Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study
2.2. Human Ethics
2.3. Quantification of Catalase Activity in Plasma by the Spectrophotometric Method
2.4. Quantification of SOD Activity in Plasma by the Spectrophotometric Method
2.5. Quantification of GPx Activity in Plasma by the Spectrophotometric Method
2.6. Methods of Analysis
3. Results
3.1. Plasma Catalase Activity
3.2. Plasma SOD Activity
3.3. Plasma GPx Activity
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Mocol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; EnNwekegwa, F.N.; Nkeh-Chungag, B.N. Free radicals, oxidative stress-related diseases and antioxidant supplementation. Altern. Ther. Health Med. 2022, 28, 114–128. [Google Scholar] [PubMed]
- Madireddy, S.; Madireddy, S. Protection from the pathogenesis of neurodegenerative disorders, including Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, Huntington’s Disease, and Parkinson’s Diseases, through the mitigation of reactive oxygen species. J. Neurosci. Neurol. Disord. 2019, 3, 148–161. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Sisein, E.A. Biochemistry of free radicals and antioxidants. Sch. Acad. J. Biosci. 2014, 2, 110–118. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Bisht, R. Antioxidants: A brief review. J. Drug Deliv. Ther. 2018, 8, 373–376. Available online: https://jddtonline.info/index.php/jddt/article/view/2116 (accessed on 8 January 2025). [CrossRef]
- Suleiman, N.; Bulama, I.; Muhammad, N.I.; Aishat, D.I.; Balarabe, S.A.; Ngaski, A.A.; Buhari, S.; Jimoh, A.A.; Abbas, A.Y.; Saidu, Y.; et al. Neurochemical effects of Vitamins C, E and DMSO combinations on oxidative stress biomarkers and severity of ischemic stroke in Wistar rats. Arch. Neurol. Neurosci. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Andonova, L.; Georgieva, M.; Zlatkov, A. Free radicals, oxidative stress, and diseases associated with them. Pharamacia 2015, 62, 26–39. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kamat, C.D.; Gadal, S.; Mhatre, M.; Williamson, K.S.; Pye, Q.N.; Hensley, K. Antioxidants in central nervous system diseases: Preclinical promise and translational challenges. J. Alzheimers Dis. 2008, 15, 473–493. [Google Scholar] [CrossRef]
- Gallego, D.; Rojas, M.; Orozco, C. Free Radicals, Neuronal Death and Neuroprotection. In Neurodegenerative Diseases—Processes, Prevention, Protection and Monitoring; Chuen-Chung Chang, R., Ed.; InTechOpen: London, UK; Rijeka, Croatia, 2011; Chapter 7. [Google Scholar] [CrossRef]
- Cherubini, A.; Polidori, M.C.; Bregnocchi, M.; Pezzuto, S.; Cecchetti, R.; Ingegni, T.; di Iorio, A.; Senin, U.; Mecocci, P. Antioxidant profile and early outcome in stroke patients. Stroke 2000, 31, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Viola, T.W.; Orso, R.; Florian, L.F.; Garcia, M.G.; Gomes, M.G.S.; Mardini, E.M.; Niederauer, J.P.O.; Zaparte, A.; Grassi-Oliveira, R. Effects of substance use disorder on oxidative and antioxidative stress markers: A systematic review and meta-analysis. Addict. Biol. 2023, 28, e13254. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Pervin, Z.; Stephen, J.M. Effect of alcohol on the central nervous system to develop neurological disorder: Pathophysiological and lifestyle modulation can be potential therapeutic options for alcohol-induced neurotoxication. AIMS Neurosci. 2021, 8, 390–413. [Google Scholar] [CrossRef]
- Crews, F.T. Alcohol-related neurodegeneration and recovery: Mechanisms from animal models. Alcohol Res. Health 2008, 31, 377–388. [Google Scholar]
- Tsermpini, E.E.; Ilješ, A.P.; Dolžan, V. Alcohol-induced oxidative stress and the role of antioxidants in alcohol use disorder: A Systematic Review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef]
