Expression of Core Hippo Pathway Proteins in Cervical Cancer and Their Association with Clinicopathologic Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Tissue Microarray (TMA) Construction and Ethical Approval
2.3. Immunohistochemical (IHC) Staining
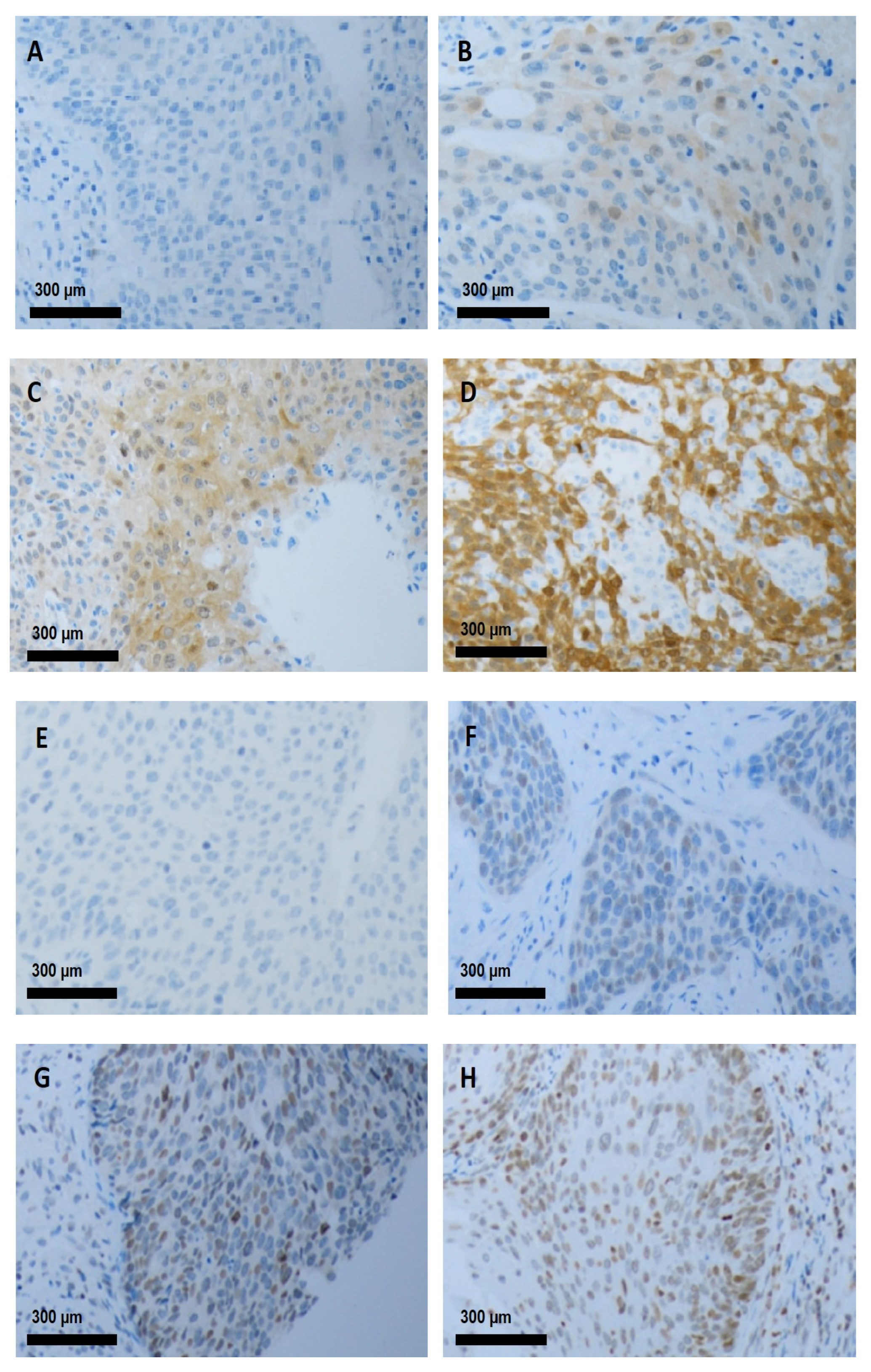
2.4. Immunostaining Evaluation
2.5. Cell Culture and siRNA-Mediated YAP Knockdown
2.6. Statistical Analysis
3. Results
3.1. Clinicopathologic Features of the Patients
3.2. IHC Staining in Cervical Cancer
3.3. Association Between Clinicopathologic Features and Protein Expression
3.4. Associations Among Hippo Core Protein Expression
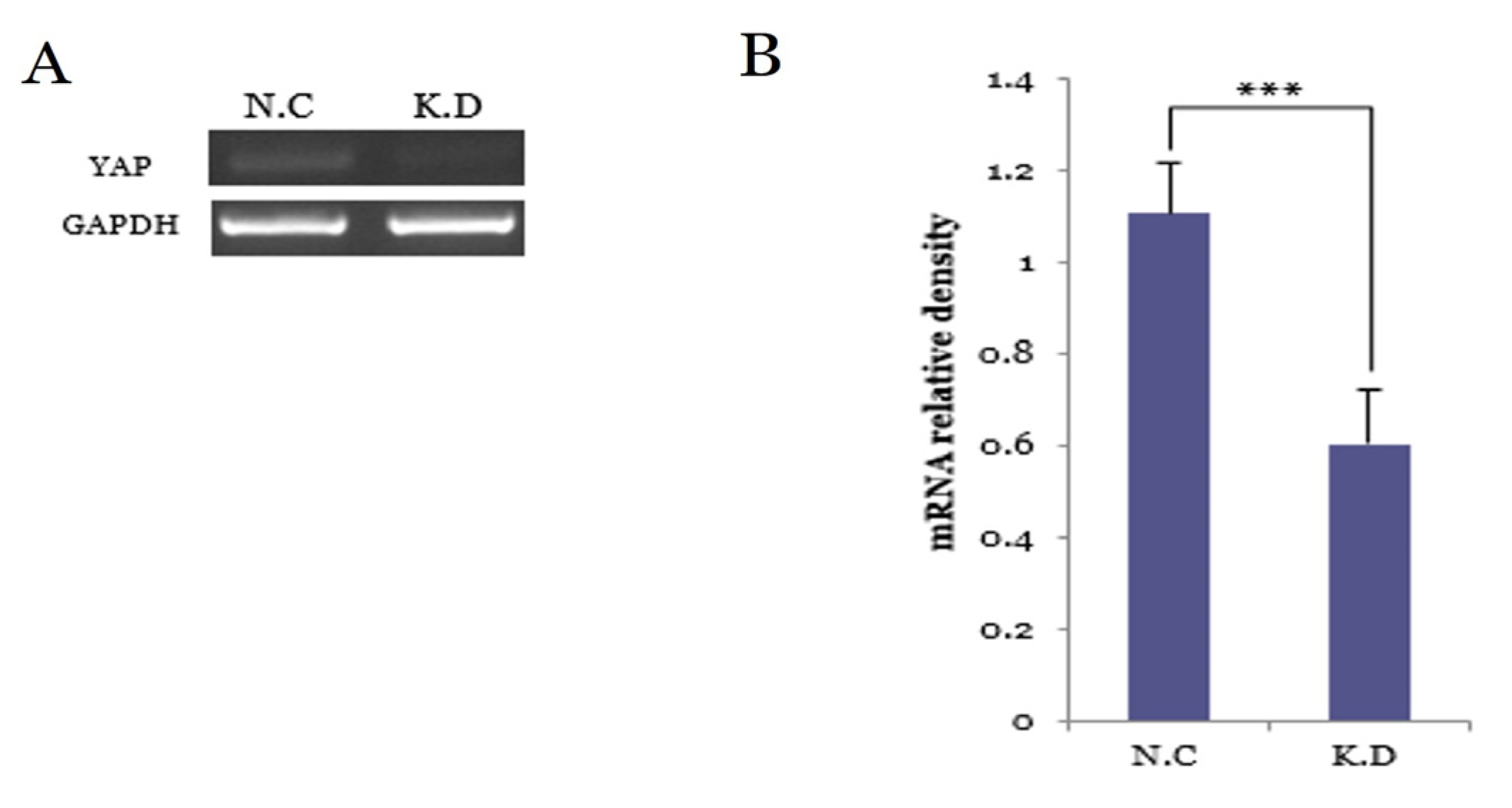
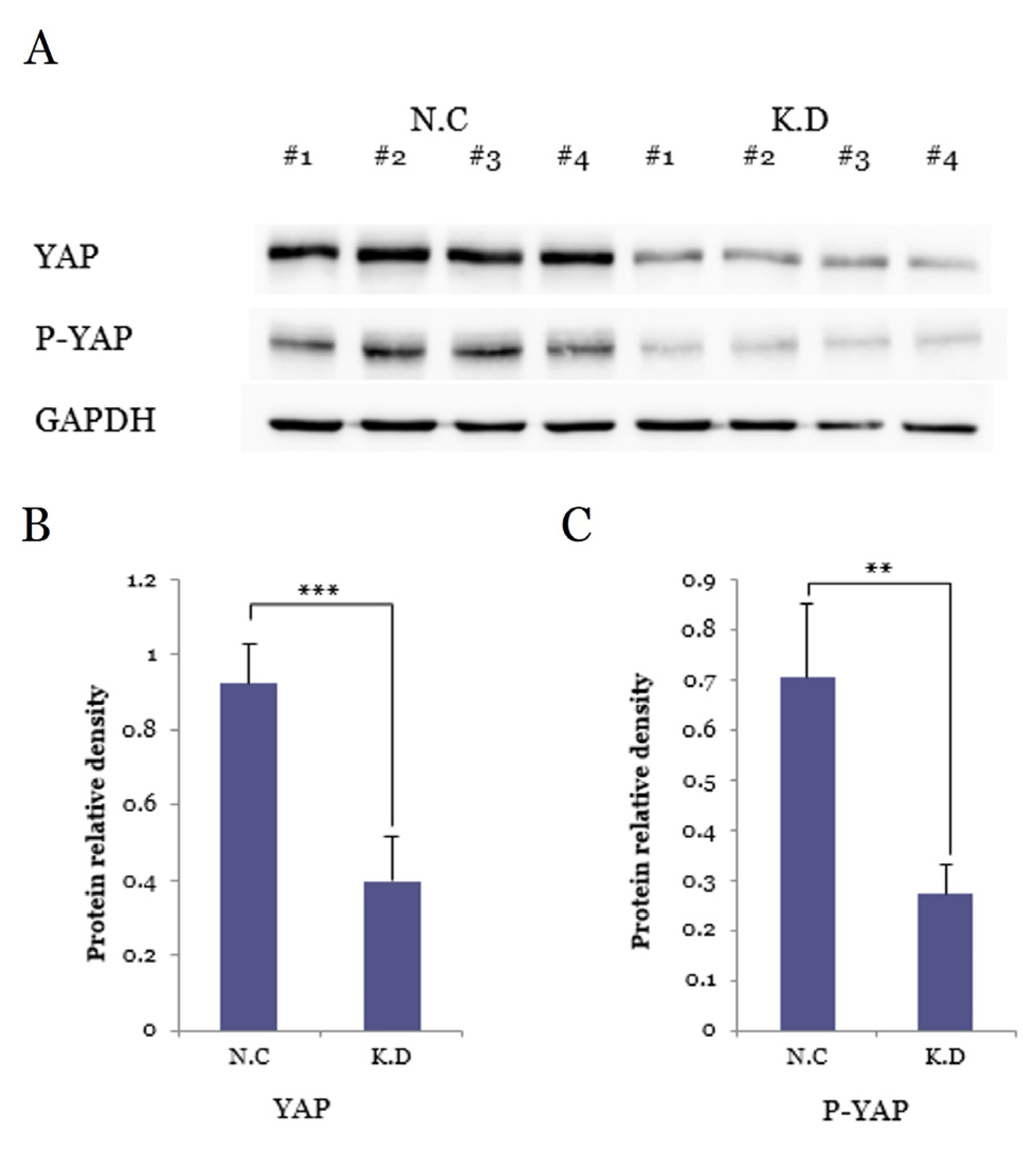
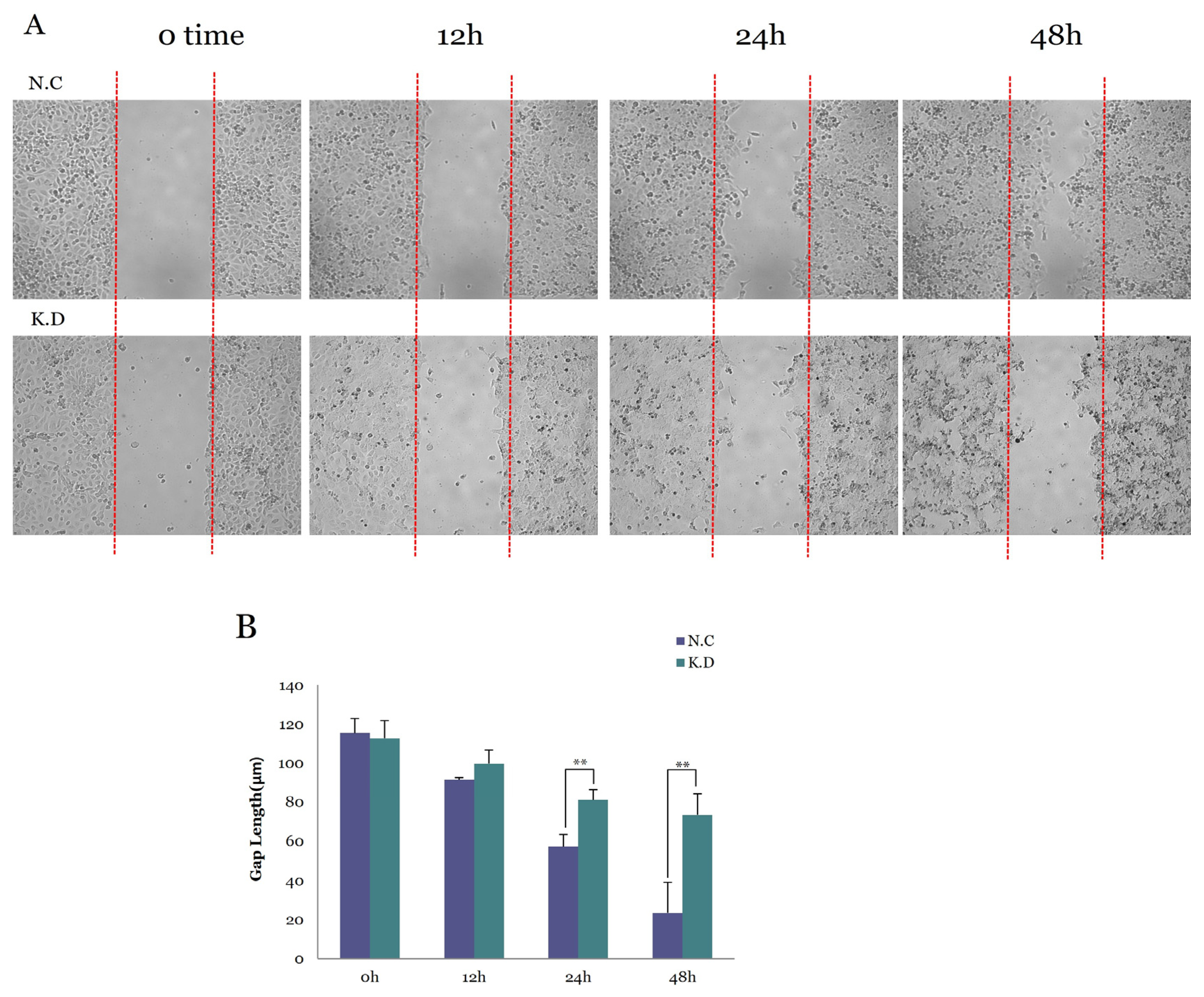
3.5. Functional Validation of YAP Knockdown in Caski Cervical Cancer Cells
3.5.1. YAP Knockdown Efficiency at the mRNA Level
3.5.2. YAP and P-YAP Suppression by SiRNA
3.5.3. Functional Consequences of YAP Knockdown on Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udan, R.S.; Kango-Singh, M.; Nolo, R.; Tao, C.; Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003, 5, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Pantalacci, S.; Tapon, N.; Leopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003, 5, 921–927. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 114, 457–467. [Google Scholar] [CrossRef]
- Park, J.A.; Kwon, Y. Hippo-YAP/TAZ signaling in angiogenesis. BMB Rep. 2018, 51, 157–162. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, B.; Guan, K. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Ni, L.; Osinski, A.; Tomchick, D.R.; Brautigam, C.A.; Luo, X. SAV1 promotes Hippo kinase activation through antagonizing the PP2A phosphatase STRIPAK. Elife 2017, 6, e30278. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef]
