Unlocking the Therapeutic Potential of Trigonella foenum-graecum and Trigonella corniculata Against High-Fat-Diet-Induced Hyperlipidemia: Antioxidant and Histopathological Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Procurement
- Inclusion Criteria:
- Exclusion Criteria:
2.2. Phytochemical Analysis
2.2.1. Chemical and Reagents
2.2.2. TPC Analysis
2.2.3. DPPH Radical Scavenging Activity Analysis
2.2.4. FRAP Analysis
2.3. In Vivo Assay
2.3.1. Animal Procurement
2.3.2. Research Design
2.3.3. Efficacy Studies and Housing of Rats
2.3.4. Standard Basal Diet Preparation
2.3.5. Treatment Diet Preparation (Pellet Formation)
2.3.6. Animal Slaughtering Protocols
2.3.7. Ethical Considerations
2.3.8. Weight Estimation
2.3.9. Liver Function Test
2.3.10. Blood Lipid Profiling
2.3.11. Hematological Examination
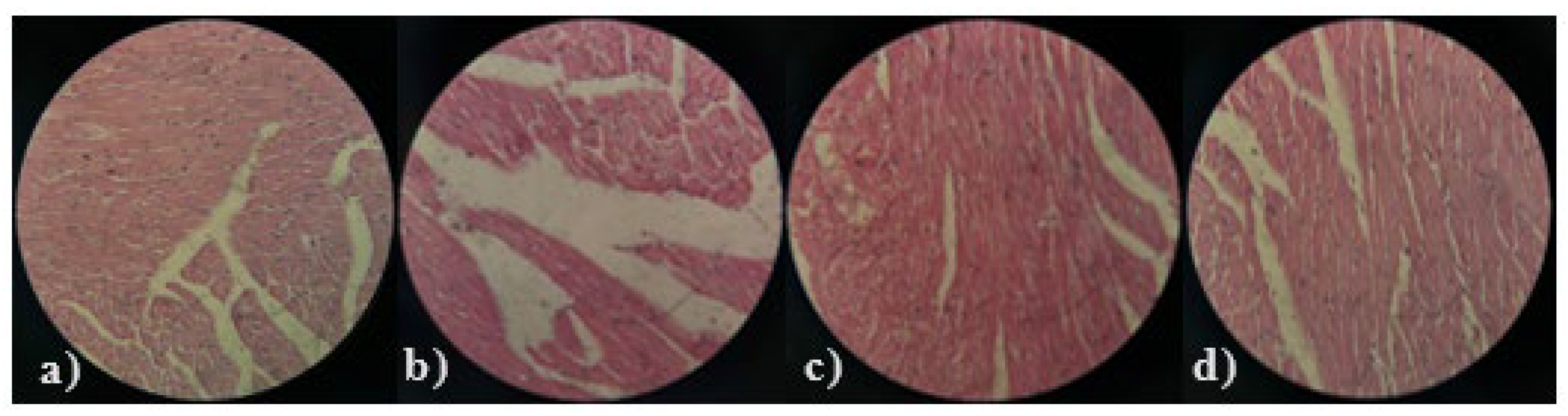
2.3.12. Histopathological Examination
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Xu, Z.; Li, C.; Yu, L.; Zhu, Q.; Li, Z.; Liu, T.; Liu, D.; Mao, C. Association Between Dietary Betaine Intake and Dyslipidemia in Chinese Children and Adolescents: A Cross-Sectional Study. Nutrients 2025, 17, 1742. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, W.; Luo, L.; Xu, C. Dyslipidemia: Causes, symptoms and treatment. Int. J. Trend Sci. Res. Dev. 2021, 5, 1013–1016. [Google Scholar]
- Muhammad, A.A.; Afzaal, M.; Khan, M.J.; Baig, A.M.; Aasim, M. Frequency and pattern of dyslipidemia and its association with other risk factors among Type-2 Diabetics. Pak. J. Med. Sci. 2025, 41, 472. [Google Scholar] [CrossRef] [PubMed]
- Uroosh, S.; Khaliq, A.; Khan, F.S.; Akram, M.; Mumtaz, N.; Kausar, S.; Rani, S. Cardiovascular risk factors in Pakistan: Current trends. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 924–930. [Google Scholar] [CrossRef]
