Long-Term Cancer Incidence Trends in Korea (2001–2020): An Age–Period–Cohort and Joinpoint Analysis with a Focus on Younger Cohorts
Abstract
1. Introduction
2. Methods
2.1. Study Data and Population
2.2. Statistical Analysis
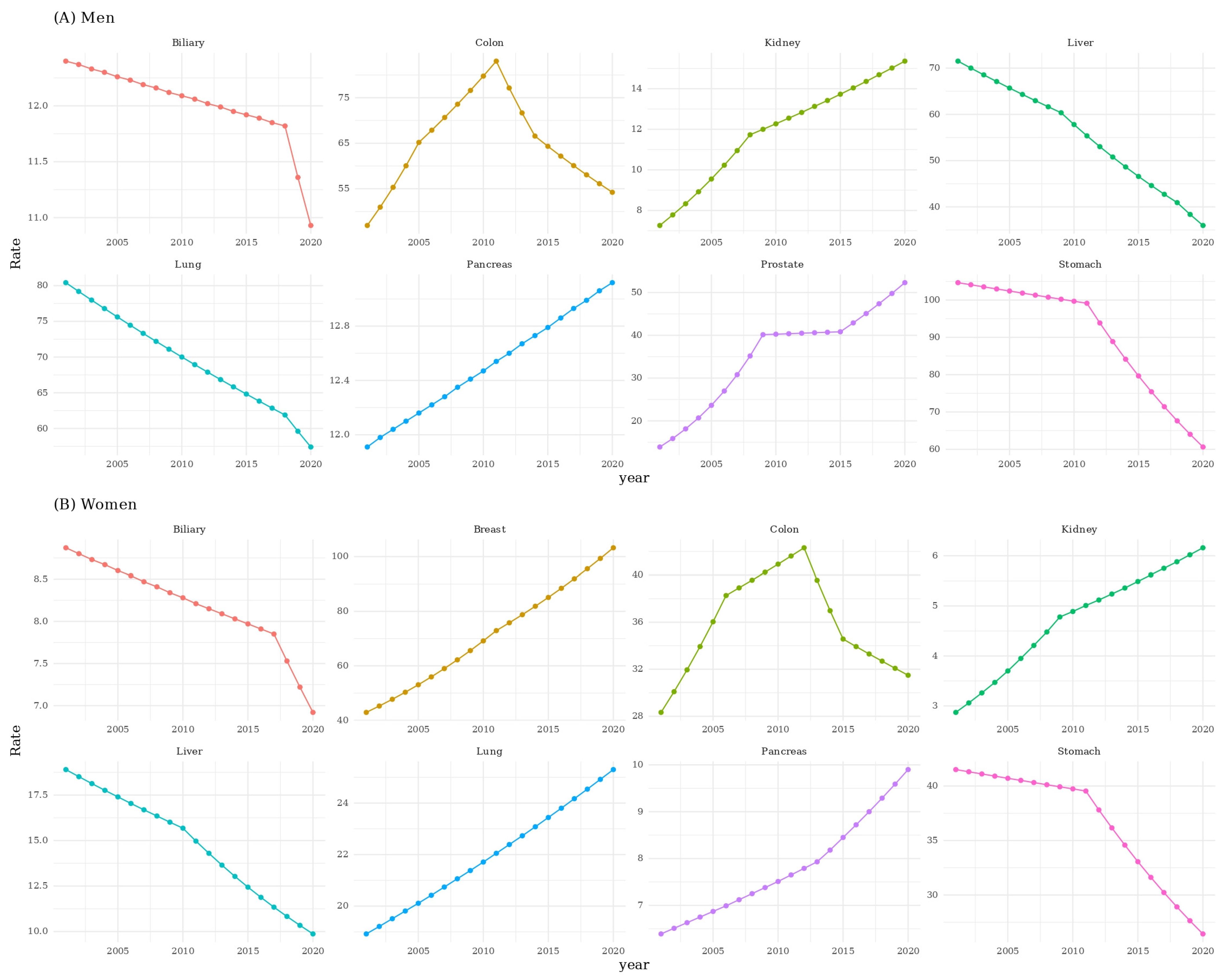
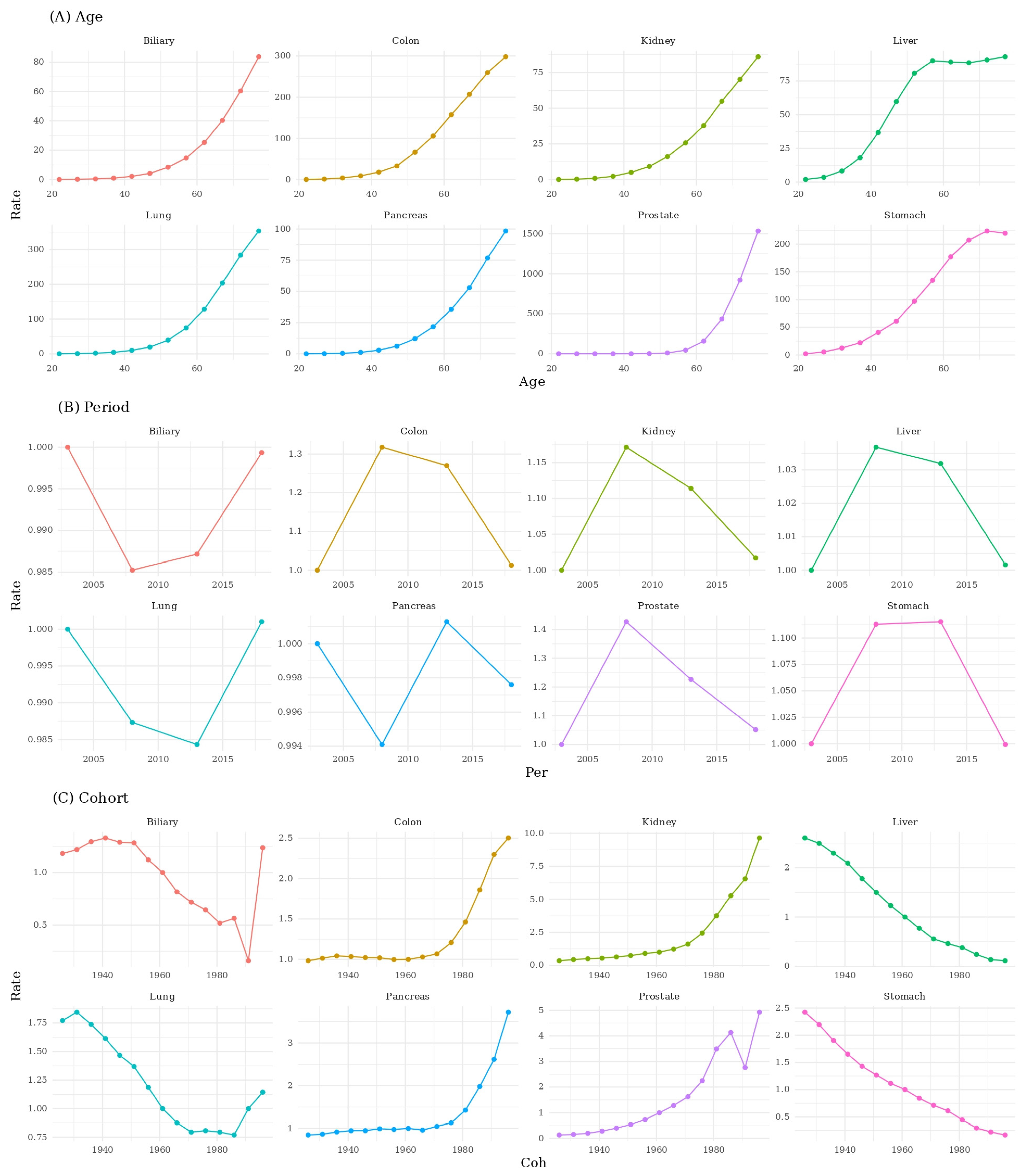
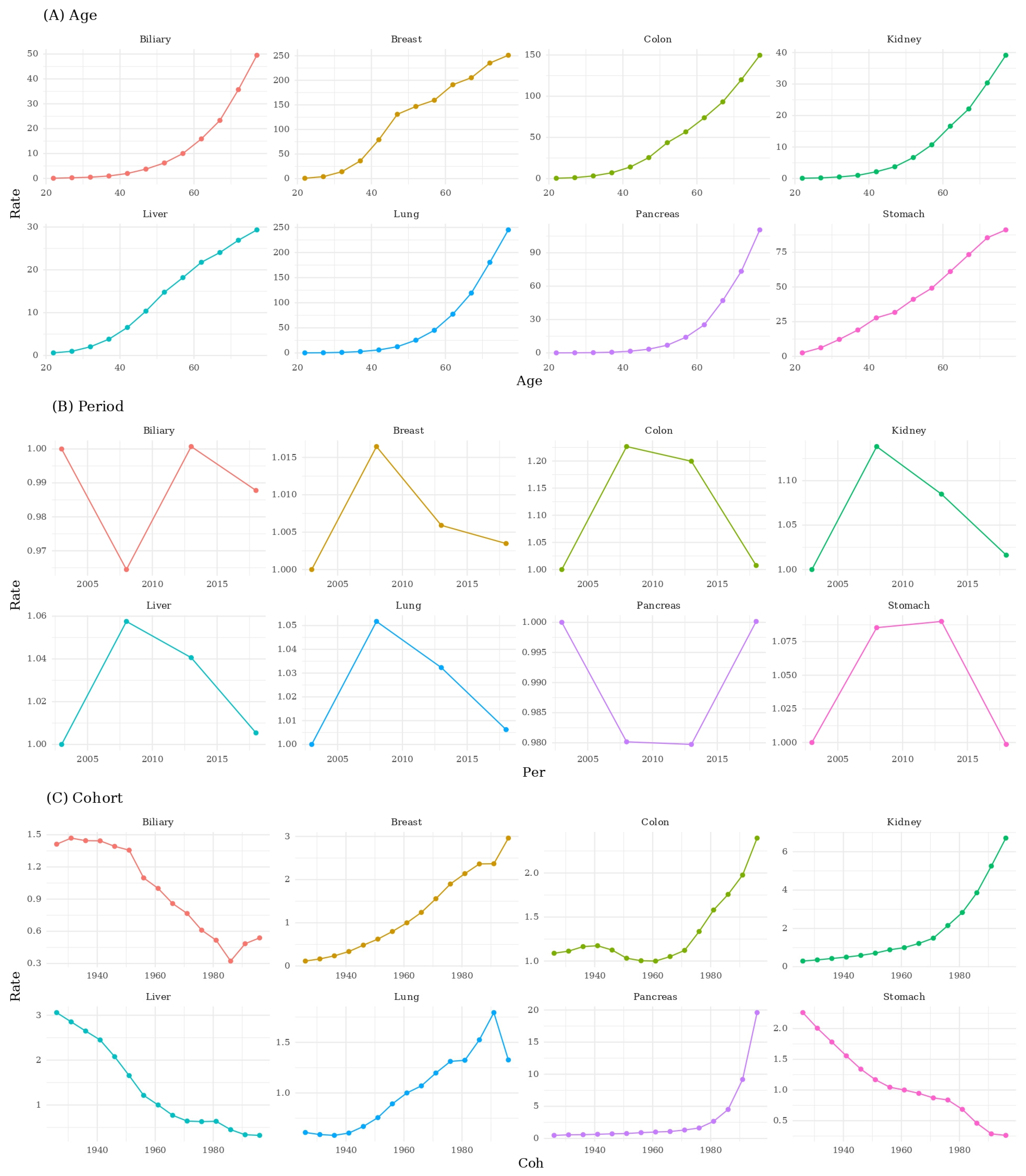
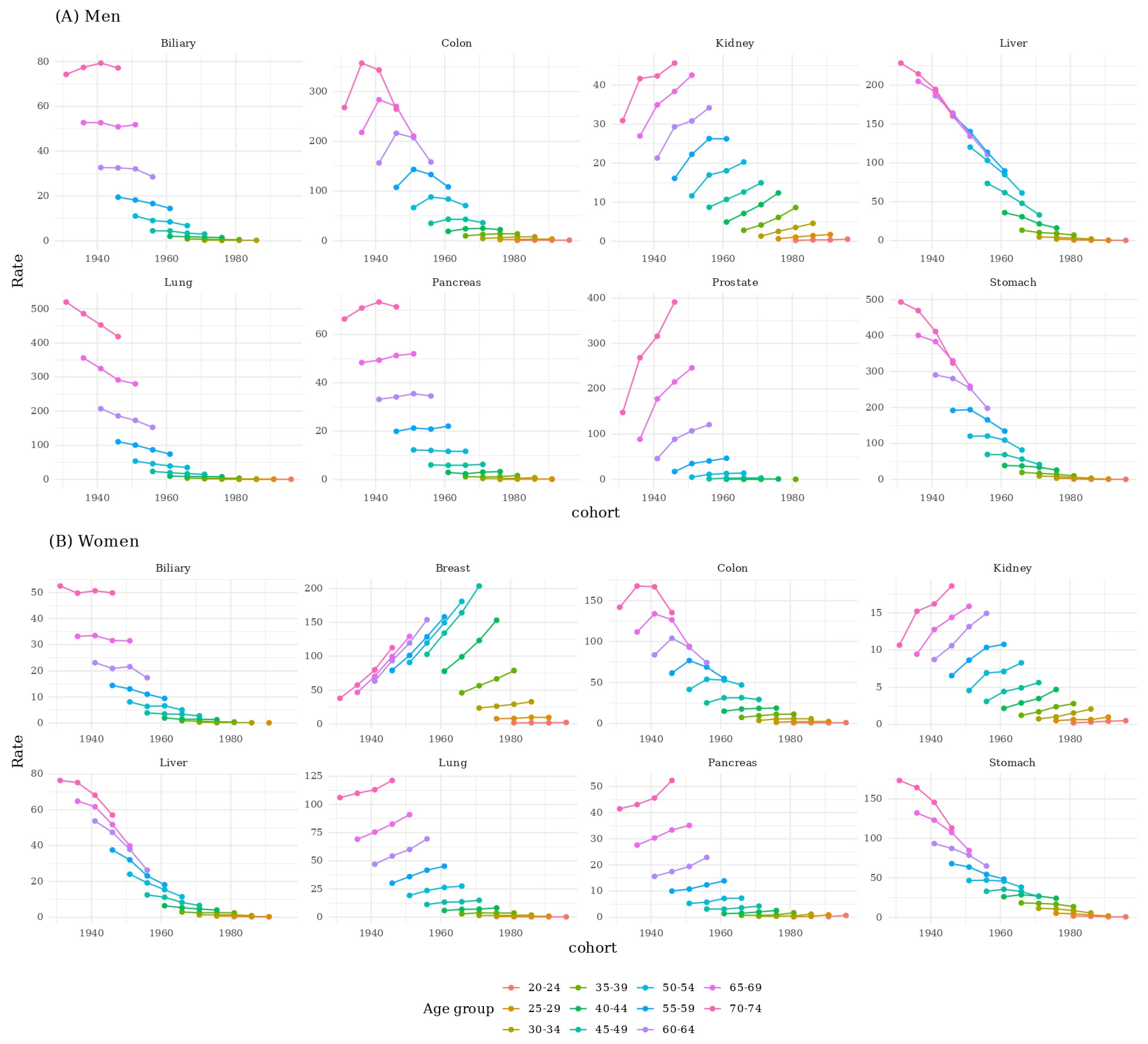
3. Results
3.1. Trends in Age-Adjusted Incidence Rates (APC Analysis)
3.2. Age–Period–Cohort Decomposition
3.3. Birth Cohort Effects by Sex
3.4. Age-Specific Cohort Comparisons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Keum, N. Global trends in early-onset and late-onset cancer incidence. J. Public Health 2025, 47, 699–709. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Kang, M.J.; Won, Y.-J.; Lee, J.J.; Jung, K.-W.; Kim, H.-J.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. CRT 2022, 54, 330–344. [Google Scholar] [CrossRef]
- Jung, K.-W.; Won, Y.-J.; Hong, S.; Kong, H.-J.; Lee, E.S. Prediction of Cancer Incidence and Mortality in Korea, 2020. CRT 2020, 52, 351–358. [Google Scholar] [CrossRef]
- Jung, K.-W.; Won, Y.-J.; Hong, S.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. CRT 2021, 53, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Joshy, G.; Thandrayen, J.; Koczwara, B.; Butow, P.; Laidsaar-Powell, R.; Rankin, N.; Canfell, K.; Stubbs, J.; Grogan, P.; Bailey, L.; et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: Population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med. 2020, 18, 372. [Google Scholar] [CrossRef]