- Kwon, Y.; Vinayagam, A.; Sun, X.; Dephoure, N.; Gygi, S.P.; Hong, P.; Perrimon, N. The Hippo signaling pathway interactome. Science 2013, 342, 737–740. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Dong, Y.; Yuan, Z. The Role and Regulatory Mechanism of Hippo Signaling Components in the Neuronal System. Front. Immunol. 2020, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ando, T.; Izumi, H.; Kobayashi, S.S.; Shintani, T.; Gutkind, J.S.; Yanamoto, S.; Miyauchi, M.; Kajiya, M. AXL activates YAP through the EGFR-LATS1/2 axis and confers resistance to EGFR-targeted drugs in head and neck squamous cell carcinoma. Oncogene 2023, 42, 2869–2877. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Mottolese, M.; Sperati, F.; Ercolani, C.; Di Lauro, L.; Pizzuti, L.; Vici, P.; Terrenato, I.; Shaaban, A.M.; Humphries, M.P.; et al. Association between AXL, Hippo transducers, and survival outcomes in male breast cancer. J. Cell Physiol. 2017, 232, 2246–2252. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chang, Y.-C.; Chien, M.-N.; Jhuang, J.-Y.; Hsu, Y.-C.; Huang, S.-Y.; Cheng, S.-P. SREBP1 promotes invasive phenotypes by upregulating CYR61/CTGF via the Hippo-YAP pathway. Endocr. Relat. Cancer. 2021, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin. Sci. 2022, 136, 197–222. [Google Scholar] [CrossRef]
- Calses, P.C.; Crawford, J.J.; Lill, J.R.; Dey, A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer. 2019, 5, 297–307. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Patterson, M.R.; Cogan, J.A.; Cassidy, R.; Theobald, D.A.; Wang, M.; Scarth, J.A.; Anene, C.A.; Whitehouse, A.; Morgan, E.L.; Macdonald, A. The Hippo pathway transcription factors YAP and TAZ play HPV-type dependent roles in cervical cancer. Nat. Commun. 2024, 15, 5809. [Google Scholar] [CrossRef]
