman, X. Ultrasonographic Pattern of a New Generation of Polymethylmethacrylate at High-Frequency and Ultra-High Frequency. Dermatol. Surg. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Sigrist, R.; Noronha, G.; Quezada, N.; Wortsman, X. Ultrasonographic Pattern of Poly- l -Lactic Acid at High-Frequency and Ultrahigh-Frequency. Dermatol. Surg. 2024, 50, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.S.F.; de Almeida, T.S.C.; Carvalho, R.M.; Machado, C.J.; Bravo, L.G.; Elias, M.C. Dermal Thickness Increase and Aesthetic Improvement with Hybrid Product Combining Hyaluronic Acid and Calcium Hydroxyapatite: A Clinical and Sonographic Analysis. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5055. [Google Scholar] [CrossRef] [PubMed]
- Casabona, G.; Kaye, K.; Cotofana, S.; Davidovic, K.; Alfertshofer, M.; Freytag, L. Histological effects of a combined collagen stimulation procedure consisting of microfocused ultrasound, soft tissue filler, and Ca-HA injections. J. Cosmet. Dermatol. 2023, 22, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jia, Q.N.; Jin, H.Z.; Li, F.; He, C.X.; Yang, J.; Zuo, Y.G.; Fu, L.Q. Long-Term Follow-Up of Longevity and Diffusion Pattern of Hyaluronic Acid in Nasolabial Fold Correction through High-Frequency Ultrasound. Plast. Reconstr. Surg. 2019, 144, 189e–196e. [Google Scholar] [CrossRef] [PubMed]
- Turlier, V.; Rouquier, A.; Black, D.; Josse, G.; Auvergnat, A.; Briant, A.; Dahan, S.; Gassia, V.; Saint-Martory, C.; Zakaria, W.; et al. Assessment of the clinical efficacy of a hyaluronic acid-based deep wrinkle filler using new instrumental methods. J. Cosmet. Laser Ther. 