Factors Associated with Para-Aortic Lymph Node Metastasis in High-Risk Endometrial Cancer
Abstract
1. Introduction
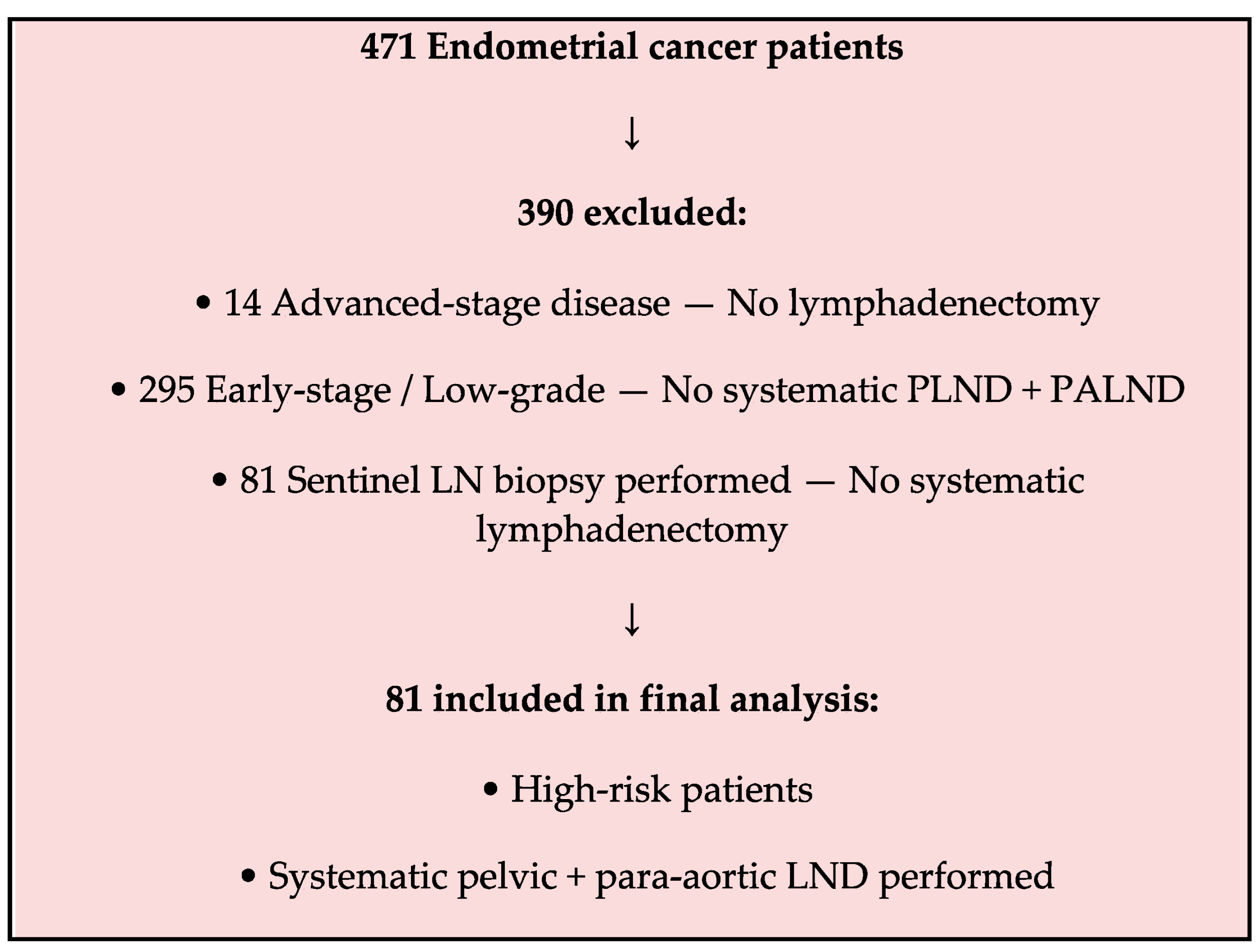
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| ESGO/ESTRO/ESP | European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology |
| FIGO | International Federation of Gynecology and Obstetrics |
| HR | Hazard ratio |
| LN | Lymph node |
| LVSI | Lymphovascular space invasion |
| MMRd | Mismatch repair deficient |
| NSMP | No specific molecular profile |
| OR | Odds ratio |
| POLE | Polymerase epsilon ultramutated subtype |
| ROC | Receiver operating characteristic |
| SLNB | Sentinel lymph node biopsy |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Torrent, A.; Amengual, J.; Sampol, C.M.; Ruiz, M.; Rioja, J.; Matheu, G.; Roca, P.; Cordoba, O. Sentinel lymph node biopsy in endometrial cancer: Dual injection, dual tracer—A multidisciplinary exhaustive approach to nodal staging. Cancers 2022, 14, 929. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)—The final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Benseler, A.; Vicus, D.; Covens, A.; Kupets, R.; Parra-Herran, C.; Gien, L.T. Assessing para-aortic nodal status in high-grade endometrial cancer patients with negative pelvic sentinel lymph node biopsy. Int. J. Gynecol. Obstet. 2025, 168, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, J.; Fu, Y.; Shen, Y.; Zhang, C.; Yao, S.; Xu, C.; Xia, M.; Lou, G.; Liu, J.; et al. Implications of isolated para-aortic lymph node metastasis in endometrial cancer: A large-scale, multicenter, and retrospective study. Front. Med. 2021, 8, 754890. [Google Scholar] [CrossRef]
- Ueno, Y.; Yoshida, E.; Nojiri, S.; Kato, T.; Ohtsu, T.; Takeshita, T.; Suzuki, S.; Yoshida, H.; Kato, K.; Itoh, M.; et al. Use of clinical variables for preoperative prediction of lymph node metastasis in endometrial cancer. Jpn. J. Clin. Oncol. 2024, 54, 38–46. [Google Scholar] [CrossRef]