- Hussain, A.; Zakria, M.; Ali, I.; Tariq, S.A.; Hussain, A.; Siraj, S. Pattern of dyslipidemia and associated factors in coronary artery disease patients in Khyber Pakhtunkhwa: A cross-sectional secondary data analysis. Pak. J. Med. Sci. 2023, 39, 1416–1421. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Youssef, A.M.; Al-Sofiani, M.E.; Amin, H.S.; AlOtaibi, O.; Mohamed, N.; Algohani, H.A.; Isnani, A.; Rafiullah, M. Medication adherence and treatment satisfaction with lipid-lowering drugs among patients with diabetes and dyslipidemia. Ann. Pharmacother. 2025, 59, 105–116. [Google Scholar] [CrossRef]
- Cheung, B.; Sikand, G.; Dineen, E.H.; Malik, S.; Barseghian El-Farra, A. Lipid-lowering nutraceuticals for an integrative approach to dyslipidemia. J. Clin. Med. 2023, 12, 3414. [Google Scholar] [CrossRef]
- Kussmann, M.; Cunha, D.H.A.; Berciano, S. Bioactive compounds for human and planetary health. Front. Nutr. 2023, 10, 1193848. [Google Scholar] [CrossRef]
- Wani, P.A. Fenugreek and its bioactive compounds: A comprehensive review on their role in diabetes and other therapeutic applications. J. Pharmacogn. Phytochem. 2025, 14, 490–500. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, K. A Comprehensive Review on Therapeutic Potential of Herbal Plants in Diabetes and Diabetes Associated Problems. J. Dis. Glob. Health 2021, 18, 7–27. [Google Scholar] [CrossRef]
- Parwez, R.; Nabi, A.; Mukarram, M.; Aftab, T.; Khan, M.M.; Naeem, M. Various Mitigation Approaches Applied to Confer Abiotic stress tolerance in Fenugreek (Trigonella foenum-graecum L.): A review. In Fenugreek; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer: Singapore, 2021; pp. 137–185. [Google Scholar]