- Wang, D.; He, J.; Dong, J.; Meyer, T.F.; Xu, T. The HIPPO pathway in gynecological malignancies. Am. J. Cancer Res. 2020, 10, 610–629. [Google Scholar] [PubMed]
- Yang, J.; Song, D.H.; Kim, C.H.; Kim, M.H.; Jo, H.C.; Kim, H.; Park, J.E.; Baek, J.C. Expression of the Hippo Pathway Core Components in Endometrial Cancer and Its Association with Clinicopathologic Features. Diagnostics 2022, 12, 2973. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Zhang, E.; Wang, D.; Cai, W.; Li, C.; Wei, Q. LncRNA PGM5-AS1 impairs the resistance of cervical cancer to cisplatin by regulating the Hippo and PI3K-AKT pathways. Biochem. Genet. 2024; online ahead of print. [Google Scholar]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Basu, P.; Kaur, P.; Bhaskar, R.; Singh, G.B.; Denzongpa, P.; Grover, R.K.; Sebastian, P.; Saikia, T.; Oswal, K.; et al. Current status of human papillomavirus vaccination in India’s cervical cancer prevention efforts. Lancet Oncol. 2019, 20, e637–e644. [Google Scholar] [CrossRef]
- He, C.; Mao, D.; Hua, G.; Lv, X.; Chen, X.; Angeletti, P.C.; Dong, J.; Remmenga, S.W.; Rodabaugh, K.J.; Zhou, J.; et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol. Med. 2015, 7, 1426–1449. [Google Scholar] [CrossRef]
- Buglioni, S.; Vici, P.; Sergi, D.; Pizzuti, L.; Di Lauro, L.; Antoniani, B.; Sperati, F.; Terrenato, I.; Carosi, M.; Gamucci, T.; et al. Analysis of the Hippo transducers TAZ and YAP in cervical cancer and its microenvironment. Oncoimmunology 2016, 5, e1160187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Wang, R.; Chen, H.; Wang, R.; Wang, W.; Yang, X. NGF Signaling Interacts with the Hippo/YAP Pathway to Regulate Cervical Cancer Progression. Front. Oncol. 2021, 11, 688794. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Ma, F.; Tian, R.; Zhou, Y.; Lan, W.; Song, Q.; Cheng, X. AJUBA increases the cisplatin resistance through Hippo pathway in cervical cancer. Gene 2018, 644, 148–154. [Google Scholar] [CrossRef]
