Abstract
Background: Sodium–glucose co-transporter-2 (SGLT2) inhibitors have emerged as vital medications for the management of type 2 diabetes mellitus (T2DM). Numerous studies have highlighted the cardioprotective and renal protective benefits of SGLT2 inhibitors. Consequently, it is essential to assess their efficacy and safety in patients with chronic diseases. Method: We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating the effects of SGLT2 inhibitors on major cardiovascular and safety outcomes in patients with T2DM, heart failure (HF), and chronic kidney disease (CKD). We searched the PubMed, Cochrane, and Embase databases for trials published between 30 September 2021 and 17 May 2023. The primary outcomes of interest included nonfatal myocardial infarction (MI), hospitalization for heart failure (HHF), cardiovascular death, and nonfatal stroke. The safety outcomes assessed were hypoglycemia, urinary tract infections (UTIs), and acute kidney injury (AKI). Result: We identified 13 RCTs involving 90,413 participants. In patients with T2DM, SGLT2 inhibitors significantly reduced the risk of nonfatal MI by 12% (hazard ratio [HR] = 0.88, 95% confidence interval [CI]: 0.78–0.98), HHF by 33% (HR = 0.67, 95% CI: 0.62–0.74), and cardiac death by 15% (HR = 0.95, 95% CI: 0.80–1.13). However, they did not significantly reduce the risk of nonfatal stroke (HR = 0.85, 95% CI: 0.75–0.95). In patients with HF, SGLT2 inhibitors reduced the risk of HHF by 28% (HR = 0.72, 95% CI: 0.66–0.77) and cardiac death by 12% (HR = 0.88, 95% CI: 0.80–0.96). For patients with CKD, SGLT2 inhibitors reduced the risk of HHF by 35% (HR = 0.65, 95% CI: 0.55–0.76) and cardiac death by 16% (HR = 0.84, 95% CI: 0.73–0.96). Regarding safety outcomes, SGLT2 inhibitors did not significantly increase the risk of hypoglycemia in patients with T2DM, HF, or CKD, nor did they increase the risk of urinary tract infections (UTIs) in patients with HF or CKD, or the risk of acute kidney injury (AKI) in patients with HF. However, they did increase the risk of UTIs by 8% (risk ratio [RR] = 1.08, 95% CI: 1.01–1.16) in patients with T2DM and reduced the risk of AKI by 22% (RR = 0.78, 95% CI: 0.67–0.89) and 19% (RR = 0.81, 95% CI: 0.69–0.97) in patients with T2DM and CKD, respectively. Conclusions: SGLT2 inhibitors have demonstrated a significant improvement in cardiovascular outcomes for patients with T2DM, HF, and CKD while also maintaining a favorable safety profile. These findings advocate for the broader application of SGLT2 inhibitors in the management of chronic diseases, particularly in reducing the incidence of nonfatal MI, HHF, and cardiac death. Further research is essential to optimize their use across diverse patient populations and stages of disease.
1. Introduction
Chronic metabolic diseases, including diabetes mellitus, hypertension, and heart failure, pose a significant global public health challenge. These conditions are associated with a rising number of patients and an increasing rate of morbidity and mortality worldwide [1]. The prevalence and impact of these diseases are expected to escalate in the future due to an aging population and changes in lifestyle habits [2]. Patients with type 2 diabetes mellitus (T2DM) face a substantially higher risk of cardiovascular complications, such as myocardial infarction, cardiac death, and chronic kidney disease [3].
Phlorizin, an O-glucoside dihydrochalcone molecule originally isolated from apple tree bark in 1835, has been shown to enhance glucose excretion in healthy individuals by inhibiting sodium–glucose co-transporters 1 and 2 (SGLT1 and 2) [4]. However, its development as an anti-hyperglycemic medication has been limited due to its poor bioavailability and gastrointestinal side effects [5,6,7]. The development of selective SGLT2 inhibitors has provided a novel therapeutic approach for chronic disease management, particularly for type 2 diabetes mellitus (T2DM) [8,9]. These agents inhibit glucose reabsorption in the proximal renal tubules, leading to increased urinary glucose excretion and reduced blood glucose levels, establishing them as a novel class of anti-diabetic drugs [10,11]. Beyond the glucose-lowering effect, SGLT2 inhibitors have been shown to reduce the risk of cardiovascular and renal diseases in patients with T2DM, heart failure (HF), or chronic kidney disease (CKD), and are currently recommended for preventing HF in diabetic patients with CKD and treating HF across the full spectrum of left ventricular ejection fraction (LVEF) [12,13,14,15,16]. These studies shifted the therapeutic landscape and positioned SGLT2 inhibitors as a cornerstone treatment in cardio-renal-metabolic care. Among the various pharmacological treatments available for patients with T2DM, which include metformin, dipeptidyl peptidase-4 (DPP-4) inhibitors, thiazolidinedione, sulfonylurea, insulin, and even herbal medications such as okra extracts, SGLT2 inhibitors demonstrate significant cardioprotective effects in patients both with and without T2DM [17,18,19]. Aiane Benevide Sereno et al. [20] demonstrated that okra improved the metabolic markers, such as insulin sensitivity, body weight loss, and lipid profiles without the direct analysis of fasting blood glucose (FBG) and glycated hemoglobin (HbA1C) in the animal model of DM, and Kabelo Mokgalaboni et al. [21] found that okra treatment reduced the levels of FBG compared to the placebo, while there was a high level of certainty in HbA1C in a systemic review and meta-analysis of the clinical evidence. Despite the growing utility, the safety profile of SGLT2 inhibitors warrant careful consideration. Commonly reported adverse events included genital mycotic infection due to glycosuria and, less frequently, urinary tract infections (UTIs) [22,23]. Given the growing utilization of SGLT2 inhibitors in chronic disease management and potential safety concerns, this study aims to conduct a systematic review and meta-analysis to assess their efficacy in improving cardiovascular and renal outcomes beyond glycemic control in patients with T2DM, HF, and CKD, as well as to evaluate the safety profile of these medications.