- Solmaz, U.; Mat, E.; Dereli, M.L.; Turan, V.; Tosun, G.; Dogan, A.; Sanci, M.; Ozdemir, I.A.; Pala, E.E. Lymphovascular space invasion and positive pelvic lymph nodes are independent risk factors for para-aortic nodal metastasis in endometrioid endometrial cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 186, 63–67. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Chen, Y.; Zheng, A.; Tan, X.; Chen, H. Paraaortic lymph node metastasis in endometrial cancer patients: A comprehensive analysis of rates, survival outcomes, and risk factors through systematic review and meta-analysis. Front. Oncol. 2024, 14, 1490347. [Google Scholar] [CrossRef]
- Altay, A.; Toptas, T.; Dogan, S.; Simsek, T.; Pestereli, E. Analysis of metastatic regional lymph node locations and predictors of para-aortic lymph node involvement in endometrial cancer patients at risk for lymphatic dissemination. Int. J. Gynecol. Cancer 2015, 25, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.M.; Tseng, J.; Denslow, S.A.; Fader, A.N.; Gehrig, P.A. High-grade endometrial cancer: Revisiting the impact of tumor size and location on outcomes. Gynecol. Oncol. 2014, 132, 44–49. [Google Scholar] [CrossRef]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Li, G.; Zheng, L.; Shang, S.; Li, J.; Guan, X.; Yang, J. Investigating the influence of primary uterine tumor site on pelvic and para-aortic lymph node metastatic pattern and evaluating the risk factors for lymph node metastases in endometrial carcinoma: A retrospective study. Medicine 2023, 102, e36100. [Google Scholar] [CrossRef]
- Todo, Y.; Takeshita, S.; Okamoto, K.; Yamashiro, K.; Kato, H. Implications of para-aortic lymph node metastasis in patients with endometrial cancer without pelvic lymph node metastasis. J. Gynecol. Oncol. 2017, 28, e59. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Seo, J.-H.; Chang, K.; Kim, H.-S.; Chen, J.; Yang, T.-S.; Chen, Y.-L.; Lee, Y.-Y. Impact of Para-Aortic Lymphadenectomy on Clinically FIGO Stage IIIC1 High-Grade Endometrial Cancer: A Retrospective Cohort Study from Two Tertiary Centers in Korea and Taiwan. Medicina 2025, 61, 1079. [Google Scholar] [CrossRef]