- Zheng, Z.; Jemal, A.; Han, X.; Guy, G.P., Jr.; Li, C.; Davidoff, A.J.; Banegas, M.P.; Ekwueme, D.U.; Yabroff, K.R. Medical financial hardship among cancer survivors in the United States. Cancer 2019, 125, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Júnior, L.C. The emerging epidemic of early-onset cancer: Global patterns, biological complexity, and urgent calls for action. Cancer Control 2025, 32, 10732748251386505. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA A Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Levine, O.; Zbuk, K. Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatr. Blood Cancer 2019, 66, e27941. [Google Scholar] [CrossRef]
- Moon, E.-K.; Park, H.J.; Oh, C.-M.; Jung, K.-W.; Shin, H.Y.; Park, B.K.; Won, Y.-J. Cancer incidence and survival among adolescents and young adults in Korea. PLoS ONE 2014, 9, e96088. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, S.H. Epidemiology of Lung Cancer in Korea: Recent Trends. Tuberc. Respir. Dis. 2016, 79, 58–69. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Shin, H.-R.; Lee, B.; Shin, A.; Jung, K.-W.; Jee, S.H.; Kim, D.H.; Yun, Y.H.; Park, S.K. Population-attributable causes of cancer in Korea: Obesity and physical inactivity. PLoS ONE 2014, 9, e90871. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Jung, K.W.; Won, Y.J.; Shin, A.; Kong, H.J.; Lee, J.S. Age-Period-Cohort Analysis of Thyroid Cancer Incidence in Korea. Cancer Res. Treat. 2015, 47, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D. Epidemiology of prostate cancer. Urology 2003, 62, 3–12. [Google Scholar] [CrossRef]
- Chung, H.W.; Noh, S.H.; Lim, J.-B. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J. Gastroenterol. 2010, 16, 256. [Google Scholar] [CrossRef]
- Xie, X.; Yin, J.; Zhou, Z.; Dang, C.; Zhang, H.; Zhang, Y. Young age increases the risk for lymph node metastasis in patients with early Colon Cancer. BMC Cancer 2019, 19, 803. [Google Scholar] [CrossRef]
- Clarke, M.A.; Joshu, C.E. Early life exposures and adult cancer risk. Epidemiol. Rev. 2017, 39, 11–27. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J. Global patterns in excess body weight and the associated cancer burden. CA A Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Berger, N.A. Young Adult Cancer: Influence of the Obesity Pandemic. Obesity 2018, 26, 641–650. [Google Scholar] [CrossRef]
- Dawson, D.W.; Hertzer, K.; Moro, A.; Donald, G.; Chang, H.H.; Go, V.L.; Pandol, S.J.; Lugea, A.; Gukovskaya, A.S.; Li, G.; et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev. Res. 2013, 6, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Kim, Y.H.; Han, K.; Jung, J.H.; Rhee, E.J.; Lee, W.Y. Obesity Fact Sheet in Korea, 2020: Prevalence of Obesity by Obesity Class from 2009 to 2018. J. Obes. Metab. Syndr. 