2010, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Urdiales-Gálvez, F.; Delgado, N.E.; Figueiredo, V.; Lajo-Plaza, J.V.; Mira, M.; Ortíz-Martí, F.; Del Rio-Reyes, R.; Romero-Álvarez, N.; Del Cueto, S.R.; Segurado, M.A.; et al. Preventing the Complications Associated with the Use of Dermal Fillers in Facial Aesthetic Procedures: An Expert Group Consensus Report. Aesthetic Plast. Surg. 2017, 41, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, L.; Li, Z.; Su, X.; Hu, J.; Chai, H. High-Frequency Ultrasound of Facial Filler Materials in the Nasolabial Groove. Aesthetic Plast. Surg. 2022, 46, 2972–2978. [Google Scholar] [CrossRef]
- Chai, H.; Su, X.; Yuan, L.; Li, Z.; Jiang, L.; Liu, Y.; Dou, M.; Hu, J. High-Frequency Ultrasound Imaging Findings of Different Mental Injectable Soft Tissue Fillers. Aesthetic Plast. Surg. 2022, 46, 2995–3002. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, L.; Su, X.; Zhen, Q.; Jia, Y.; Hu, J.; Chai, H. The Application of High-frequency Ultrasonic Imaging in Identifying Fillers in the Temporal Region. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5269. [Google Scholar] [CrossRef]
- Sigrist, R.; Desyatnikova, S.; Chammas, M.C.; Vasconcelos-Berg, R. Best Practices for the Use of High-Frequency Ultrasound to Guide Aesthetic Filler Injections-Part 1: Upper Third of the Face. Diagnostics 2024, 14, 1718. [Google Scholar] [CrossRef]
- Weiner, S.F. Ultrasound for the Aesthetic Injector. Plast. Aesthetic Nurs. 2022, 42, 88–98. [Google Scholar] [CrossRef]
- Velthuis, P.J.; Jansen, O.; Schelke, L.W.; Moon, H.J.; Kadouch, J.; Ascher, B.; Cotofana, S. A Guide to Doppler Ultrasound Analysis of the Face in Cosmetic Medicine. Part 1: Standard Positions. Aesthetic Surg. J. 2021, 41, NP1621–NP1632. [Google Scholar] [CrossRef]