- Kosciuczuk, U.; Jakubow, P.; Tarnowska, K.; Rynkiewicz-Szczepanska, E. Opioid Therapy and Implications for Oxidative Balance: A clinical study of total oxidative capacity (TOC) and total antioxidative capacity (TAC). J. Clin. Med. 2024, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Kadkhodaee, M.; Zahmatkesh, M.; Sefi, B.; Bakhshi, E.; Akhondzadeh, S.; Adeli, S.; Askari, H.; Arbabi, M. Opioid Use Disorder Induces Oxidative Stress and Inflammation: The Attenuating Effect of Methadone Maintenance Treatment. Iran. J. Psychiatry 2018, 13, 46–54. [Google Scholar] [PubMed]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, J.; Mahajan, J.; Kumar, R.; Arora, S. Oxidative stress-implications, source and its prevention. Environ. Sci. Pollut. Res. Int. 2014, 21, 1599–1613. [Google Scholar] [CrossRef]
- Moraes, L.; Dries, S.S.; Seibert, B.S.; Linden, R.; Perassolo, M.S. Evaluation of oxidative stress markers in ethanol users. Braz. J. Med. Biol. Res. 2023, 56, e12465. [Google Scholar] [CrossRef]
- Nordmann, R. Alcohol and antioxidant systems. Alcohol Alcohol. 1994, 29, 513–522. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, C.Y.; Huang, T.L.; Tsai, M.C. Brain-derived neurotrophic factor and glutathione peroxidase as state biomarkers in alcohol use disorder patients undergoing detoxification. Medicine 2020, 99, e19938. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Kattimani, S.; Sridhar, M.G. Oxidative stress during alcohol Withdrawal and its relationship with withdrawal severity. Indian J. Psychol. Med. 2015, 37, 175–180. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, X.; Tan, X.; Huang, X.; Yuan, L.; Zhang, Z.; Yang, Y.; Xu, M.; Wan, Y.; Li, Z. The Status of Oxidative Stress in Patients with Alcohol Dependence: A Meta-Analysis. Antioxidants 2022, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Temel, I.; Özerol, E.; Bay, A.; Çigli, A.; Akyol, Ö. Erythrocyte Catalase Activities in Alcohol Consumption, Medications and Some Diseases. Inönü Üniversitesi Tip Fakültesi Derg. 2002, 9, 11–14. Available online: https://hdl.handle.net/11616/42453 (accessed on 8 January 2025).