2021, 30, 141–148. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Shin, D.W.; Chang, D.; Jung, J.H.; Han, K.; Kim, S.Y.; Choi, K.S.; Lee, W.C.; Park, J.H.; Park, J.H. Disparities in the Participation Rate of Colorectal Cancer Screening by Fecal Occult Blood Test among People with Disabilities: A National Database Study in South Korea. Cancer Res. Treat. 2020, 52, 60–73. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, S.J.; Lee, Y.C.; Yang, H.J.; Park, S.Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.H.; Nam, S.Y.; et al. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver 2021, 15, 168–195. [Google Scholar] [CrossRef]
- Yim, S.Y.; Kim, J.H. The epidemiology of hepatitis B virus infection in Korea. Korean J. Intern. Med. 2019, 34, 945–953. [Google Scholar] [CrossRef]
- Kim, H.; Shin, A.R.; Chung, H.H.; Kim, M.K.; Lee, J.S.; Shim, J.-J.; Kim, B.-H. Recent trends in hepatitis B virus infection in the general Korean population. Korean J. Intern. Med. 2013, 28, 413. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shin, S.W. Overdiagnosis and screening for thyroid cancer in Korea. Lancet 2014, 384, 1848. [Google Scholar] [CrossRef]
- Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2020. Minist. Health Welf. 2022. [Google Scholar]
- Lin, Y.; Wu, Y. Trends in incidence and overdiagnosis of thyroid cancer in China, Japan, and South Korea. Cancer Sci. 2023, 114, 4052–4062. [Google Scholar] [CrossRef]