- Fotopoulou, C.; El-Balat, A.; du Bois, A.; Sehouli, J.; Harter, P.; Muallem, M.Z.; Krätschell, R.W.; Traut, A.; Heitz, F. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch. Gynecol. Obstet. 2015, 292, 1321–1327. [Google Scholar] [CrossRef]
- Venigalla, S.; Chowdhry, A.K.; Shalowitz, D.I. Survival implications of staging lymphadenectomy for non-endometrioid endometrial cancers. Gynecol. Oncol. 2018, 149, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Renz, M.; Marjon, N.; Devereaux, K.; Raghavan, S.; Folkins, A.; Karam, A. Immediate intraoperative sentinel lymph node analysis by frozen section is predictive of lymph node metastasis in endometrial cancer. J. Robot. Surg. 2020, 14, 35–40. [Google Scholar] [CrossRef]
- Pavone, M.; Jochum, F.; Lecointre, L.; Fanfani, F.; Scambia, G.; Querleu, D.; Akladios, C. Therapeutic role of para-aortic lymphadenectomy in patients with intermediate-and high-risk endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2024, 34, 519–527. [Google Scholar] [CrossRef]
- Siegenthaler, F.; Imboden, S.; Büchi, C.; Christe, L.; Solass, W.; Saner, F.; Rauh, C.; Hofer, S.; Schlatter, B.; Wampfler, J.; et al. Added prognostic value of sentinel lymph node mapping in endometrial cancer to molecular subgroups. Gynecol. Oncol. 2025, 193, 12–19. [Google Scholar] [CrossRef]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Britton, H.; Huang, L.; Lum, A.; Leung, S.; Shum, K.; Kale, M.; Burleigh, A.; Senz, J.; Yang, W.; McConechy, M.; et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol. Oncol. 2019, 153, 487–495. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 81) |
|---|---|
| Age (years), mean ± SD | 63.5 ± 12.3 |
| BMI (kg/m2), mean ± SD | 31.2 ± 6.4 |
| Histologic subtype (n, %) | |
| Mixed | 26 (32.1) |
| Serous | 21 (25.9) |
| Clear cell | 10 (12.3) |
| Undifferentiated | 11 (13.6) |
| Carcinosarcoma | 7 (8.6) |
| Grade 3 Endometrioid | 6 (7.4) |
| LVSI positive (n, %) | 29 (35.8) |