2. Materials and Methods
2.1. Database Searches and Identification of Eligible Papers
This study followed the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [24]. PubMed was first searched for primary reference for this study in using the following syntax: (Sodium–Glucose Transporter 2 OR Canagliflozin or Dapagliflozin OR Empagliflozin OR Ertugliflozin OR Sotagliflozin) and (Cardiovascular Outcomes) and (Meta-analysis). The meta-analysis published by Dario Giugliano et al. [25] in 2021 was chosen as the primary reference of this study. Next, further searches were conducted using PubMed, Cochrane, and Embase databases. The search period was from the end date of the primary reference search (30 September 2021 to 17 May 2023). The following syntax was used in PubMed and resulted in 318 articles: (“Sodium-Glucose Transporter 2” [Mesh] OR Canagliflozin OR Dapagliflozin OR Empagliflozin OR Ertugliflozin OR Sotagliflozin) and (“Diabetes Mellitus, Type 2” [Mesh] OR “Heart Failure” [Mesh] OR “Chronic kidney failure” [Mesh]) therapy/narrow [filter] 2021: 2023 [dp] AND (Randomized Controlled Trial). The following syntax was used for a search conducted in Cochrane and restricted to the date range between 30 September 2021 and 17 May 2023, which resulted in a total of 4 articles: (Sodium–Glucose Transporter 2 Inhibitors OR Canagliflozin OR Dapagliflozin OR Empagliflozin OR Ertugliflozin OR Sotagliflozin) AND (Type 2 Diabetes Mellitus OR Heart Failure OR Chronic Kidney Diseases) AND (Randomized Controlled Trial) AND (CVOT). A search use PICOS (Population: non-insulin dependent diabetes mellitus OR heart failure OR chronic kidney failure, Intervention: sodium glucose co-transporter 2 inhibitor, Comparison: placebo, Outcome: cardiovascular disease, Study design: randomized controlled trial) in Embase database was conducted and limited to the search dates from 30 September 2021 to 17 May 2023, which resulted in 248 articles. EndNote 20.4 was introduced to automatically delete duplicate articles.
2.2. Inclusion and Exclusion Criteria
Studies that met the following inclusion criteria were included and reviewed systematically: (1) studies comparing the use of SGLT2 inhibitors to the placebo; (2) large-scale randomized controlled trials (RCTs) with a sample size greater than 1000 participants; (3) a follow-up duration of at least 6 months or more; (4) participants diagnosed with T2DM, HF, or CKD; and (5) studies with relevant efficacy and safety data. Studies were excluded if there were no publicly available full-text data for extraction.
2.3. Data Screening and Extraction
Database search was completed by YJL. Each article was independently reviewed based on its title, abstract, and, when necessary, full text by two blinded reviewers selected from ICL and HHC. Discrepancies were resolved through discussion or, if required, by involving a third reviewer from other authors. CCC and CMP evaluated the risk of bias and extracted data. The Cochrane Handbook for Systematic Reviews of Interventions tool was used to assess the risk of bias for each RCT [26]. The evaluated domains included random sequence generation, allocation concealment, the blinding of participants and personnel, the blinding of outcome assessment, incomplete outcome data, and selective reporting.
2.4. Primary Outcomes
The primary outcomes were as follows: (1) Nonfatal MI: the patient survives but still experiences symptoms of MI after treatment. (2) Nonfatal stroke: the patient survives but still experiences symptoms of the cerebrovascular accident after treatment. (3) HHF: this includes both acute and chronic heart failure requiring hospitalization. The duration of treatment can vary depending on the severity of the patient’s condition and treatment efficacy. (4) Cardiac death: examples include death after MI, fatal arrhythmias, the rupture of aortic aneurysm, death following HF, etc.
2.5. Safety Outcomes
The safety outcomes were as follows: (1) Adverse events: diseases, symptoms, results of physical examination, or laboratory test results, as well as responses to drug treatment or preventive measures. These events may have adverse effects on the patient’s health. (2) Hypoglycemia: blood glucose concentration below the normal range, typically defined as blood glucose concentration below 70 mg/dL. Hypoglycemia can lead to several symptoms such as cold sweating, palpitation, hunger, shaking, dizziness, irritability or confusion, etc. (3) UTIs: symptoms and signs of various urinary reproductive system infections such as cystitis, urethritis, pyelonephritis, etc. These infections may cause discomfort symptoms such as frequent urination, urgency, dysuria, and, in severe cases, may lead to kidney damage. (4) Acute kidney injury (AKI): symptoms and diseases such as renal failure, kidney injury, etc. AKI can be caused by various factors such as drug toxicity, renal vascular diseases, etc., and patients may experience symptoms such as swelling, hematuria, etc.
2.6. Statistical Analysis and PROSPERO Registry
Given the variability in medications, dosages, and baseline risks of the included participants, a random-effects model was utilized to combine and analyze the data. The meta-analysis was conducted using R statistical software (Version 4.2.3, R Foundation of Statistical Computing, Vienna, Austria). Hazard ratios (HRs) with 95% confidence intervals (CIs) were selected to assess primary outcomes, while risk ratios (RRs) with a 95% CI were used to evaluate safety outcomes. Subgroup analyses for different chronic conditions, including T2DM, HF, and CKD, were performed based on variations in the target populations. The degree of heterogeneity among studies was assessed using the I2 statistic proposed by Higgins and colleagues [27]. An I2 value of less than 25%, between 25% and 75%, and greater than 75% indicates low, moderate, and high heterogeneity, respectively. This study is registered with PROSPERO (CRD42024591070).
3. Results
3.1. Study Search and Characteristics of Included Patients
The PRISMA flowchart detailing the literature review process is shown in Figure 1. After removing duplicate records and excluding irrelevant articles based on the title and abstract screening, we identified 13 RCTs involving 90,413 participants. These studies evaluated the cardiovascular and renal outcomes, as well as the adverse effects, of SGLT2 inhibitors in patients with T2DM, HF, and CKD. Details of the 13 selected studies, including trial names, publication years, study drugs, sample sizes, mean participant ages, and follow-up durations, are summarized in Table 1. Quality assessments conducted using the Cochrane Risk of Bias tool indicated that all included RCTs were at low risk of bias (Table 2).