- Prokopieva, V.D.; Vetlugina, T.P. Features of oxidative stress in alcoholism. Biomeditsinskaya Khimiya 2023, 69, 83–96. Available online: http://pbmc.ibmc.msk.ru/en/issues-en/PBMC-2023-69-2/ (accessed on 8 January 2025). [CrossRef] [PubMed]
- Berríos-Cárcamo, P.; Quezada, M.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules. Antioxidants 2020, 9, 830. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
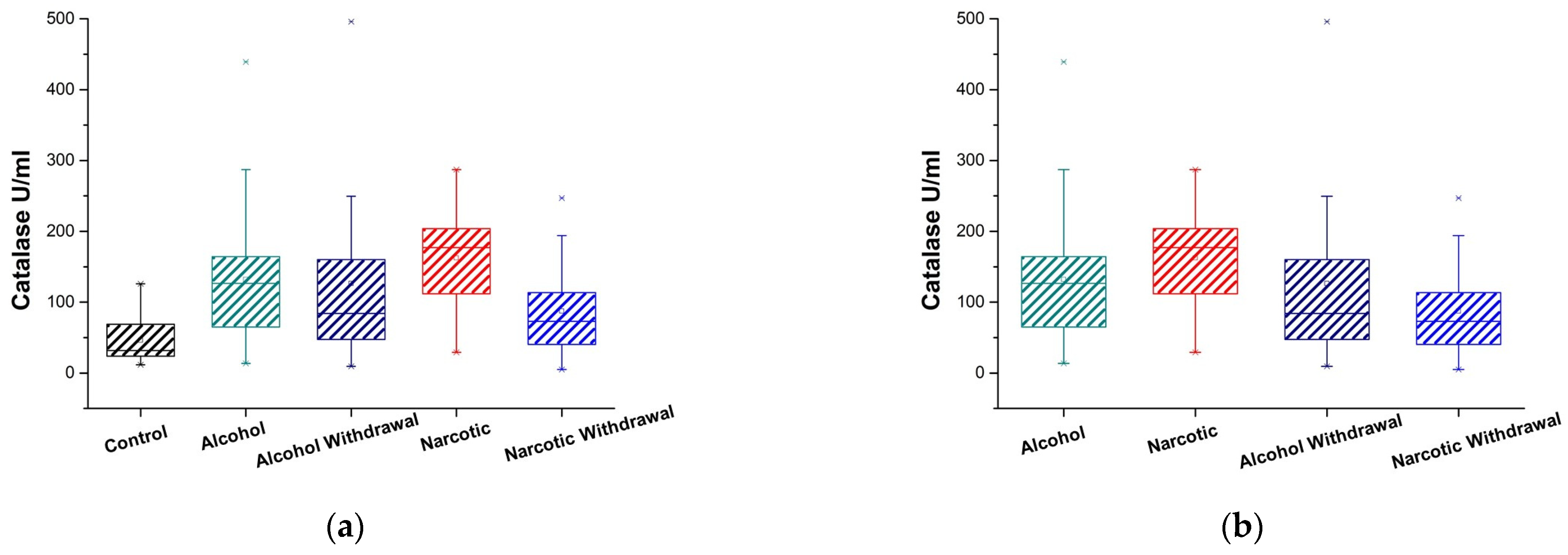
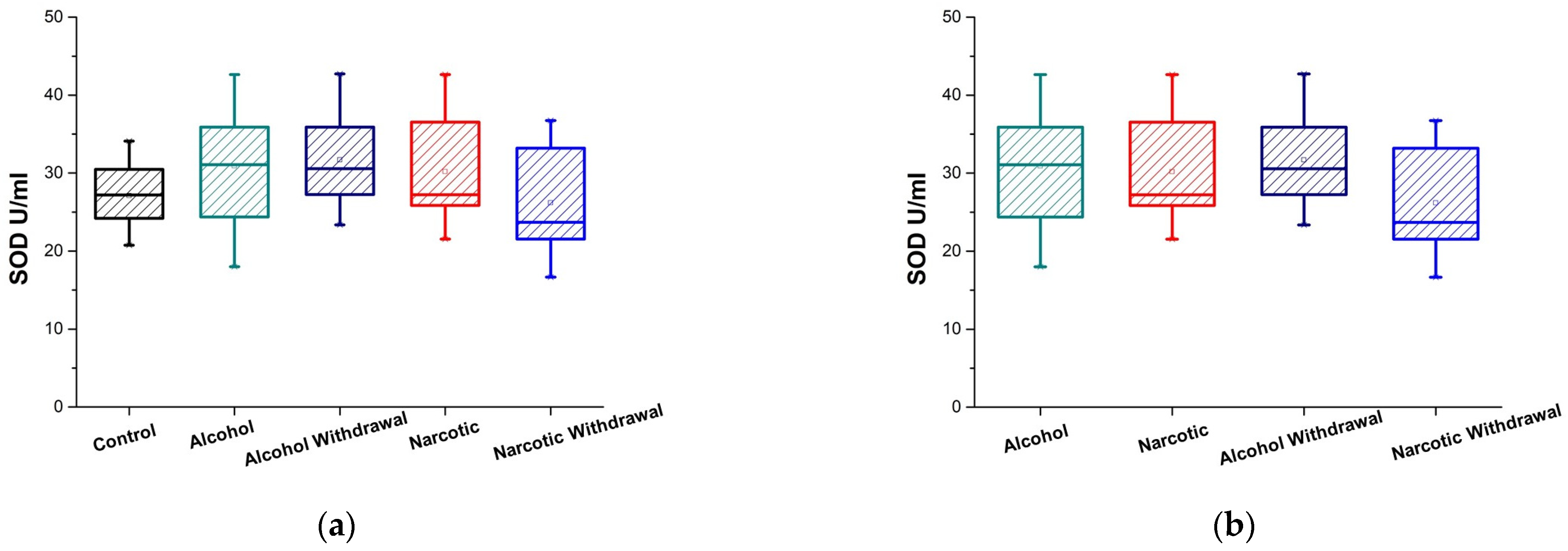
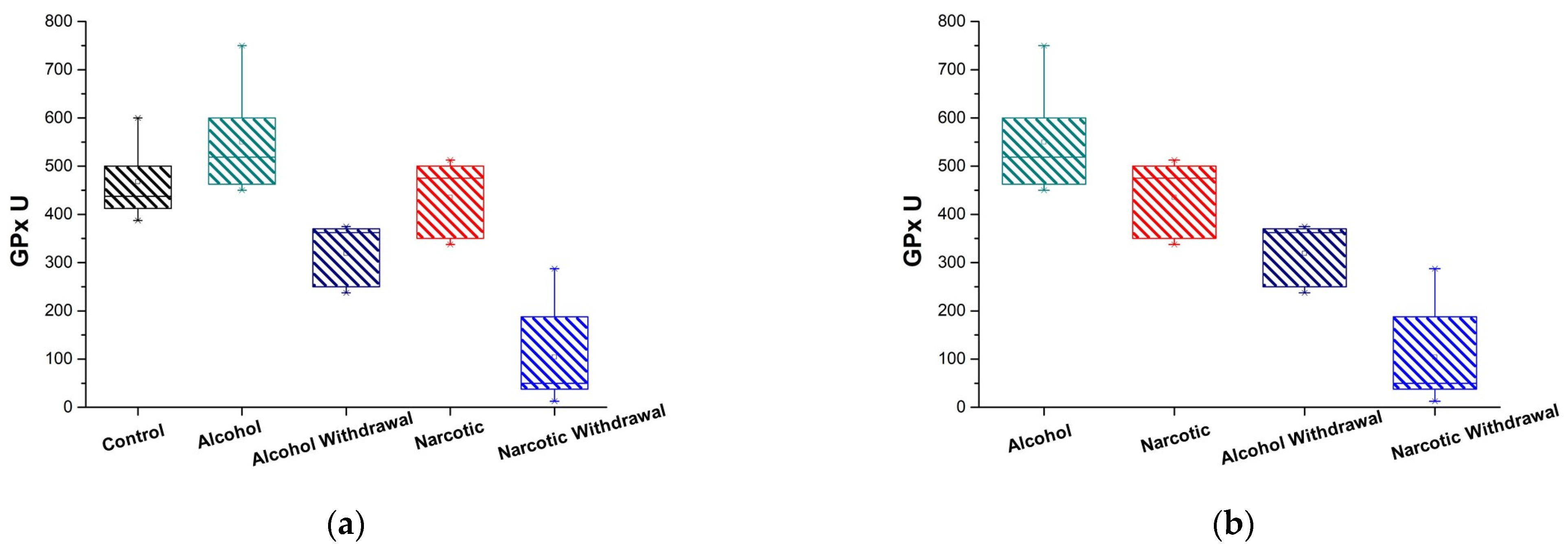
| Antioxidant Enzyme Activity | Control and Alcohol | Control and Alcohol Withdrawal | Control and Narcotic | Control and Narcotic Withdrawal |
|---|---|---|---|---|
| Catalase U/mL | 0.0001 (are significantly different p < 0.01) | 0.0058 (are significantly different p < 0.01) | 0.00001 (are significantly different p < 0.01) | 0.034 (are marginally significant p < 0.05) |
| SOD U/mL | 0.038 (are marginally significant p < 0.05) | 0.028 (are marginally significant p < 0.05) | 0.20 (are not significantly different p ≥ 0.05) | 0.55 (are not significantly different p ≥ 0.05) |
| GPx U | 0.20 (are not significantly different p ≥ 0.05) | 0.012 (are significantly different at p < 0.05) | 0.76 (are not significantly different p ≥ 0.05) | 0.008 (are significantly different p < 0.01) |
| Enzymes | Control | Alcohol | Alcohol Withdrawal | Narcotic | Narcotic Withdrawal |
|---|---|---|---|---|---|
| Catalase U/mL | 46.13 ± 18.05 = (28.07, 64.18) | 131.87 ± 22.60 = (109.27, 154.47) | 126.41 ± 42.49 = (83.92, 168.89) | 162.30 ± 28.79 = (133.51, 191.09) | 87.30 ± 24.28 = (63.02, 111.58) |
| SOD U/mL | 27.121 ± 2.13 = (24.99, 29.25) | 30.91 ± 2.311 = (28.59, 33.22) | 31.71 ± 2.49 = (29.22, 34.19) | 30.18 ± 2.55 = (27.62, 32.73) | 26.18 ± 3.19 = (22.98, 29.37) |