- Kitahara, C.M. The growing global burden of thyroid cancer overdiagnosis. Lancet Diabetes Endocrinol. 2024, 12, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Robertson, C.; Boyle, P. Age-period-cohort analysis of chronic disease rates. I: Modelling approach. Stat. Med. 1998, 17, 1305–1323. [Google Scholar] [CrossRef]
- Kim, D.-M.; Kim, K.-H. The Changes in Obesity Prevalence and Dietary Habits in Korean Adults by Residential Area during the Last 10 Years–Based on the 4 th (2007–2009) and the 7 th (2016–2018) Korea National Health and Nutrition Examination Survey Data. Korean J. Community Nutr. 2021, 26, 37–47. [Google Scholar] [CrossRef]
- Kim, Y.; Nho, S.J.; Woo, G.; Kim, H.; Park, S.; Kim, Y.; Park, O.; Oh, K. Trends in the prevalence and management of major metabolic risk factors for chronic disease over 20 years: Findings from the 1998-2018 Korea National Health and Nutrition Examination Survey. Epidemiol. Health 2021, 43, e2021028. [Google Scholar] [CrossRef]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar]
- Randi, G.; Malvezzi, M.; Levi, F.; Ferlay, J.; Negri, E.; Franceschi, S.; La Vecchia, C. Epidemiology of biliary tract cancers: An update. Ann. Oncol. 2009, 20, 146–159. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, H.-Y.; Lim, D.; Cho, H.-J.; Khang, Y.-H. Long-term trends in smoking prevalence and its socioeconomic inequalities in Korea, 1992–2016. Int. J. Equity Health 2019, 18, 148. [Google Scholar] [CrossRef]