- He, C.; Lv, X.; Liu, J.; Ruan, J.; Chen, P.; Huang, C.; Angeletti, P.C.; Hua, G.; Moness, M.L.; Shi, D.; et al. HPV-YAP1 oncogenic alliance drives malignant transformation of fallopian tube epithelial cells. EMBO Rep. 2024, 25, 4542–4569. [Google Scholar] [CrossRef]
- Wloszek, E.; Krupa, K.; Skrok, E.; Budzik, M.P.; Deptala, A.; Badowska-Kozakiewicz, A. HPV and Cervical Cancer-Biology, Prevention, and Treatment Updates. Curr. Oncol. 2025, 32, 122. [Google Scholar] [CrossRef]
- Olmedo-Nieva, L.; Muñoz-Bello, J.O.; Manzo-Merino, J.; Lizano, M. New insights in Hippo signalling alteration in human papillomavirus-related cancers. Cell Signal. 2020, 76, 109815. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2025 update. Int. J. Gynaecol. Obstet. 2025, 171 (Suppl. 1), 87–108. [Google Scholar] [CrossRef]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical cancer: Screening, diagnosis and staging. JBUON 2016, 21, 320–325. [Google Scholar] [PubMed]
- Laengsri, V.; Kerdpin, U.; Plabplueng, C.; Treeratanapiboon, L.; Nuchnoi, P. Cervical Cancer Markers: Epigenetics and microRNAs. Lab. Med. 2018, 49, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cells 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Mesrouze, Y.; Chene, P. Study of the TEAD-binding domain of the VGLL1, VGLL2 and VGLL3 proteins from vertebrates. Arch. Biochem. Biophys. 2024, 760, 110136. [Google Scholar] [CrossRef]