- Rao, N.R.; Bhuvaneswar, A.V.; Sri, A.B.; Kumar, P.S.; Sravani, K.; Krupanidhi, G.; Chiranjeevi, M.; Baniyan, R.J.; Aditya, N.V. Decoding Fenugreek’s Cholesterol-Lowering Mechanism: A Critical Review of its Therapeutic Potential. Arch. Curr. Res. Int. 2025, 25, 716–730. [Google Scholar] [CrossRef]
- Abd El-Megeid, A.E.; Salleh El Marasy, S.; Ibrahim Mahmoud, S. A comparative study on fenugreek seed and its oil’s in hyperlipidemic rats. J. Home Econ. 2025, 41, 1–30. [Google Scholar] [CrossRef]
- Jahan, I.; Hassan, S.H.; Alimullah, M.; Haque, A.U.; Fakruddin, M.; Subhan, N.; Khan, F.; Ahmed, K.S.; Akramuddaula, K.; Hossain, H.; et al. Evaluation of fenugreek (Trigonella foenum-graecum L.) powder supplementation on metabolic syndrome, oxidative stress and inflammation in high fat diet fed rats. Pharmacol. Res.-Nat. Prod. 2024, 5, 100116. [Google Scholar] [CrossRef]
- Asad, M.; Jabeen, F.; Ayaz, S. Assessment of ameliorative effect of Trigonella foenum-graecum against CuO-NPs induced toxicity in Oreochromis mossambicus. Pak. J. Pharm. Sci. 2021, 34, 387. [Google Scholar] [PubMed]
- Yılmaz, E. Trigonella foenum-graecum L. In Medicinal Plants of Turkey, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 362–374. [Google Scholar]
- Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and therapeutic potentialities. Plants 2022, 11, 1450. [Google Scholar] [CrossRef]
- Kassaee, S.M.; Goodarzi, M.T.; Kassaee, S.N. Ameliorative effect of Trigonella foenum-graecum L. on lipid profile, liver histology and LDL-receptor gene expression in high cholesterol-fed hamsters. Acta Endocrinol. 2021, 17, 7–13. [Google Scholar] [CrossRef]