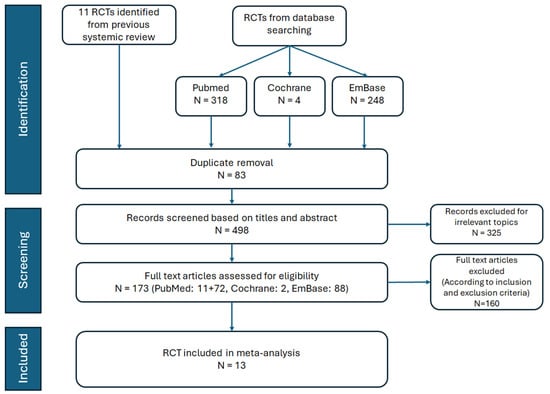
Figure 1.
Flow chart of study selection.

Table 1.
Summary of included studies.

Table 2.
Quality assessment conducted using the Cochrane Risk of Bias tool.
3.2. Type 2 Diabetic Mellitus
In the subgroup analysis of patients with T2DM, this study included data from seven RCTs [28,29,30,31,34,36,37].
3.2.1. Nonfatal Myocardial Infarction, Nonfatal Stroke, and Hospitalization for Heart Failure and Cardiac Death
Data from five trials were included to assess the HR for nonfatal MI and nonfatal stroke, comparing the efficacy of SGLT2 inhibitors to the placebo in T2DM patients. In the pooled analysis of the five trials, SGLT2 inhibitors significantly led to a 12% reduction in nonfatal MI compared to the placebo (HR = 0.88, 95% CI: 0.78–0.98, I2 = 38%, Figure 2a). However, no significant difference was observed between the SGLT2 inhibitors and placebo groups regarding the risk of nonfatal stroke (HR = 0.95, 95% CI: 0.80–1.13, I2 = 54%, Figure 2b). All seven included RCTs reported the HR for HHF and cardiac death. In the pooled analysis of these seven trials, SGLT2 inhibitors significantly reduced the risk of HHF by 33% compared to the placebo (HR = 0.67, 95% CI: 0.62–0.74, I2 = 0%, Figure 2c) and cardiac death by 15% compared to the placebo (HR = 0.85, 95% CI: 0.75–0.95, I2 = 48%, Figure 2d).
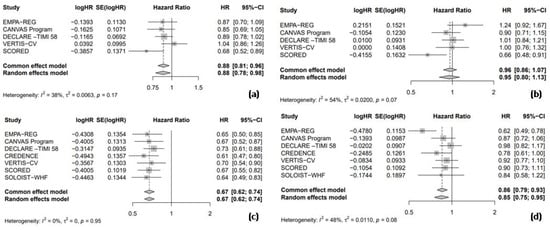
Figure 2.
Forest plot of five included trials: (a) Incidence of non-fatal myocardial infarction; (b) incidence of non-fatal stroke; (c) incidence of hospitalization due to heart failure; and (d) incidence of cardiac death. CI: confidence interval.
3.2.2. Safety Outcomes
Among the seven RCTs, six trials provided data on the RR for adverse events, enabling a pooled analysis to compare the safety outcomes, including any adverse events, hypoglycemia, UTIs, and AKI between SGLT2 inhibitors and placebo groups. The results indicated that SGLT2 inhibitors were associated with a nearly statistically significant 2% reduction in the risk of any adverse events compared to the placebo (RR = 0.98, 95% CI = 0.96–1.00, I2 = 61%, Figure 3a). However, the use of SGLT2 inhibitors did not significantly increase the risk of hypoglycemic events compared to the placebo (RR = 0.92, 95% CI: 0.83–1.02, I2 = 24%, Figure 3b). In a pooled analysis of six trials, SGLT2 inhibitors were associated with a significantly increased risk of UTIs compared to the placebo (RR = 1.08, 95% CI: 1.01–1.16, I2 = 0%, Figure 3c). Finally, in a pooled analysis of five trials investigating AKI, SGLT2 inhibitors significantly reduced the risk by 22% compared to the placebo (RR = 0.78, 95% CI: 0.67–0.89, I2 = 0%, Figure 3d).
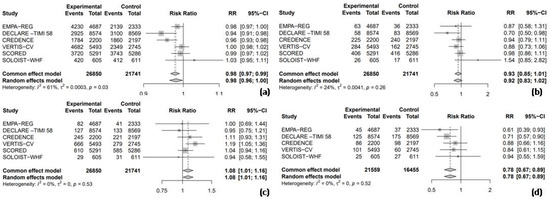
Figure 3.
Forest plot of six included trials: (a) incidence of any adverse events; (b) incidence of hypoglycemic events; (c) incidence of urinary tract infections; and (d) incidence of acute kidney injury. CI: confidence interval.
3.3. Heart Failure
In the subgroup analysis of patients with HF, this study included data from five RCTs [32,35,38,39,40].
3.3.1. Hospitalization for Heart Failure and Cardiac Death
All five RCTs provided HR data for HHF, enabling a combined analysis to evaluate the efficacy of SGLT2 inhibitors compared to the placebo. The pooled analysis showed that SGLT2 inhibitors were associated with a significant 28% reduction in the risk of HHF compared to the placebo (HR = 0.72, 95% CI: 0.66–0.77, I2 = 0%; Figure 4a). Furthermore, SGLT2 inhibitors significantly reduced the risk of cardiac death by 12% compared to the placebo (HR = 0.88, 95% CI: 0.80–0.96, I2 = 0%; Figure 4b).