| GPx U | 465.7 ± 74.55 = (392.95, 542.05) | 550 ± 91.89 = (458.11, 641.89) | 319 ± 60.46 = (258.54, 379.46) | 435 ± 74.07 = (360.93, 509.07) | 104.17 ± 87.35 = (16.82, 191.51) |
| Antioxidant Enzyme Activity | Alcohol and Alcohol Withdrawal | Narcotic and Narcotic Withdrawal | Alcohol and Narcotic | Alcohol Withdrawal and Narcotic Withdrawal |
|---|---|---|---|---|
| Catalase U/mL | 0.33 (are not significantly different p ≥ 0.05) | 0.00094 (are significantly different p < 0.01) | 0.044 (are marginally significant p < 0.05) | 0.29 (are not significantly different p ≥ 0.05) |
| SOD U/mL | 0.67 (are not significantly different p ≥ 0.05) | 0.023 (are marginally significant p < 0.05) | 0.93 (are not significantly different p ≥ 0.05) | 0.0093 (are significantly different p < 0.01) |
| GPx U | 0.008 (are significantly different p < 0.01) | 0.008 (are significantly different p < 0.01) | 0.27 (are not significantly different p ≥ 0.05) | 0.023 (are marginally significant p < 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asatiani, N.; Sapojnikova, N.; Kartvelishvili, T.; Asanishvili, L.; Sichinava, N.; Chikovani, Z. Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients. Medicina 2025, 61, 204. https://doi.org/10.3390/medicina61020204
Asatiani N, Sapojnikova N, Kartvelishvili T, Asanishvili L, Sichinava N, Chikovani Z. Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients. Medicina. 2025; 61(2):204. https://doi.org/10.3390/medicina61020204
Chicago/Turabian StyleAsatiani, Nino, Nelly Sapojnikova, Tamar Kartvelishvili, Lali Asanishvili, Nestan Sichinava, and Zaza Chikovani. 2025. "Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients" Medicina 61, no. 2: 204. https://doi.org/10.3390/medicina61020204
APA StyleAsatiani, N., Sapojnikova, N., Kartvelishvili, T., Asanishvili, L., Sichinava, N., & Chikovani, Z. (2025). Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients. Medicina, 61(2), 204. https://doi.org/10.3390/medicina61020204