- Mesrouze, Y.; Aguilar, G.; Bokhovchuk, F.; Martin, T.; Delaunay, C.; Villard, F.; Meyerhofer, M.; Zimmermann, C.; Fontana, P.; Wille, R.; et al. A new perspective on the interaction between the Vg/VGLL1-3 proteins and the TEAD transcription factors. Sci. Rep. 2020, 10, 17442. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Ashayeri, N.; Baghaie, L.; Sambi, M.; Satari, K.; Baluch, N.; Bosykh, D.A.; Szewczuk, M.R.; Chakraborty, S. The Hippo Pathway Effectors YAP/TAZ-TEAD Oncoproteins as Emerging Therapeutic Targets in the Tumor Microenvironment. Cancers 2023, 15, 3468. [Google Scholar] [CrossRef]
- Pobbati, A.V.; Kumar, R.; Rubin, B.P.; Hong, W. Therapeutic targeting of TEAD transcription factors in cancer. Trends Biochem. Sci. 2023, 48, 450–462. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Gao, H.; Meng, F.; Yang, S.; Lou, G. Clinical Significance of Yes-Associated Protein Overexpression in Cervical Carcinoma: The Differential Effects Based on Histotypes. Int. J. Gynecol. Cancer 2013, 23, 735–742. [Google Scholar] [CrossRef]
- Tsujiura, M.; Mazack, V.; Sudol, M.; Kaspar, H.G.; Nash, J.; Carey, D.J.; Gogoi, R. Yes-Associated Protein (YAP) Modulates Oncogenic Features and Radiation Sensitivity in Endometrial Cancer. PLoS ONE 2014, 9, e100974. [Google Scholar] [CrossRef] [PubMed]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Integrin Signaling in Cancer: Bidirectional Mechanisms and Therapeutic Opportunities. Nat. Rev. Cancer 2023, 23, 455–474. [Google Scholar]
- Wu, Y.; Zhou, D.; Xu, H.; Cheng, J.; Tong, X.; Chen, L. Sustained Adrenergic Activation of YAP1 Induces Anoikis Resistance in Cervical Cancer Cells. Cells 2021, 10, 3347. [Google Scholar]
- Amano, Y.; Hata, M.; Kato, K.; Kato, Y.; Nakayama, E.; Saito, Y. The Significance of Phosphorylated Yes-Associated Protein (YAP) (Ser127) Expression in Oral Squamous Cell Carcinoma. Anticancer Res 2025, 45, 3835–3845. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, W.H.; Koo, J.S. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014, 7, 3224–3234. [Google Scholar]