- Ali, A.M.; ElNour, M.E. Antioxidant activity, total phenolic, flavonoid and tannin contents of callus and seeds extracts of fenugreek (Trigonella foenum-graecum L.). Int. J. Sci. Res. 2014, 3, 1268–1272. [Google Scholar]
- Anupama, G.; Hegde, L.N.; Hegde, N.K.; Devappa, V.; Mastiholi, A.B.; Nishani, S. Effect of nitrogen and spacing levels on physiological and yield parameters of Kasuri methi (Trigonella corniculata L.) var. Pusa Kasuri. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 723–733. [Google Scholar] [CrossRef][Green Version]
- Aslam, M.A.; Ahmed, S.; Saleem, M.; Sardar, R.; Shah, A.A.; Siddiqui, M.H.; Shabbir, Z. Mitigation of chromium-induced phytotoxicity in 28-homobrassinolide treated Trigonella corniculata L. by modulation of oxidative biomarkers and antioxidant system. Ecotoxicol. Environ. Saf. 2023, 263, 115354. [Google Scholar] [CrossRef]
- Pasricha, V.; Gupta, R.K. Nutraceutical potential of methi (Trigonella foenum-graecum L.) and Kasuri methi (Trigonella corniculata L.). J. Pharmacogn. Phytochem. 2014, 3, 47–57. [Google Scholar]
- Akbari, S.; Nour, A.H.; Yunus, R.M. Determination of phenolics and saponins in fenugreek seed extracted via microwave-assisted extraction method at the optimal condition. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022024. [Google Scholar] [CrossRef]
- Chaubey, P.S.; Somani, G.; Kanchan, D.; Sathaye, S.; Varakumar, S.; Singhal, R.S. Evaluation of debittered and germinated fenugreek (Trigonella foenum-graecum L.) seed flour on the chemical characteristics, biological activities, and sensory profile of fortified bread. J. Food Process. Preserv. 2018, 42, e13395. [Google Scholar] [CrossRef]