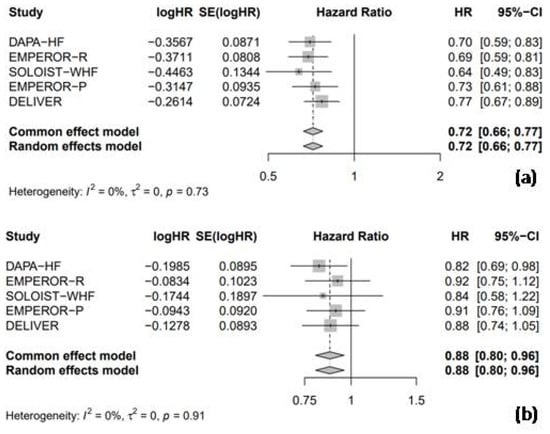
Figure 4.
Forest plot of five included trials: (a) incidence of hospitalization due to heart failure; and (b) incidence of cardiac death. CI: confidence interval.
3.3.2. Safety Outcomes
All five randomized RCTs reported RR data for adverse events, facilitating a combined analysis to compare the safety profile of SGLT2 inhibitors with the placebo. The pooled analysis revealed no significant difference in the overall adverse event rates between SGLT2 inhibitors and the placebo (RR = 0.97, 95% CI: 0.94–1.01, I2 = 68%; Figure 5a). Similarly, the use of SGLT2 inhibitors was not associated with a significant increase in the risk of hypoglycemic events (RR = 1.01, 95% CI: 0.80–1.29, I2 = 0%; Figure 5b), UTI (RR = 1.13, 95% CI: 0.99–1.29, I2 = 1%; Figure 5c), or AKI (RR = 0.94, 95% CI: 0.83–1.06, I2 = 0%; Figure 5d).
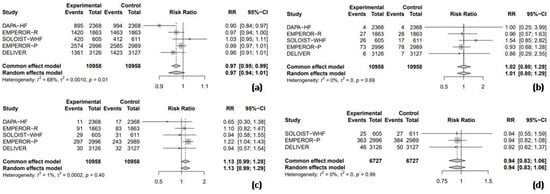
Figure 5.
Forest plot of five included trials: (a) incidence of overall adverse events; (b) incidence of hypoglycemic events; (c) incidence of urinary tract infections; and (d) incidence of acute kidney injury. CI: confidence interval.
3.4. Chronic Kidney Disease
In the subgroup analysis of patients with CKD, this study included data from four RCTs [31,33,36,40].
3.4.1. Hospitalization for Heart Failure and Cardiac Death
Among the four RCTs included in this analysis, two trials evaluated the HR for HHF, comparing the efficacy of SGLT2 inhibitors with the placebo in patients with CKD. The pooled analysis demonstrated that SGLT2 inhibitors were associated with a significant 35% reduction in the risk of HHF compared to the placebo (HR = 0.65, 95% CI: 0.55–0.76, I2 = 0%, Figure 6a). Moreover, all four RCTs reported the HR for cardiac death. The pooled analysis of these trials revealed that SGLT2 inhibitors significantly reduced the risk of cardiac death by 16% compared to the placebo (HR = 0.84, 95% CI: 0.73–0.96, I2 = 0%; Figure 6b).
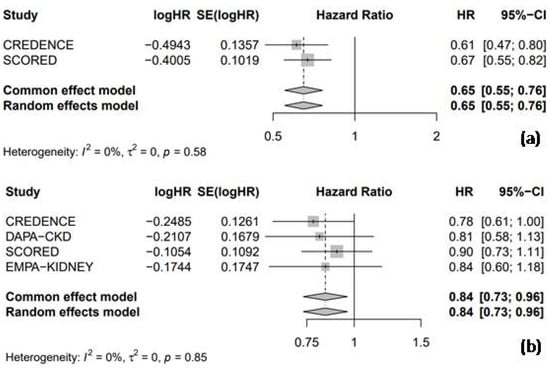
Figure 6.
Forest plot of 4 included trials: (a) incidence of hospitalization due to heart failure; and (b) incidence of cardiac death. CI: confidence interval.
3.4.2. Safety Outcomes
All four RCTs reported RR data for adverse events, allowing for a combined analysis to compare the safety profile of SGLT2 inhibitors with placebo. The results indicated that SGLT2 inhibitors significantly reduced the risk of adverse events by 5% compared to the placebo (RR = 0.95, 95% CI: 0.91–0.99, I2 = 75%, Figure 7a). However, the use of SGLT2 inhibitors was not associated with a significant increase in the risk of hypoglycemic events (RR = 0.94, 95% CI: 0.82–1.07, I2 = 28%, Figure 7b), nor UTIs (RR = 1.06, 95% CI: 0.97–1.16, I2 = 0%, Figure 7c). Three of the four RCTs were pooled to assess the risk of AKI, and the results demonstrated that SGLT2 inhibitors significantly reduced the risk of AKI by 19% compared to the placebo (RR = 0.81, 95% CI: 0.69–0.97, I2 = 0%, Figure 7d).
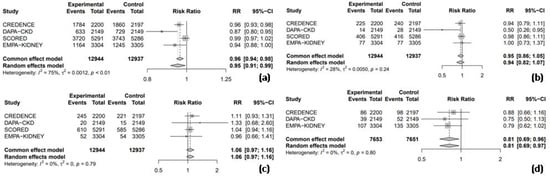
Figure 7.
Forest Plot of 4 included trials: (a) incidence of overall adverse events; (b) incidence of hypoglycemic events; (c) incidence of urinary tract infections; and (d) incidence of acute kidney injury. CI: confidence interval.
3.4.3. Subgroup Analysis by the Estimated Glomerular Filtration Rate
A subgroup analysis based on the estimated glomerular filtration rate (eGFR) in CKD patients, using a threshold of 45 mL/min/1.73 m2, revealed a significant difference in the risk of adverse events (Figure 8). SGLT2 inhibitors were more effective in reducing the risk of adverse events in patients with an eGFR greater than 45 mL/min/1.73 m2 compared to those with an eGFR less than 45 mL/min/1.73 m2.