| Variable | Value | |
|---|---|---|
| Age, years (mean [range]) | 52.6 [26–82] | |
| <50 years, n (%) ≥50 years, n (%) | 45 (45.5%) | |
| 54 (54.5%) | ||
| High-risk HPV, n (%) | No | 5 (5.1%) |
| Yes (16 18 31 33 51 52 56 58) | 36 (36.3%) | |
| Non-informative | 58 (58.6%) | |
| High parity (≥3), n (%) | No | 60 (60.6%) |
| Yes | 39 (39.4%) | |
| SCC antigen (mean [range]) | 4.73 [0.5–40.8] | |
| <3.0 ng/mL | 64 (64.6) | |
| ≥3.0 ng/mL | 35 (35.4) | |
| Pap smear | Negative | 2 (2.0%) |
| ASC-US | 16 (16.2%) | |
| LSIL | 2 (2.0%) | |
| HSIL | 32 (32.3%) | |
| SCC | 18 (18.2%) | |
| Non-informative | 29 (29.3%) | |
| FIGO stage, n (%) | IA1 | 5 (5.1%) |
| IA2 | 11 (11.1%) | |
| IB1 | 18 (18.2%) | |
| IB2 | 21 (21.2%) | |
| IB3 | 11 (11.1%) | |
| IIA1 | 10 (10.1%) | |
| IIIA2 | 1 (1.0%) | |
| IIIC1 | 19 (19.2%) | |
| IVB | 2 (2.0%) | |
| Tumor size, n (%) | <2 cm | 32 (32.3%) |
| 2≥, <4 cm | 41 (41.4%) | |
| ≥4 cm | 26 (26.3%) | |
| LVSI, n (%) | Negative | 51 (51.5%) |
| Positive | 27 (27.3%) | |
| Non-informative | 21 (21.2%) | |
| Stromal invasion, n (%) | <1/2 | 58 (58.6%) |
| ≥1/2 | 41 (41.4%) | |
| Surgical approaches, n (%) | Laparotomy | 27 (27.3%) |
| Laparoscopy | 72 (72.7%) | |
| Surgical type, n (%) | Modified radical | 63 (63.6%) |
| Radical | 36 (36.4%) |
| Signal Intensity | YAP | p-YAP | MST1 | LATS1 |
|---|---|---|---|---|
| Negative | 25 (25.3%) | 34 (34.3%) | 15 (15.2%) | 76 (76.8%) |
| Mild | 64 (64.7%) | 34 (34.3%) | 54 (54.5%) | 14 (14.1%) |
| Moderate | 7 (7.1%) | 24 (24.3%) | 26 (26.3%) | 7 (7.1%) |
| Strong | 0 (0%) | 4 (4.0%) | 2 (2.0%) | 1 (1.0%) |
| Non-informative | 3 (3.0%) | 3 (3.0%) | 2 (2.0%) | 1 (1.0%) |