- Vasconcelos-Berg, R.; Izidoro, J.F.; Wenz, F.; Müller, A.; Navarini, A.A.; Sigrist, R.M.S. Doppler Ultrasound-Guided Filler Injections: Useful Tips to Integrate Ultrasound in Daily Practice. Aesthetic Surg. J. 2023, 43, 773–783. [Google Scholar] [CrossRef]
- Desyatnikova, S.; Barrera, P. High-Resolution Ultrasound for Diagnosis and Treatment of Filler-Related Septal Necrosis. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5630. [Google Scholar] [CrossRef] [PubMed]
- Desyatnikova, S.; Mangieri, L. Nitrous Oxide Improves Tissue Perfusion in Vascular Occlusion Management. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5154. [Google Scholar] [CrossRef]
- Schelke, L.; Lowrey, N.; Mojallal, A.; Rowland-Warmann, M.J.; Wortsman, X.; Sigrist, R.M.; Velthuis, P.J.; Cotofana, S. Post-Treatment Displacement of Facial Soft Tissue Fillers-A Retrospective Ultrasound-based Investigation of 382 Zygomatic Regions. Dermatol. Surg. 2024, 50, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Mlosek, R.K.; Skrzypek, E.; Skrzypek, D.M.; Malinowska, S. High-frequency ultrasound-based differentiation between nodular dermal filler deposits and foreign body granulomas. Ski. Res. Technol. 2018, 24, 417–422. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Migda, B.; Skrzypek, E.; Słoboda, K.; Migda, M. The use of high-frequency ultrasonography for the diagnosis of palpable nodules after the administration of dermal fillers. J. Ultrason. 2021, 20, e248–e253. [Google Scholar] [CrossRef]
- Cerón Bohórquez, J.M.; Desyatnikova, S. Ultrasound-guided Treatment of Polycaprolactone Granuloma. Plast Reconstr Surg Glob Open. 2024, 12, e5610. [Google Scholar] [CrossRef]