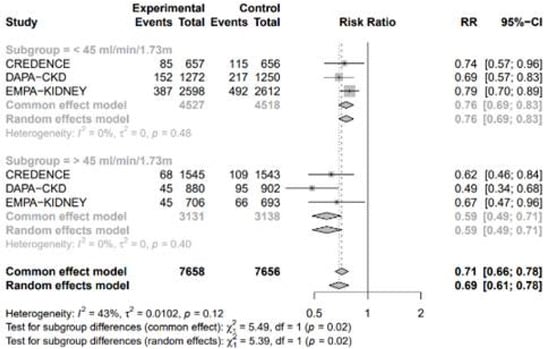
Figure 8.
Forest plot of 4 included trials grouped by eGFR 45 mL/min/1.73 m2.
4. Discussion
This systematic review and meta-analysis of 13 RCTs examined the efficacy and safety of SGLT2 inhibitors in patients with chronic conditions, including T2DM, HF, and CKD. In T2DM patients, SGLT2 inhibitors significantly reduced the risk of nonfatal MI by 12%, HHF by 33%, and cardiac death by 15%. In HF patients, the risk of HHF decreased by 28% and cardiac death by 12%. In CKD patients, SGLT2 inhibitors reduced the risk of HHF by 35% and cardiac death by 16%. In terms of safety outcomes, SGLT2 inhibitors significantly reduced the risk of AKI by 22%, increased the risk of UTIs by 8%, and there were no significant differences observed in the risks of adverse events and hypoglycemia in T2DM patients.
In HF patients, SGLT2 inhibitors had no significant effect on the risks of adverse events, hypoglycemia, UTIs, and AKI. In CKD patients, SGLT2 inhibitors reduced the risk of adverse events by 5% and AKI by 19%, but there were no significant differences in the risks of hypoglycemia or UTIs. The clinical application of SGLT2 inhibitors has been extended to the treatment of HF and CKD. The UTIs may differ in non-diabetic patients. Josip A. Borovac et al. also demonstrated that the use of SGLT2 inhibitors was comparable to the placebo regarding the risk of UTI events in HF patients (relative risk [RR]: 1.09; 95% confidence interval [CI]: 0.94–1.26; p = 0.24) [41]. Similar findings were reported in CKD patients, where Xiutian Chen et al. [42] found the risk of UTIs to be very low (I2 = 0%; p = 0.87), with an odds ratio of 1.06 (95% CI: 0.96–1.17; p = 0.22), which was not statistically significant. Therefore, the use of SGLT2 inhibitors does not appear to increase the incidence of UTIs in patients with HF or CKD.
The results of this meta-analysis demonstrate that SGLT2 inhibitors are an effective and generally well-tolerated treatment option for patients with T2DM, HF, and CKD.
In 2021, McGuire, D.K. et al. [43] conducted a meta-analysis using the PubMed database to assess the cardiovascular and renal effects of SGLT2 inhibitors in patients with T2DM. Their findings showed that SGLT2 inhibitors significantly reduced the risk of hospitalization for HF and cardiovascular death. Our work incorporated data from two other databases (Cochrane and Embase) and included two recently published large-scale RCTs, yielding similar results [36,37]. Notably, McGuire’s analysis reported significant heterogeneity in the risk ratios for cardiovascular death, while this study did not find such heterogeneity.
Whereas the previous research focused solely on patients with T2DM, the present study expanded the scope to include patients with HF and CKD. A study that was performed by Pandey, A.K. et al. [44] in 2022 conducted a meta-analysis to investigate the cardiovascular outcomes of using SGLT2 inhibitors in patients with HF. The study demonstrated that SGLT2 inhibitors significantly reduced the RR of HHF and cardiovascular death, consistent with the results of our study. This study incorporated an additional large RCT published in 2022, including 6263 more participants. The inclusion of this new data did not alter the effect of SGLT2 inhibitors in reducing the risk ratio of cardiovascular death, but it did narrow the confidence interval by 0.1% on either side.
In 2022, Li, N. et al. [45] published a meta-analysis on the use of SGLT2 inhibitors in patients with CKD, focusing on cardiovascular outcomes. Their findings demonstrated that SGLT2 inhibitors significantly reduced the RR of HHF or cardiovascular death, consistent with the results of our study.
In 2019, Donnan, J.R. et al. [46] conducted a meta-analysis evaluating the safety of SGLT2 inhibitors in T2DM patients. Their study found that SGLT2 inhibitors significantly lowered the risk of AKI, aligning with our findings. However, unlike this study, which reported an increased risk of UTIs with SGLT2 inhibitor use, Donnan’s analysis did not find a significant difference. This discrepancy may be due to variations in the specific SGLT2 inhibitors analyzed.
Li, N. et al.’s study also reported a significant reduction in adverse events with SGLT2 inhibitors, consistent with our study [45]. However, their analysis primarily included patients with moderate to severe renal failure (stages 3 to 4), while this study focused on patients with mild to moderate renal failure (stages 2 to 3). A subgroup analysis in this study, using an eGFR threshold of 45 mL/min/1.73 m2, showed that SGLT2 inhibitors were more effective in reducing adverse events in CKD patients with an eGFR greater than 45 mL/min/1.73 m2 compared to those with lower eGFR values.
SGLT2 inhibitors primarily function by inhibiting the coupled reabsorption of sodium and glucose from the proximal tubules in kidneys and lowering cardiac preload and afterload through osmotic diuresis [47]. SGLT2 inhibitors also exerted beneficial effects of cardiac energy metabolism [48,49]. In patients with heart failure, the heart is “energy starved” secondary to mitochondria dysfunction and a decrease in oxidation metabolism [50,51]. Ketone metabolism is increased in this scenario as a “starved fuel” and considered an adaptive metabolism [52,53,54]. SGLT2 could mobilize fatty acid and increase the concentration of circulating ketone bodies, enhancing cardiac ketone metabolism and therefore improving energy supply to the “starving” heart [55]. Consequently, they confer partial cardioprotective effects [56,57]. The potential cardiovascular risk reduction benefits of SGLT2 inhibitors may be associated with their ability to improve the balance of pro-inflammatory and anti-inflammatory cytokines in the body and reduce cardiac fibrosis [58,59]. The association between heart failure and markers of inflammation is evident in patients both with reduced and preserved heart failure [60]. SGLT2 inhibitors empagliflozin, canagliflozin, and dapagliflozin have been shown to reduce markers of inflammation in patients with diabetes [61,62,63]. The exact pathway that SGLT2 inhibitors used to regulate inflammation remained unclear. It was also proposed that empagliflozin reduced NLRP3 inflammasomes, possibly secondary to inhibition by increased circulating ketone metabolites, β-hydroxybutyrate [64]. Furthermore, the diuretic effect of SGLT2 inhibitors may have secondary effects, such as increasing red blood cell concentration, which further protects the heart [65,66].