| Clinicopathologic Features | YAP Expression | p-YAP Expression | LATS1 Expression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 25) | Positive (n = 71) | p-Value | Negative (n = 34) | Positive (n = 62) | p-Value | Negative (n = 76) | Positive (n = 22) | p-Value | ||
| Age (years) | <50 | 8 (32.0%) | 36 (50.7%) | 0.161 | 14 (41.2%) | 29 (46.8%) | 0.671 | 37 (48.7%) | 8 (36.4%) | 0.341 |
| ≥50 | 17 (68.0%) | 35 (49.3%) | 20 (58.8%) | 33 (53.2%) | 39 (51.3%) | 14 (63.6%) | ||||
| High-risk HPV | No | 1 (80.0%) | 4 (60.6%) | >0.99 | 1 (70.6%) | 4 (61.3%) | 0.384 | 49 (64.5%) | 14 (63.6%) | >0.99 |
| Yes | 5 (20.0%) | 28 (39.4%) | 10 (29.4%) | 24 (38.7%) | 27 (35.5%) | 8 (36.4%) | ||||
| High SCC antigen (ng/mL) | <3.0 | 15 (60.0%) | 47 (66.2%) | 0.631 | 18 (52.9%) | 43 (69.4%) | 0.125 | 50 (65.8%) | 13 (59.1%) | 0.618 |
| ≥3.0 | 10 (40.0%) | 24 (33.8%) | 16 (47.1%) | 19 (30.6%) | 26 (34.2%) | 9 (40.9%) | ||||
| High parity | <3 | 14 (56.0%) | 44 (62.0%) | 0.640 | 18 (52.9%) | 40 (64.5%) | 0.284 | 47 (61.8%) | 12 (54.5%) | 0.623 |
| ≥3 | 11 (44.0%) | 27 (38.0%) | 16 (47.1%) | 22 (35.5%) | 29 (38.2%) | 10 (45.5%) | ||||
| FIGO | ≤IB1 | 3 (12.0%) | 30 (42.3%) | 0.007 | 0 (0%) | 29 (46.8%) | <0.001 | 23 (30.3%) | 10 (45.5%) | 0.207 |
| ≥1B2 | 22 (88.0%) | 41 (57.7%) | 34 (100%) | 33 (53.2%) | 53 (69.7%) | 12 (54.5%) | ||||
| Stromal invasion > 1/2 | No | 14 (56.0%) | 43 (60.6%) | 0.814 | 15 (44.1%) | 41 (66.1%) | 0.051 | 45 (59.2%) | 12 (54.5%) | 0.807 |
| Yes | 11 (44.0%) | 28 (39.4%) | 19 (55.9%) | 21 (33.9%) | 31 (40.8%) | 10 (45.5%) | ||||
| Tumor size (cm) | <2 | 3 (12.0%) | 28 (39.4%) | 0.013 | 5 (14.7%) | 25 (40.3%) | 0.011 | 23 (30.3%) | 9 (40.9%) | 0.440 |
| ≥2 | 22 (88.0%) | 43 (60.6%) | 29 (85.3%) | 37 (59.7%) | 53 (69.7%) | 13 (59.1%) | ||||