- Loha, M.; Mulu, A.; Abay, S.M.; Ergete, W.; Geleta, B. Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 5702159. [Google Scholar] [CrossRef] [PubMed]
- Olayode, O.A.; Daniyan, M.O.; Olayiwola, G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J. Tradit. Complement. Med. 2020, 10, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Aguiar, C.; Alsayed, N.; Chibber, Y.S.; ElBadawi, H.; Ezhov, M.; Farnier, M. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis 2023, 383, 117312. [Google Scholar] [CrossRef] [PubMed]
- Shabil, M.; Bushi, G.; Bodige, P.K.; Maradi, P.S.; Patra, B.P.; Padhi, B.K.; Khubchandani, J. Effect of fenugreek on hyperglycemia: A systematic review and meta-analysis. Medicina 2023, 59, 248. [Google Scholar] [CrossRef]
- Zahid, N.; Shaheen, S.; Memon, Z.; Ali, A.; Zafar, U.; Agha, F. Comparative Analysis of Antidyslipidemic Effects of Fenugreek Seed Extract and Standard Pharmacological Therapy in Diet Induced Animal Model of Dyslipidemia: An Experimental Study. J. Pharm. Res. Int. 2020, 32, 143–151. [Google Scholar] [CrossRef]
- Roberts, K.T. The potential of fenugreek (Trigonella foenum-graecum) as a functional food nutraceutical its effects on glycemia lipidemia. J. Med. Food 2011, 14, 1485–1489. [Google Scholar] [CrossRef]
- Sekhar, M.G.; Shanmugam, K.R.; Prasad, K.S.; Chilakala, R. Amelioration of lipid metabolic profiles by trigonelline a bioactive compound of Trigonella foenum-graecum in alcohol induced albino rats. Indian J. Pharm. Educ. Res. 2024, 58, 172–177. [Google Scholar] [CrossRef]
- Radini, I.A.; Hasan, N.; Malik, M.A.; Khan, Z. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol. B Biol. 2018, 183, 154–163. [Google Scholar] [CrossRef]