The primary mechanism by which SGLT2 inhibitors slow the progression of renal dysfunction is through the inhibition of glucose and sodium reabsorption in the proximal tubules [47]. This results in an increase in sodium concentration, causing afferent arteriolar constriction via tubuloglomerular feedback, decreasing intraglomerular pressure and thereby restoring normal renal perfusion [67,68,69]. Our research revealed that SGLT2 inhibitors were more effective in reducing the incidence of adverse events in patients with an eGFR greater than 45 mL/min/1.73 m2 compared to those with an eGFR below this threshold. This outcome may be attributed to the diminished glucose-lowering efficacy in patients with lower eGFR levels, likely due to reduced glucose filtration [70]. SGLT2 inhibitors also could reduce inflammatory responses and renal fibrosis, potentially through the activation of adenosine monophosphate-activated protein kinase [71,72]. In patients with CKD, and even those with concomitant HF, excessive sodium and fluid retention is a common issue. The use of SGLT2 inhibitors, with their natriuretic and diuretic effects, can alleviate renal interstitial edema, thereby protecting the kidneys. Hyperglycemia increases the filtration of glucose and facilitates glucose reabsorption in the proximal tubules. The process increases the oxygen consumption and depletes oxygen supply to distal regions, especially renal medulla [67,73]. SGLT2 inhibitors may preserve oxygen supply by reducing the glucose reuptake and may reduce the production of reactive oxygen species. In animal studies, SGLT2 inhibitors were shown to alter the signaling pathway of hypoxia-inducible factors (HIFs), reducing HIF-1 activity and promote HIF-2α activity, leading to a decrease in pro-inflammatory cytokines and fibrotic factors [74,75].
Initially, SGLT2 inhibitors were thought to exert detrimental effects on the incidence of AKI [76]. However, further studies did not support the conclusion. In a study conducted by GN Nadkarni et al. [77], there was no association of AKI and the use of SGLT2 inhibitors. The characteristic dip of eGFR may be attributed to the effect of proximal tubular natriuresis on tubuloglomerular feedback, causing reversible intrarenal hemodynamics including afferent arteriole vasoconstriction [78]. In large scale RCTs, the risk of AKI was reduced in users of canagliflozin and empagliflozin [28,29]. Our study coincides with previous research revealing that SGLT2 inhibitors may reduce the incidence of AKI in patients with chronic diseases. The mechanism by which SGLT2 inhibitors reduce the incidence of AKI is associated with several factors. First, SGLT2 inhibitors decrease sodium reabsorption in the proximal convoluted tubules, resulting in increased sodium delivery to the macula densa. This process reactivates the tubuloglomerular feedback mechanism; specifically, the elevated sodium delivery is detected by macula densa cells, mediating constriction of the afferent glomerular arteriole via adenosine. This action prevents vasodilation of the afferent arteriole and subsequently reduces intraglomerular pressure [79]. Second, SGLT2 inhibitors may enhance renal cortical oxygenation by decreasing sodium and glucose reabsorption in the proximal convoluted tubules, promoting erythropoietin production, and inducing hemoconcentration [80]. Third, multiple lines of evidence from basic research indicate that the use of SGLT2 inhibitors contributes to metabolic reprogramming and the regulation of hypoxia, inflammation, and oxidative stress in conditions such as sepsis, heart failure, and nephrotoxin- or contrast media-induced acute kidney injury [81,82].
This study has three main limitations. First, the sample limitation: the included literature primarily involves Caucasian and Asian subjects, with an average participant age of 66 years. Results may differ across other races or age groups. Additionally, the studies varied in inclusion criteria, participant baseline risks, and medication types and doses. Future research should expand sample sizes or diversify the populations to improve representativeness. Second, while efficacy and safety definitions were generally consistent, some heterogeneity remained. Third, as this is a study-level meta-analysis using published data, methodological limitations may affect the results.
Despite these limitations, this study has several strengths. It conducted a comprehensive literature search, applied robust meta-analytical methods, and included a diverse population with chronic conditions. All 13 large-scale RCTs were assessed to have a low risk of bias, providing a strong foundation for analysis. To account for variations in drug use, dosage, and participant risks, a random-effects model was appropriately used in the meta-analysis.
5. Conclusions
For patients with multiple chronic diseases such as T2DM, HF, and CKD, treatment with SGLT2 inhibitors significantly reduces the risk of nonfatal MI, HHF, and cardiac death, particularly in those with both T2DM and CKD. SGLT2 inhibitors also reduce the incidence of AKI, although there is a slight increase in the incidence of UTIs. Subgroup analyses reveal that, among CKD patients with an eGFR above 45 mL/min/1.73 m2, SGLT2 inhibitors are more effective in mitigating adverse renal outcomes compared to patients with lower eGFR levels. Overall, this study confirmed the cardiovascular and renal protective benefits of SGLT2 inhibitors, highlighting their value beyond blood sugar control. These benefits span various patient groups, including those with HF and CKD, without a notable increase in adverse events, supporting the broader application in enhancing outcomes for chronic disease management.