| YAP | p-YAP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | p | Univariable OR (95% CI) | Negative | Positive | p | Univariable OR (95% CI) | ||
| p-YAP | Negative | 15 | 19 | 0.003 | 4.342 (1.695–11.614) | - | |||
| Positive | 10 | 55 | |||||||
| MST1 | Negative | 5 | 10 (77.8%) | 0.520 | 1.6 (0.454–5.085) | 4 | 11 | 0.569 | 0.654 (0.169–2.102) |
| Positive | 20 | 64 (22.2%) | 30 | 54 | |||||
| LAST1 | Negative | 22 | 54 (80.5%) | 0.172 | 2.716 (0.823–12.348) | 28 | 48 | 0.454 | 1.652 (0.607–5.021) |
| Positive | 3 | 20 (19.5%) | 6 | 17 | |||||
| YAP | Negative | - | 15 | 10 | 0.003 | 4.342 (1.695–11.614) | |||
| Positive | 19 | 55 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Yang, J.; Song, D.H.; Kim, C.H.; Shin, J.K.; Choi, W.J.; Baek, J.C. Expression of Core Hippo Pathway Proteins in Cervical Cancer and Their Association with Clinicopathologic Parameters. Medicina 2025, 61, 2134. https://doi.org/10.3390/medicina61122134
Kim MH, Yang J, Song DH, Kim CH, Shin JK, Choi WJ, Baek JC. Expression of Core Hippo Pathway Proteins in Cervical Cancer and Their Association with Clinicopathologic Parameters. Medicina. 2025; 61(12):2134. https://doi.org/10.3390/medicina61122134
Chicago/Turabian StyleKim, Min Hye, Juseok Yang, Dae Hyun Song, Cho Hee Kim, Jeong Kyu Shin, Won Jun Choi, and Jong Chul Baek. 2025. "Expression of Core Hippo Pathway Proteins in Cervical Cancer and Their Association with Clinicopathologic Parameters" Medicina 61, no. 12: 2134. https://doi.org/10.3390/medicina61122134
APA StyleKim, M. H., Yang, J., Song, D. H., Kim, C. H., Shin, J. K., Choi, W. J., & Baek, J. C. (2025). Expression of Core Hippo Pathway Proteins in Cervical Cancer and Their Association with Clinicopathologic Parameters. Medicina, 61(12), 2134. https://doi.org/10.3390/medicina61122134