- Reduan, F.H.; Shaari, R.M.; Sayuti, N.S.A.; Mustapha, N.M.; Abu Bakar, M.Z.; Sithambaram, S.; Hamzah, H. Acute and subacute dermal toxicity of ethanolic extract of Melastoma malabathricum leaves in Sprague-Dawley rats. Toxicol. Res. 2020, 36, 203–210. [Google Scholar] [CrossRef]
- Nagamma, T.; Konuri, A.; Bhat, K.M.; Udupa, P.E.; Nayak, Y. Trigonella foenum-graecum L. seed extract modulates biochemical and histomorphological changes in therapeutic model of high-fat diet-fed ovariectomized rats. 3 Biotech 2023, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, Z.; Hassandokht, M.; Naghavi, M.R.; Rezadoost, H.; Mirjalili, M.H. Fatty acid and nutrient profiles, diosgenin and trigonelline contents, mineral composition, and antioxidant activity of the seed of some Iranian Trigonella L. species. BMC Plant Biol. 2024, 24, 669. [Google Scholar] [CrossRef]
- Verma, P.P.; Siddiqui, S.; Nayyer, M.A.; Singh, S.; Kumar, D.; Padalia, R.C. Exploration of Industrial, Traditional, and Pharmaceutical Applications of Diversity Rich Genus Trigonella: A Comprehensive Review. Curr. Agric. Res. J. 2024, 12, 63–80. [Google Scholar] [CrossRef]
- Fatima, H.; Shahid, M.; Pruitt, C.; Pung, M.A.; Mills, P.J.; Riaz, M.; Ashraf, R. Chemical fingerprinting, antioxidant, and anti-inflammatory potential of hydroethanolic extract of Trigonella foenum-graecum. Antioxidants 2022, 11, 364. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Lohvina, H.; Sándor, M.; Wink, M. Effect of ethanol solvents on total phenolic content and antioxidant properties of seed extracts of fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic composition by HPLC-ESI-MS. Diversity 2021, 14, 7. [Google Scholar] [CrossRef]
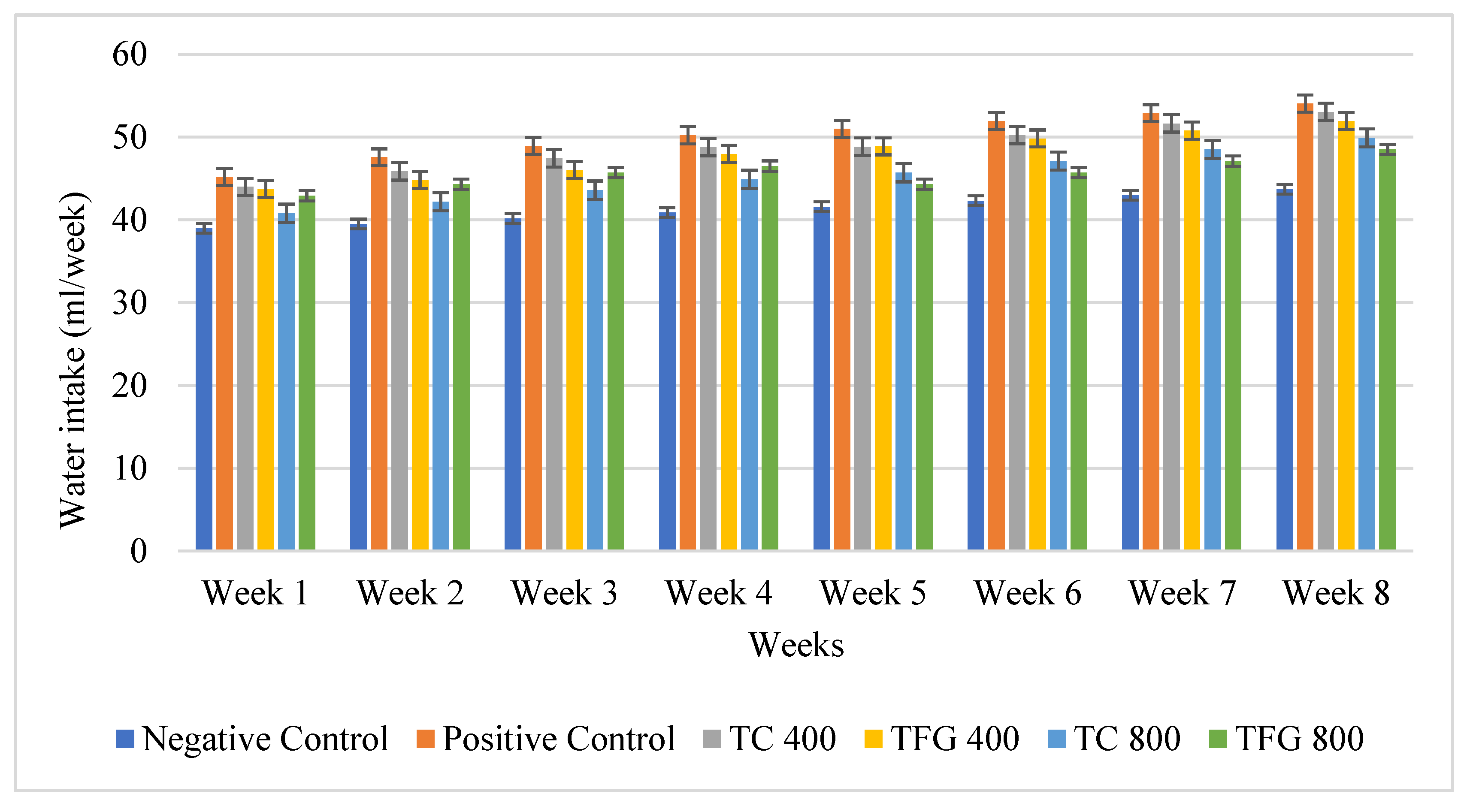
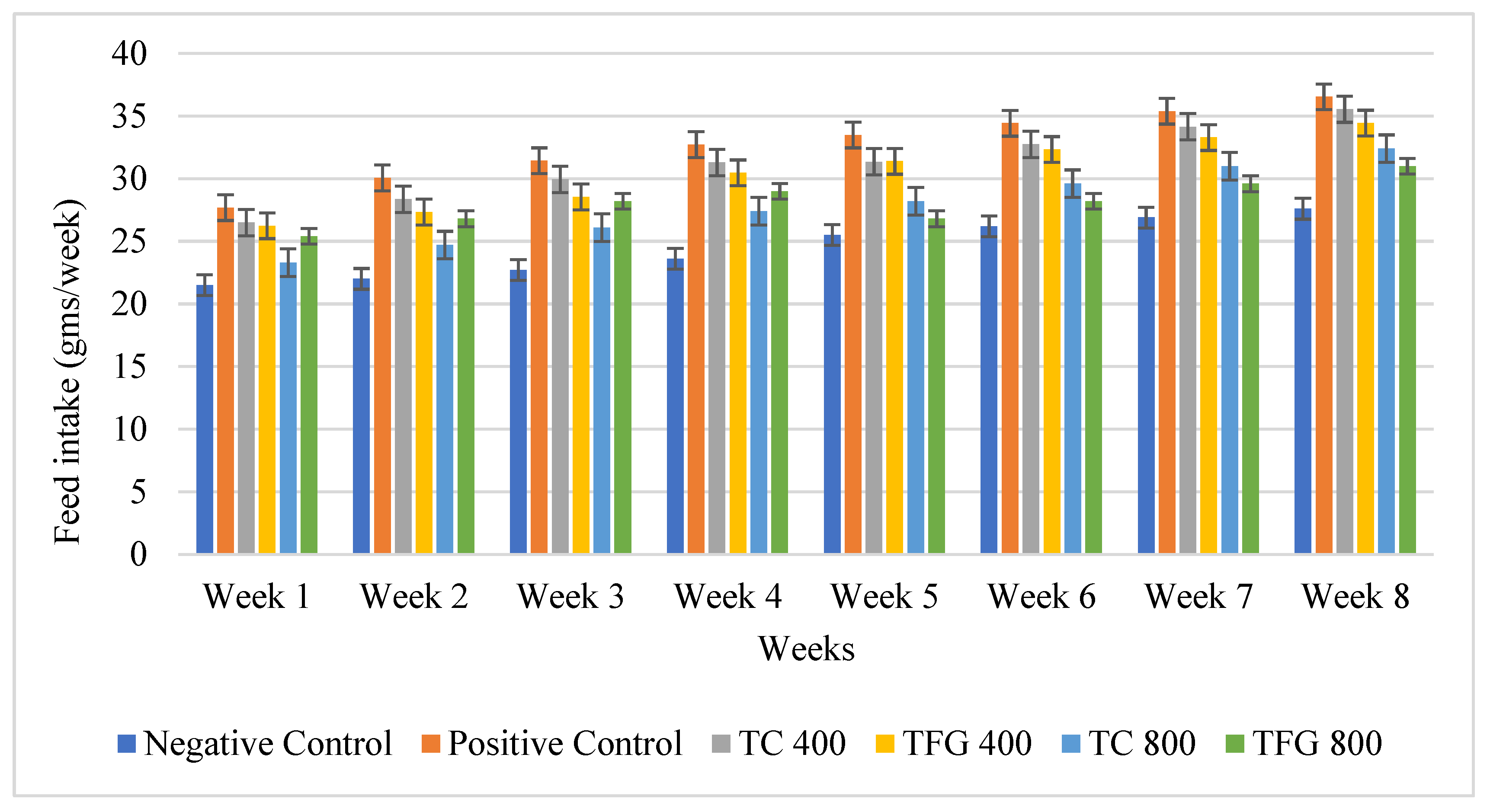
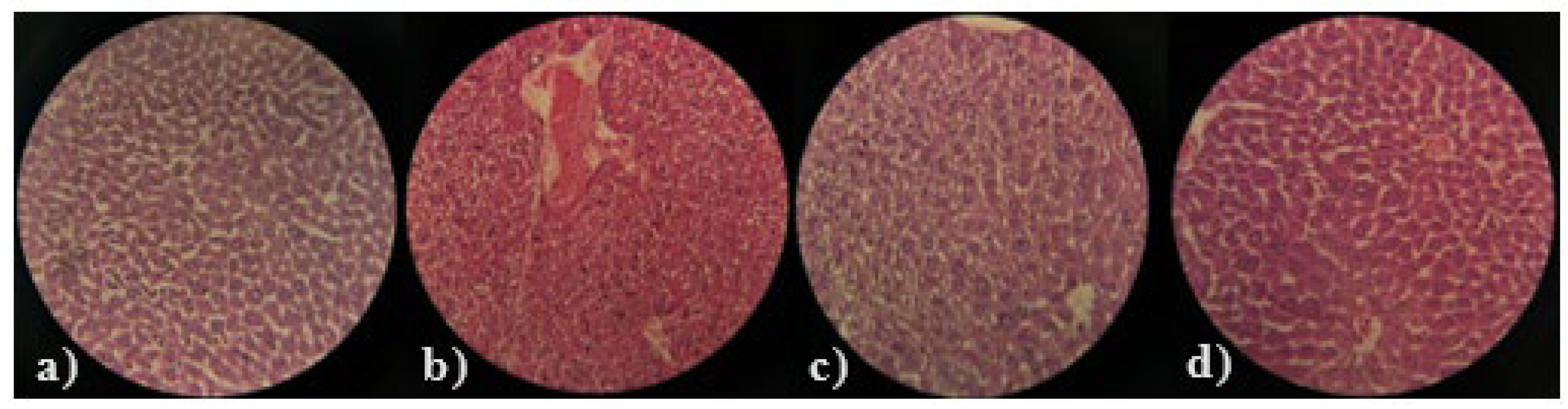