- Gatica, J.L.; Ortega, R.; Aragón-Caqueo, D.; Wortsman, X.; Sazunic, I.; Loubies, R. Granulomatous Reaction Secondary to Fractional Radiofrequency Microneedling with Clinical, Ultrasonographic, and Histologic Correlation. Facial Plast. Surg. Aesthetic Med. 2024, 26, 529–531. [Google Scholar] [CrossRef]
- Gonzalez, C.; Rengifo, J.; Macias-Arias, P.; Duque-Clavijo, V.; Noreña-Rengifo, B.D. High-Resolution Ultrasound for Complications of Botulinum Toxin Use: A Case Series and Literature Review. Cureus 2024, 16, e63232. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, D.; Kenkel, J.M. Practice Advisory on Gluteal Fat Grafting. Aesthetic Surg. J. 2022, 42, 1019–1029. [Google Scholar] [CrossRef]
- Tillo, O.; Nassab, R.; Pacifico, M.D. The British Association of Aesthetic Plastic Surgeons (BAAPS) Gluteal Fat Grafting Safety Review and Recommendations. Aesthetic Surg. J. 2023, 43, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Laureano, N.; Huerta, C.T.; Perez, E.A.; Earle, S.A. Augmented Safety Profile of Ultrasound-Guided Gluteal Fat Transfer: Retrospective Study with 1815 Patients. Aesthetic Surg. J. 2024, 44, NP263–NP270. [Google Scholar] [CrossRef] [PubMed]
- Elsaftawy, A.; Ostrowski, P.; Bonczar, M.; Stolarski, M.; Gabryszuk, K.; Bonczar, T. Buttock Augmentation with Ultrasonic Liposuction and Ultrasound-Guided Fat Grafting: A Retrospective Analysis Based on 185 Patients. J. Clin. Med. 2024, 13, 1526. [Google Scholar] [CrossRef]
- Elsaftawy, A.; Ostrowski, P.; Bonczar, M.; Stolarski, M.; Gabryszuk, K.; Bonczar, T. Enhancing Buttock Contours: A Safer Approach to Gluteal Augmentation with Ultrasonic Liposuction, Submuscular Implants, and Ultrasound-Guided Fat Grafting. J. Clin. Med. 2024, 13, 2856. [Google Scholar] [CrossRef]