Author Contributions
Conceptualization: I.-C.L., H.-H.C., Y.-J.L. and C.-F.H.; methodology: I.-C.L., H.-H.C., Y.-J.L., C.-M.C. and C.-M.P.; formal analysis and investigation: I.-C.L., H.-H.C., Y.-J.L. and D.-C.C.; writing—original draft preparation: I.-C.L., H.-H.C. and Y.-J.L.; writing—review and editing: C.-H.S., C.-M.P., D.-C.C. and C.-C.H.; funding acquisition: C.-M.C., C.-F.H., C.-M.P. and C.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Council (113-2320-B-030-007-MY3), Cardinal Tien Hospital (CTH113A-NDMC-2223), Cathay General Hospital (107-CGH-FJU-05), and Taoyuan Armed Forces General Hospital (TYAFGH-A-114020).
Data Availability Statement
Data are available upon reasonable request. The data supporting the findings of this study can be obtained from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SGLT2 | sodium–glucose co-transporter-2 |
| T2DM | Type 2 diabetes mellitus |
| RCT | randomized controlled trials |
| HF | heart failure |
| CKD | chronic kidney disease |
| MI | myocardial infarction |
| HHF | hospitalization for heart failure |
| UTI | urinary tract infection |
| AKI | acute kidney injury |
| HR | hazard ratio |
| CI | confidence interval |
| RR | risk ratio |
| LVEF | left ventricular ejection fraction |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| eGFR | estimated glomerular filtration rate |
References
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Ansah, J.P.; Chiu, C.-T. Projecting the chronic disease burden among the adult population in the United States using a multi-state population model. Front. Public Health 2023, 10, 1082183. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444. [Google Scholar] [CrossRef]
- Idris, I.; Donnelly, R. Sodium–glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug. Diabetes Obes. Metab. 2009, 11, 79–88. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef]
- Haider, K.; Pathak, A.; Rohilla, A.; Haider, M.R.; Ahmad, K.; Yar, M.S. Synthetic strategy and SAR studies of C-glucoside heteroaryls as SGLT2 inhibitor: A review. Eur. J. Med. Chem. 2019, 184, 111773. [Google Scholar] [CrossRef]
- Ferrannini, E. Sodium–glucose transporter-2 inhibition as an antidiabetic therapy. Nephrol. Dial. Transplant. 2010, 25, 2041–2043. [Google Scholar] [CrossRef]
- Madaan, T.; Akhtar, M.; Najmi, A.K. Sodium glucose CoTransporter 2 (SGLT2) inhibitors: Current status and future perspective. Eur. J. Pharm. Sci. 2016, 93, 244–252. [Google Scholar] [CrossRef]
- Choi, C.-I. Sodium-glucose cotransporter 2 (SGLT2) inhibitors from natural products: Discovery of next-generation antihyperglycemic agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef]
- Oranje, P.; Gouka, R.; Burggraaff, L.; Vermeer, M.; Chalet, C.; Duchateau, G.; van der Pijl, P.; Geldof, M.; de Roo, N.; Clauwaert, F.; et al. Novel natural and synthetic inhibitors of solute carriers SGLT1 and SGLT2. Pharmacol. Res. Perspect. 2019, 7, e00504. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2024. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Obley, A.J.; Shamliyan, T.; Hicks, L.A.; Harrod, C.S.; Crandall, C.J.; Clinical Guidelines Committee of the American College of Physicians; Balk, E.M.; Cooney, T.G.; Cross, J.T.; et al. Newer pharmacologic treatments in adults with type 2 diabetes: A clinical guideline from the American College of Physicians. Ann. Intern. Med. 2024, 177, 658–666. [Google Scholar] [CrossRef] [PubMed]
- O’hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 inhibitors beyond glycaemic control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef]
- Gajewska, A.; Wasiak, J.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. SGLT2 Inhibitors in Kidney Diseases—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4959. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 diabetes mellitus and heart failure: A scientific statement from the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019, 140, e294–e324. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S158–S178. [Google Scholar] [CrossRef]
- Bahari, H.; Shahraki Jazinaki, M.; Rahnama, I.; Aghakhani, L.; Amini, M.R.; Malekahmadi, M. The cardiometabolic benefits of okra-based treatment in prediabetes and diabetes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2024, 11, 1454286. [Google Scholar] [CrossRef]
- Nikpayam, O.; Saghafi-Asl, M.; Safaei, E.; Bahreyni, N.; Sadra, V.; Asgharian, P. The effect of Abelmoschus esculentus L. (Okra) extract supplementation on glycaemic control, inflammation, kidney function and expression of PPAR-α, PPAR-γ, TGF-β and Nrf-2 genes in patients with diabetic nephropathy: A triple-blind, randomised, placebo-controlled trial. Br. J. Nutr. 2024, 131, 648–657. [Google Scholar]
- Sereno, A.B.; Pinto, C.D.; Andrade, F.A.; da Silva, M.A.B.; Garcia, A.C.; Krüger, C.C.H.; Reason, I.J.d.M. Effects of okra (Abelmoschus esculentus (L.) Moench) on glycemic markers in animal models of diabetes: A systematic review. J. Ethnopharmacol. 2022, 298, 115544. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Lebelo, S.L.; Modjadji, P.; Ghaffary, S. Okra ameliorates hyperglycaemia in pre-diabetic and type 2 diabetic patients: A systematic review and meta-analysis of the clinical evidence. Front. Pharmacol. 2023, 14, 1132650. [Google Scholar] [CrossRef] [PubMed]
- Adimadhyam, S.; Schumock, G.T.; Calip, G.S.; Smith Marsh, D.E.; Layden, B.T.; Lee, T.A. Increased risk of mycotic infections associated with sodium–glucose co-transporter 2 inhibitors: A prescription sequence symmetry analysis. Br. J. Clin. Pharmacol. 2019, 85, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, I.R.; Rose, S.C.P.; Freire, N.B.; Patrocínio, M.S.; Pierdoná, N.; Bittencourt, R.J. Use of sodium-glucose cotransporter-2 inhibitors and urinary tract infections in type 2 diabetes patients: A systematic review. Rev. Da Assoc. Médica Bras. 2019, 65, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Giugliano, D.; Longo, M.; Scappaticcio, L.; Bellastella, G.; Maiorino, M.I.; Esposito, K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: A meta-analysis of 11 CVOTs. Cardiovasc. Diabetol. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Higgins, J. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Cochrane Collab. 2011. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089. [Google Scholar] [CrossRef]