| Groups | Treatments | Dosing Regimen |
|---|---|---|
| G0 | Positive control | Disease + standard basal diet |
| G1 | Negative control | Standard basal diet |
| G2 | TC (400 mg/kg B.W) | SBD supplemented with TC 400 mg/kg/day |
| G3 | TFG (400 mg/kg B.W) | SBD supplemented with TFG 400 mg/kg/day |
| G4 | TC (800 mg/kg B.W) | SBD supplemented with TC 800 mg/kg/day |
| G5 | TFG (800 mg/kg B.W) | SBD supplemented with TFG 800 mg/kg/day |
| Ingredients | Percentage |
|---|---|
| Corn starch | 68% |
| Casein | 11% |
| Wheat Bran | 7% |
| Cellulose | 5% |
| Soybean oil | 4% |
| Mineral Mix | 2% |
| Vitamin Mix | 1% |
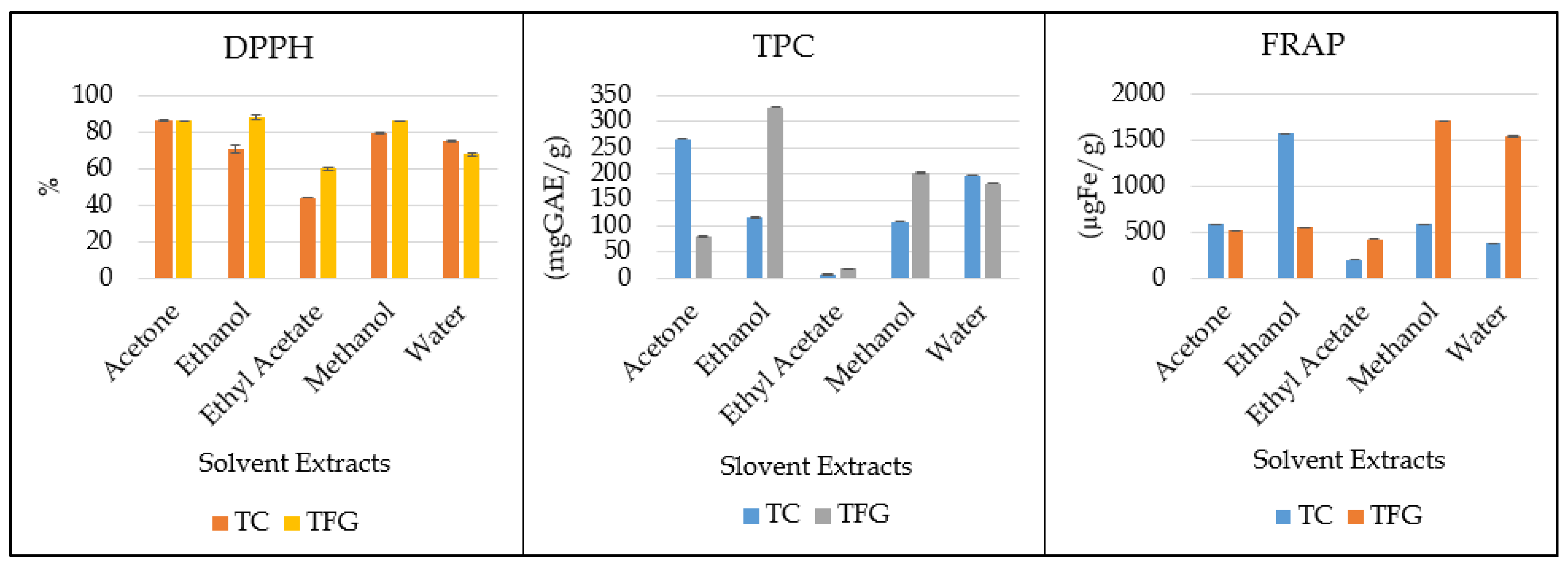
| Plant | Solvent | DPPH (%) | TPC (mgGAE/g) | FRAP (µgFe/g) |
|---|---|---|---|---|
| TC | Acetone | 86.77 ± 0.42 a | 266.52 ± 0.08 b | 584.8 ± 0.30 c |
| Ethanol | 70.96 ± 2.27 cd | 116.73 ± 0.71 f | 1576.5 ± 0.40 b | |
| Ethyl Acetate | 44.13 ± 0.04 f | 6.44 ± 0.19 j | 199.6 ± 1.69 g | |
| Methanol | 79.87 ± 0.53 b | 108.02 ± 0.02 g | 584.7 ± 1.14 c | |
| Water | 75.37 ± 0.55 bc | 196.60 ± 0.12 d | 375.4 ± 1.99 f | |
| TFG | Acetone | 86.37 ± 0.17 a | 80.07 ± 0.82 h | 522.5 ± 0.25 d |
| Ethanol | 88.48 ± 1.44 a | 328.28 ± 0.10 a | 550.0 ± 2.89 d | |
| Ethyl Acetate | 60.16 ± 0.71 e | 17.23 ± 0.48 i | 425.1 ± 0.25 e | |
| Methanol | 86.34 ± 0.05 a | 201.87 ± 0.37 c | 1712.9 ± 1.11 a | |
| Water | 68.04 ± 0.97 d | 181.78 ± 0.08 e | 1549.0 ± 1.58 b |
| Parameters | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
| Body Weight (g) | 37.10 ± 0.53 a | 30.30 ± 0.36 d | 33.67 ± 0.87 b | 32.20 ± 0.2 c | 32.20 ± 0.36 c | 31.07 ± 0.60 d |
| Liver (g) | 1.475 ± 0.03 a | 1.39 ± 0.02 b | 1.38 ± 0.04 bc | 1.36 ± 0.06 c | 1.33 ± 0.05 d | 1.31 ± 0.01 d |