- Wang, B.; He, P.; Zhao, R. B-ultrasound-assisted gluteal fat grafting in Asians: A prospective study of quantitative results from three-dimensional imaging and B-ultrasound analysis. J. Plast. Reconstr. Aesthetic Surg. 2024, 94, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Durán Vega, H.C.; Manzaneda, R.; Flores, E.; Manfrim, C.; Morelli, H. Deep Back Liposuction: Ultrasound-Guided Deep Fat Liposuction of the Subiliac Crest. Aesthetic Surg. J. 2024, 44, 296–301. [Google Scholar] [CrossRef]
- Gagliardi, F.; Russo, A.; Scharf, C.; Pinto, A.; Faenza, M.; D’Ippolito, E.; Argenziano, G.; Troiani, T.; Reginelli, A.; Nardone, V. All for one: Collaboration between dermatologist, radiation oncologist and radiologist in the clinical management of “difficult to treat” non melanoma skin cancer. Clin. Transl. Radiat. Oncol. 2024, 46, 100774. [Google Scholar] [CrossRef] [PubMed]
- Sechi, A.; Patrizi, A.; Vincenzi, C.; Savoia, F.; Tartari, F.; Leuzzi, M.; Di Altobrando, A.; Besagni, F.; Merli, Y.; Neri, I. Sonographic features of vaccination granulomas in children with delayed-type hypersensitivity to aluminum. Pediatr. Dermatol. 2019, 36, 1012–1016. [Google Scholar] [CrossRef]
- Gonzalez, C.; Wortsman, X. How to Start on Dermatologic Ultrasound: Basic Anatomical Concepts, Guidelines, Technical Considerations, and Best Tips. Semin. Ultrasound CT MRI 2024, 45, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanchez, A.; Bambekova, P.G.; Owen, J.L.; Mader, M.; Wortsman, X.; Soni, N.J. Barriers to dermatologic ultrasound: A national survey of dermatologists in the US Veterans Affairs health care system. JAAD Int. 2022, 9, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Hoppmann, R.A.; Mladenovic, J.; Melniker, L.; Badea, R.; Blaivas, M.; Montorfano, M.; Abuhamad, A.; Noble, V.; Hussain, A.; Prosen, G.; et al. International consensus conference recommendations on ultrasound education for undergraduate medical students. Ultrasound J. 2022, 14, 31. [Google Scholar] [CrossRef]
- Tagliati, C.; Rizzetto, G.; Molinelli, E.; De Simoni, E.; Fogante, M.; Argalia, G.; Lanni, G.; Rebonato, A.; Burroni, L.; Giuseppetti, G.M.; et al. Does dermatoradiology exist? Acta Dermatovenerol. Alp. Pannonica Adriat. 2024, 33, 107–108. [Google Scholar] [CrossRef]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, I.; Carrera, C.; Palou, J.; Alos, L.; Malvehy, J.; Puig, S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br. J. Dermatol. 2014, 170, 802–808. [Google Scholar] [CrossRef]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. High-frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013188. [Google Scholar] [CrossRef] [PubMed]
- Alfageme, F.; Minguela, E.; Martínez, C.; Salgüero, I.; Calvo, A.; León, F.; Álvarez, L.; de Vicente, O.; Panadero, F.J.; Salguero, O.L.; et al. Dermatologic Ultrasound in Primary Care: A New Modality of Teledermatology: A Prospective Multicenter Validation Study. J. Ultrasound Med. 2021, 40, 351–356. [Google Scholar] [CrossRef]
- Khan, R.; Khachemoune, A. Ultrasound Utility in the Management of Morphea: A Comprehensive Review. Indian Dermatol. Online J. 2024, 15, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Ali, F.; Al-Niaimi, F. Ultrasonography in diagnostic dermatology: A primer for clinicians. Arch. Dermatol. Res. 2023, 315, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Yang, Z.G.; Qi, W.H.; Zhang, H.; Bi, Y.; Shan, Y.; Cui, X.W.; Jiang, F. A preliminary study on ultrasound techniques applied to evaluate the curative effect of botulinum toxin type a in hypertrophic scars. Heliyon 2024, 10, e34723. [Google Scholar] [CrossRef]