- EMPA-Kidney Collaborative Group. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Borovac, J.A.; Kurir, T.; Mustapic, I.; Kumric, M.; Bozic, J.; Glavas, D.; D’amario, D. SGLT2 inhibitors and the risk of urinary tract infections in patients with heart failure: A pooled analysis examining safety endpoints. Pol. Heart J. 2022, 80, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Lin, Y.; Yao, K.; Xie, Y.; Zhou, T. Cardiovascular outcomes and safety of SGLT2 inhibitors in chronic kidney disease patients. Front. Endocrinol. 2023, 14, 1236404. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Dhingra, N.K.; Hibino, M.; Gupta, V.; Verma, S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: A meta-analysis. ESC Heart Fail. 2022, 9, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, G.; Zheng, Y.; Lv, D.; Zhu, X.; Wei, P.; Zheng, M.; Liu, S.; Zhou, E.; Sun, W.; et al. Effects of SGLT2 inhibitors on cardiovascular outcomes in patients with stage 3/4 CKD: A meta-analysis. PLoS ONE 2022, 17, e0261986. [Google Scholar] [CrossRef]
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; Marra, C.A.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cherney, D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107. [Google Scholar] [CrossRef]
- Garcia-Ropero, A.; Santos-Gallego, C.G.; Zafar, M.U.; Badimon, J.J. Metabolism of the failing heart and the impact of SGLT2 inhibitors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 275–285. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The failing heart relies on ketone bodies as a fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Ian, A.; Margulies, K.B.; et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Zhang, L.; Wagg, C.; Al Batran, R.; Gopal, K.; Levasseur, J.; Leone, T.; Dyck, J.R.B.; Ussher, J.R.; Muoio, D.M.; et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 2019, 115, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin increases cardiac energy production in diabetes: Novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Raskin, P. Sodium–glucose cotransporter inhibition: Therapeutic potential for the treatment of type 2 diabetes mellitus. Diabetes/Metab. Res. Rev. 2013, 29, 347–356. [Google Scholar] [CrossRef]
- Kaplan, A.; Abidi, E.; El-Yazbi, A.; Eid, A.; Booz, G.W.; Zouein, F.A. Direct cardiovascular impact of SGLT2 inhibitors: Mechanisms and effects. Heart Fail. Rev. 2018, 23, 419–437. [Google Scholar] [CrossRef]
- Fathi, A.; Vickneson, K.; Singh, J.S. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail. Rev. 2021, 26, 623–642. [Google Scholar] [CrossRef]
- Briasoulis, A.; Androulakis, E.; Christophides, T.; Tousoulis, D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail. Rev. 2016, 21, 169–176. [Google Scholar] [CrossRef]
- Iannantuoni, F.; de Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Wu, M.; Pan, H.; Lei, X.; Chen, L.; Wu, Q.; Ouyang, X.; Liang, Z. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann. Transl. Med. 2019, 7, 429. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Sano, M.; Takei, M.; Shiraishi, Y.; Suzuki, Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J. Clin. Med. Res. 2016, 8, 844. [Google Scholar] [CrossRef]
- Lee, H.K. Cardiorenal protective effect of sodium–glucose cotransporter 2 inhibitors and mitochondrial function. J. Diabetes Investig. 2019, 10, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Fujita, Y.; Inagaki, N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: Clinical data and mechanism of action. J. Diabetes Investig. 2014, 5, 265–275. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kitada, K. Possible renoprotective mechanisms of SGLT2 inhibitors. Front. Med. 2023, 10, 1115413. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kameda, H.; Yamashita, K.; Nakamura, A.; Kurihara, Y. Protective effect of sodium–glucose cotransporter 2 inhibitors in patients with rapid renal function decline, stage G3 or G4 chronic kidney disease and type 2 diabetes. J. Diabetes Investig. 2019, 10, 1510–1517. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, T. The anticipated renoprotective effects of sodium-glucose cotransporter 2 inhibitors. Intern. Med. 2018, 57, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Z.; Ye, Y.-J.; Cheng, Z.-Y.; Hu, J.-J.; Zhang, C.-B.; Qian, L.; Lu, X.-H.; Cai, X.-R. Non-invasive assessment of early stage diabetic nephropathy by DTI and BOLD MRI. Br. J. Radiol. 2020, 93, 20190562. [Google Scholar] [CrossRef]
- Packer, M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: Modulation by SGLT2 inhibitors and hypoxia mimetics. Am. J. Kidney Dis. 2021, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ke, Q.; Fang, Y.; Wen, P.; Chen, H.; Yuan, Q.; Luo, J.; Zhang, Y.; Sun, Q.; Lv, Y.; et al. Sodium–glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020, 11, 390. [Google Scholar] [CrossRef]
- Bailey, C.J. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol. Sci. 2011, 32, 63–71. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Ferrandino, R.; Chang, A.; Surapaneni, A.; Chauhan, K.; Poojary, P.; Saha, A.; Ferket, B.; Grams, M.E.; Coca, S.G. Acute kidney injury in patients on SGLT2 inhibitors: A propensity-matched analysis. Diabetes Care 2017, 40, 1479–1485. [Google Scholar] [CrossRef]
- Sridhar, V.S.; Tuttle, K.R.; Cherney, D.Z. We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 76, 454–456. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal protection with SGLT2 inhibitors: Effects in acute and chronic kidney disease. Curr. Diabetes Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, S.; Wen, Y.; Tang, T.; Wang, B.; Liu, B. Cardiorenal protection of SGLT2 inhibitors-perspectives from metabolic reprogramming. EBioMedicine 2022, 83, 104215. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Cesaro, A.; Gallinoro, E.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in diabetic patients with acute myocardial infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT registry. Diabetes Res. Clin. Pract. 2023, 202, 110766. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).