| Cervical stromal invasion (n, %) | 23 (28.4) |
| Myometrial invasion ≥ 50% (n, %) | 38 (46.9) |
| Tumor size ≥ 3.55 cm (n, %) | 38 (46.9) |
| Variable | Para-Aortic Metastasis (+) (n = 17) | Para-Aortic Metastasis (−) (n = 64) | p-Value |
|---|---|---|---|
| Pelvic LN ≥ 1 positive | 15 (88.2%) | 8 (12.5%) | <0.001 |
| LVSI positive | 13 (76.5%) | 16 (25.0%) | 0.005 |
| Cervical stromal invasion | 10 (58.8%) | 13 (20.3%) | 0.008 |
| Myometrial invasion ≥ 50% | 13 (76.5%) | 25 (39.1%) | 0.030 |
| Tumor size ≥ 3.55 cm | 13 (76.5%) | 25 (39.1%) | 0.030 |
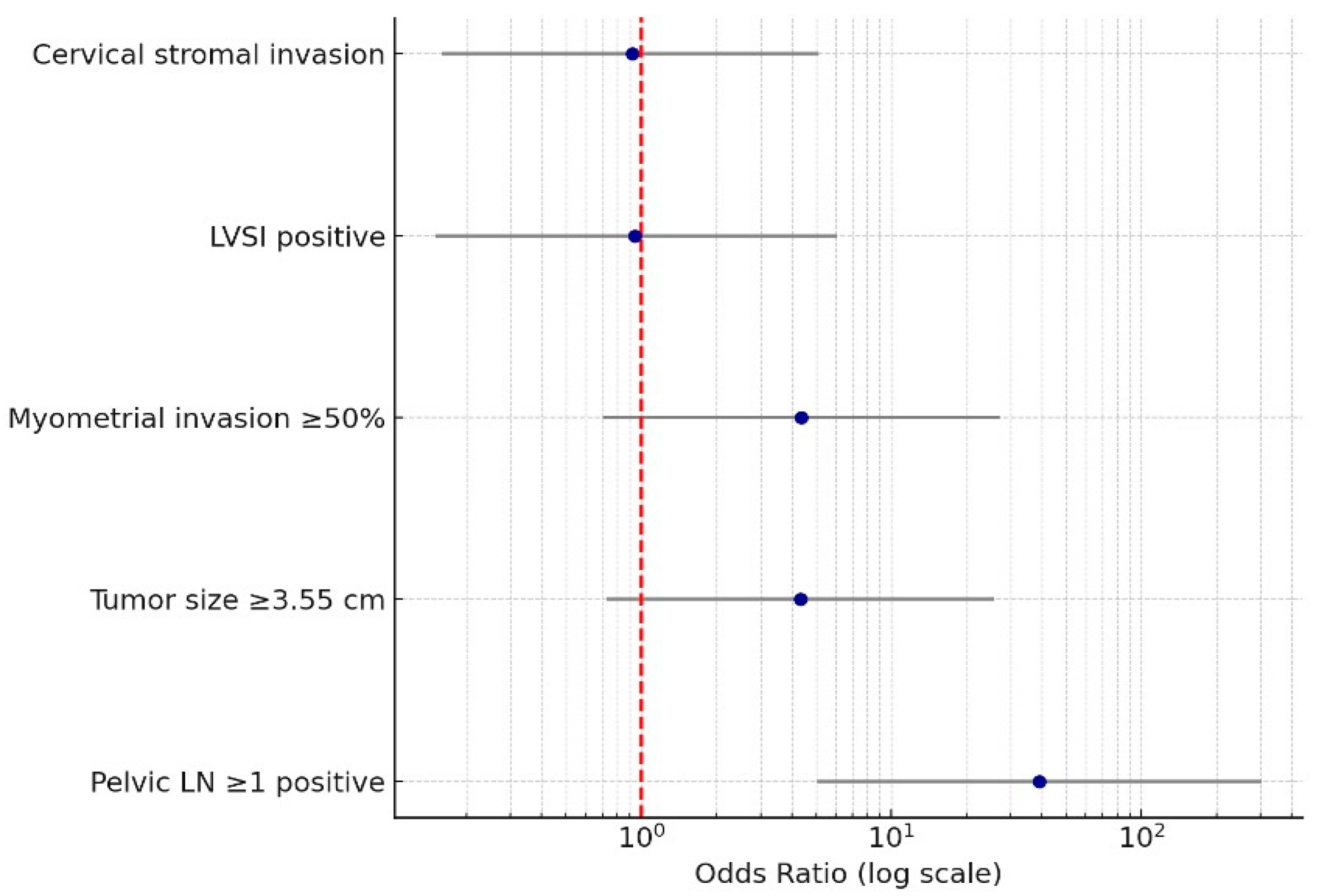
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Pelvic LN ≥ 1 positive | 39.0 (5.06–301.46) | <0.001 |
| LVSI positive | 0.94 (0.15–6.11) | 0.95 |
| Cervical stromal invasion | 0.92 (0.16–5.15) | 0.93 |
| Myometrial invasion ≥ 50% | 4.35 (0.70–27.06) | 0.11 |
| Tumor size ≥ 3.55 cm | 4.32 (0.73–25.74) | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güner, F.C.; Iltar, E.; Ateş Tıkız, M.; Doğan, S.; Doğan, N.U.; Tuncer, H.A.; Şimşek, T. Factors Associated with Para-Aortic Lymph Node Metastasis in High-Risk Endometrial Cancer. Medicina 2025, 61, 2189. https://doi.org/10.3390/medicina61122189
Güner FC, Iltar E, Ateş Tıkız M, Doğan S, Doğan NU, Tuncer HA, Şimşek T. Factors Associated with Para-Aortic Lymph Node Metastasis in High-Risk Endometrial Cancer. Medicina. 2025; 61(12):2189. https://doi.org/10.3390/medicina61122189
Chicago/Turabian StyleGüner, Fatma Ceren, Elif Iltar, Müge Ateş Tıkız, Selen Doğan, Nasuh Utku Doğan, Hasan Aykut Tuncer, and Tayup Şimşek. 2025. "Factors Associated with Para-Aortic Lymph Node Metastasis in High-Risk Endometrial Cancer" Medicina 61, no. 12: 2189. https://doi.org/10.3390/medicina61122189
APA StyleGüner, F. C., Iltar, E., Ateş Tıkız, M., Doğan, S., Doğan, N. U., Tuncer, H. A., & Şimşek, T. (2025). Factors Associated with Para-Aortic Lymph Node Metastasis in High-Risk Endometrial Cancer. Medicina, 61(12), 2189. https://doi.org/10.3390/medicina61122189