- Lee, H.-E.; Zaitsu, M.; Kim, E.-A.; Kawachi, I. Cancer incidence by occupation in Korea: Longitudinal analysis of a nationwide cohort. Saf. Health Work 2020, 11, 41–49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, M.; Song, G.; Joung, J.Y.; Seo, H.; Yoon, J.-H.; Chung, J. Long-Term Cancer Incidence Trends in Korea (2001–2020): An Age–Period–Cohort and Joinpoint Analysis with a Focus on Younger Cohorts. Medicina 2025, 61, 2179. https://doi.org/10.3390/medicina61122179
Lee H, Kim M, Song G, Joung JY, Seo H, Yoon J-H, Chung J. Long-Term Cancer Incidence Trends in Korea (2001–2020): An Age–Period–Cohort and Joinpoint Analysis with a Focus on Younger Cohorts. Medicina. 2025; 61(12):2179. https://doi.org/10.3390/medicina61122179
Chicago/Turabian StyleLee, Hyungho, Mingyu Kim, Geehyun Song, Jae Young Joung, Hokyung Seo, Jin-Ha Yoon, and Jinsoo Chung. 2025. "Long-Term Cancer Incidence Trends in Korea (2001–2020): An Age–Period–Cohort and Joinpoint Analysis with a Focus on Younger Cohorts" Medicina 61, no. 12: 2179. https://doi.org/10.3390/medicina61122179
APA StyleLee, H., Kim, M., Song, G., Joung, J. Y., Seo, H., Yoon, J.-H., & Chung, J. (2025). Long-Term Cancer Incidence Trends in Korea (2001–2020): An Age–Period–Cohort and Joinpoint Analysis with a Focus on Younger Cohorts. Medicina, 61(12), 2179. https://doi.org/10.3390/medicina61122179