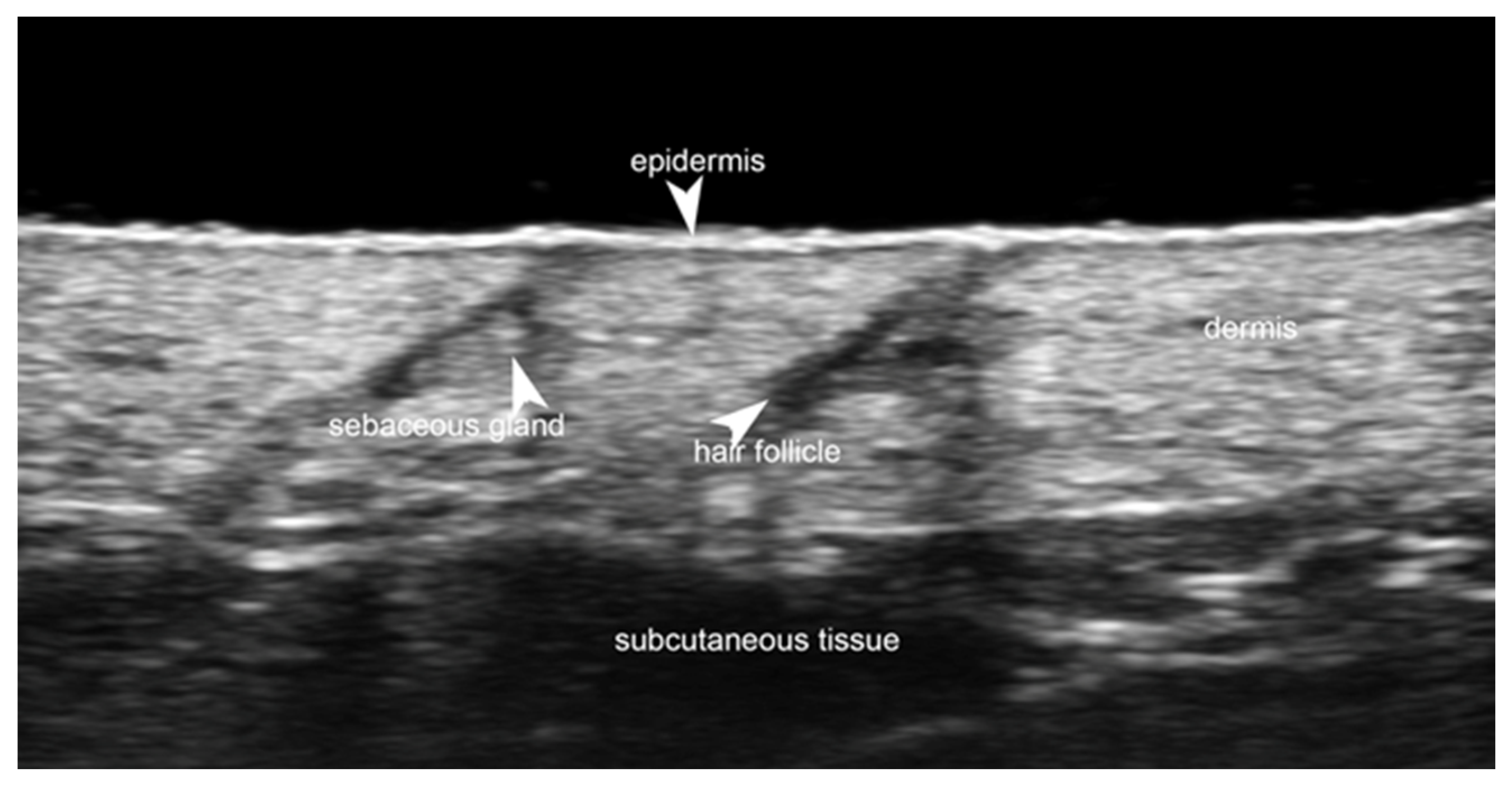

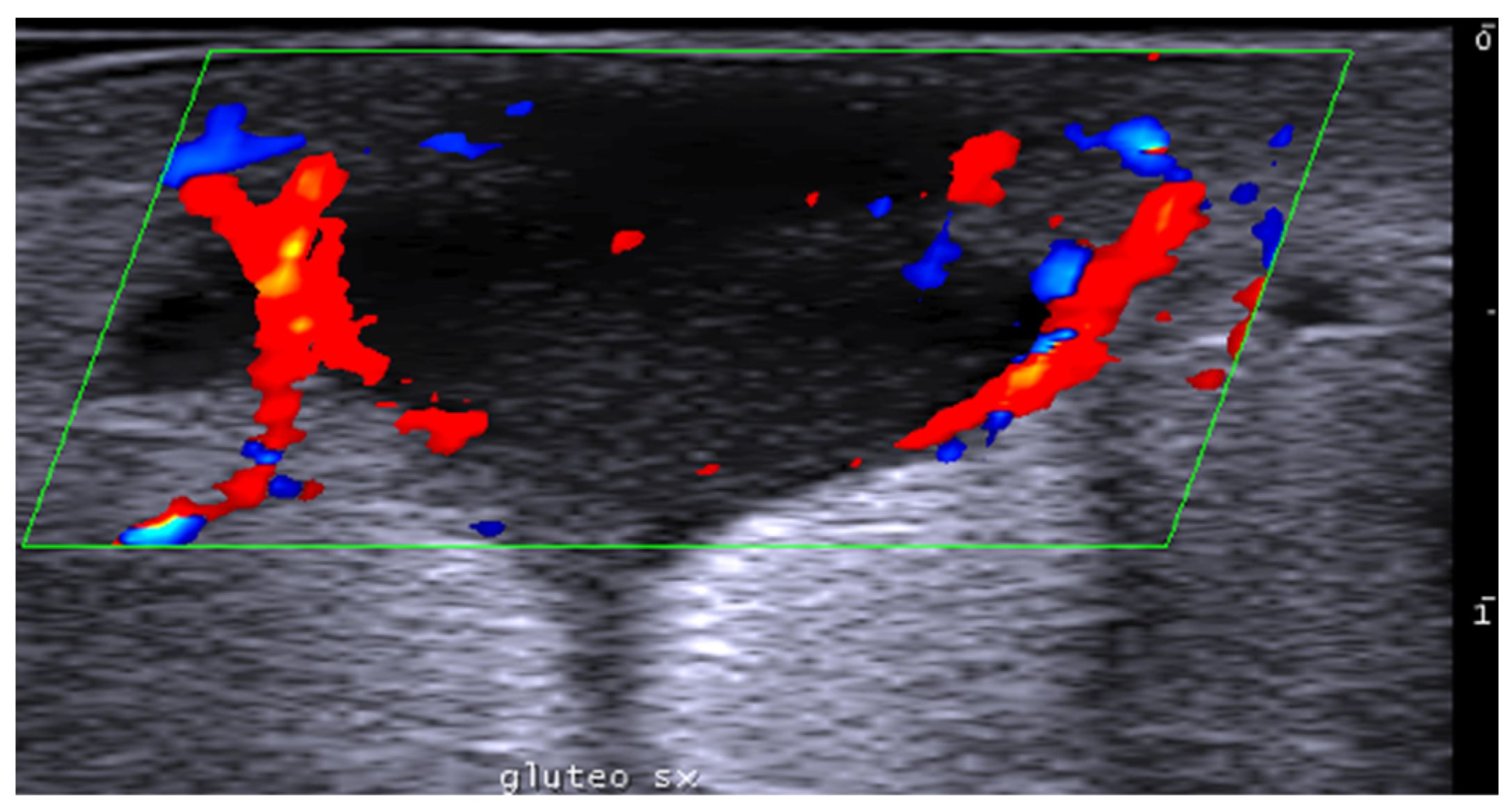



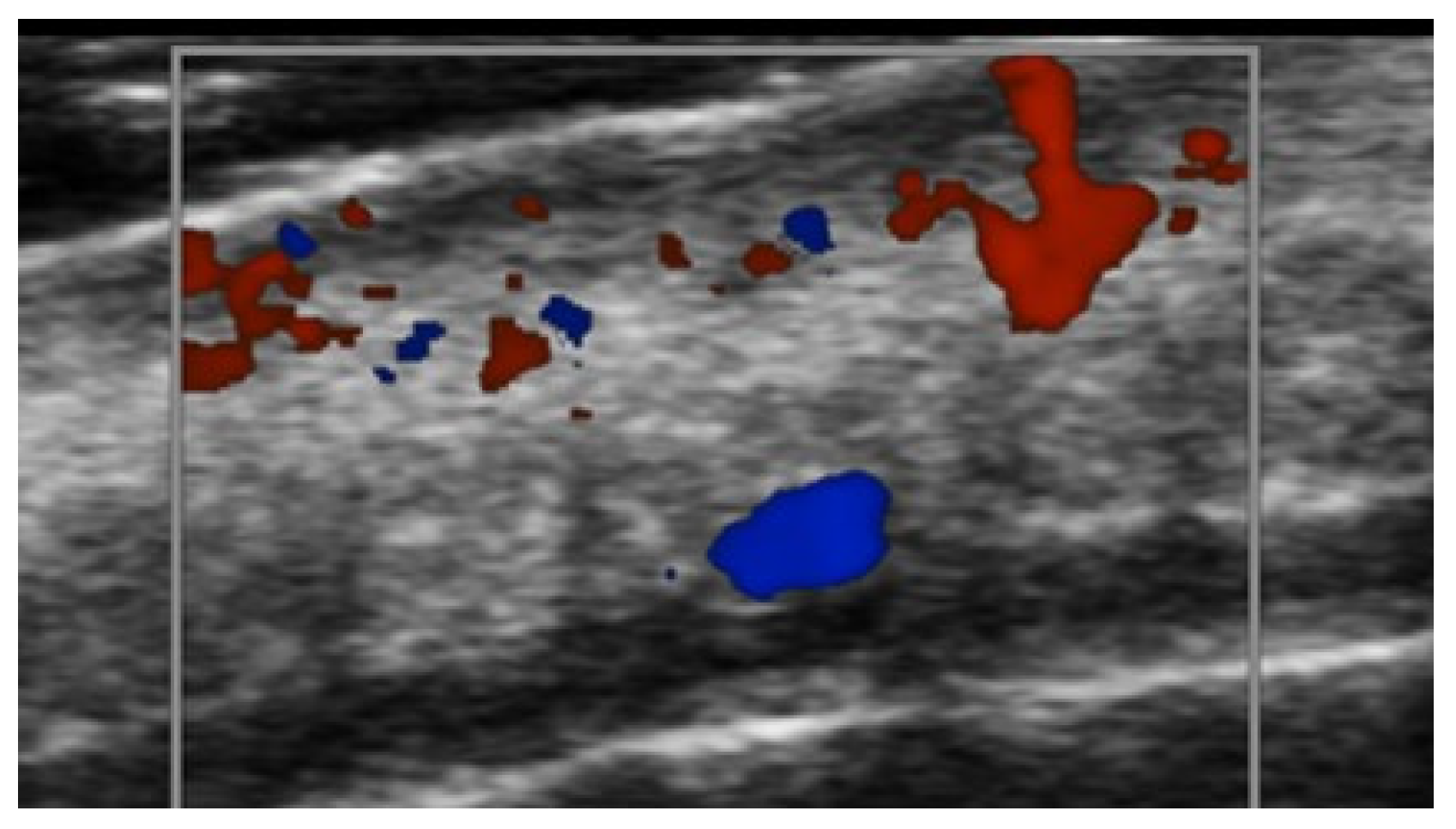
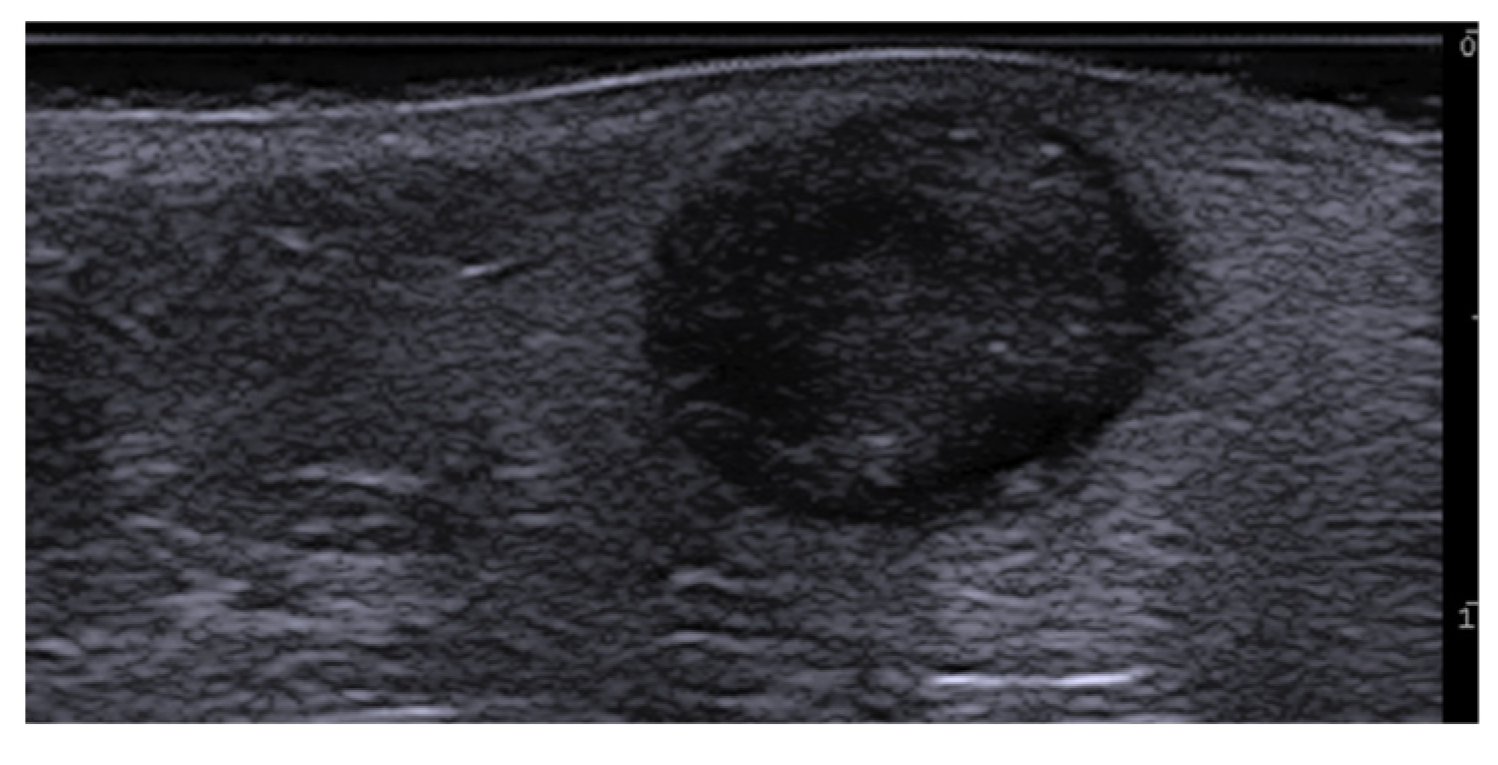


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argalia, G.; Reginelli, A.; Molinelli, E.; Russo, A.; Michelucci, A.; Sechi, A.; Marzano, A.V.; Desyatnikova, S.; Fogante, M.; Patanè, V.; et al. High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine. Medicina 2025, 61, 220. https://doi.org/10.3390/medicina61020220
Argalia G, Reginelli A, Molinelli E, Russo A, Michelucci A, Sechi A, Marzano AV, Desyatnikova S, Fogante M, Patanè V, et al. High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine. Medicina. 2025; 61(2):220. https://doi.org/10.3390/medicina61020220
Chicago/Turabian StyleArgalia, Giulio, Alfonso Reginelli, Elisa Molinelli, Anna Russo, Alessandra Michelucci, Andrea Sechi, Angelo Valerio Marzano, Stella Desyatnikova, Marco Fogante, Vittorio Patanè, and et al. 2025. "High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine" Medicina 61, no. 2: 220. https://doi.org/10.3390/medicina61020220
APA StyleArgalia, G., Reginelli, A., Molinelli, E., Russo, A., Michelucci, A., Sechi, A., Marzano, A. V., Desyatnikova, S., Fogante, M., Patanè, V., Granieri, G., Tagliati, C., Rizzetto, G., De Simoni, E., Matteucci, M., Candelora, M., Lanza, C., Ventura, C., Carboni, N., ... Simonetti, O. (2025). High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine. Medicina, 61(2), 220. https://doi.org/10.3390/medicina61020220