| Heart (g) | 0.19 ± 0.01 a | 0.17 ± 0.03 b | 0.17 ± 0.01 bc | 0.17 ± 0.01 c | 0.17 ± 0.01 d | 0.16 ± 0.02 d |
| Right Kidney (g) | 0.14 ± 0.04 a | 0.12 ± 0.02 c | 0.12 ± 0.04 cd | 0.11 ± 0.05 de | 0.11 ± 0.03 ef | 0.11 ± 0.01 f |
| Left Kidney (g) | 0.15 ± 0.01 a | 0.13 ± 0.02 b | 0.13 ± 0.02 bc | 0.13 ± 0.01 cd | 0.13 ± 0.01 de | 0.13 ± 0.01 e |
| Spleen (g) | 0.11 ± 0.00 a | 0.11 ± 0.02 b | 0.10 ± 0.02 bc | 0.10 ± 0.02 cd | 0.10 ± 0.03 de | 0.09 ± 0.06 e |
| Lungs (g) | 0.41 ± 0.04 a | 0.38 ± 0.07 b | 0.37 ± 0.01 bc | 0.37 ± 0.30 cd | 0.36 ± 0.03 de | 0.36 ± 0.02 e |
| Lipid Test Parameters | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
| TG (mg/dL) | 85.67 ± 3.51 a | 46.00 ± 1.53 e | 71.0 ± 1.0 b | 68.00 ± 1.0 c | 64.67 ± 0.58 d | 62.00 ± 1.0 d |
| TC-c (mg/dL) | 143.0 ± 2.42 a | 112.00 ± 0.75 d | 129.00 ± 0.71 b | 126.67 ± 0.68 b | 122.00 ± 0.56 c | 122.00 ± 1.92 c |
| HDL-c (mg/dL) | 16.23 ± 0.57 e | 27.51 ± 0.9 a | 18.24 ± 0.91 d | 19.87 ± 0.79 c | 21.59 ± 0.41 b | 22.69 ± 0.58 b |
| LDL-c (mg/dL) | 105.96 ± 0.32 a | 72.66 ± 0.64 e | 93.46 ± 0.54 b | 89.98 ± 0.98 c | 85.44 ± 1.08 d | 83.86 ± 0.80 d |
| VLDL-c (mg/dL) | 17.13 ± 0.702 a | 9.20 ± 0.31 e | 14.20 ± 0.2 b | 13.60 ± 0.2 c | 12.93 ± 0.12 d | 12.40 ± 0.2 d |
| Liver Blood Test Parameters | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
| Bilirubin (mg/dL) | 0.42 ± 0.01 a | 0.22 ± 0.01 f | 0.35 ± 0.010 c | 0.31 ± 0.01 d | 0.27 ± 0.010 e | 0.23 ± 0.010 f |
| ALT (IU/L) | 194.0 ± 2.0 a | 167.0 ± 3.79 e | 189.0 ± 1.0 b | 183.67 ± 1.53 c | 178.33 ± 3.06 d | 176.33 ± 2.08 d |
| AST (IU/L) | 279.3 ± 5.03 a | 184.0 ± 5.3 e | 229.67 ± 4.7 b | 216.0 ± 4.58 c | 195.33 ± 5.03 d | 188.0 ± 4.0 de |
| ALP (IU/L) | 191.0 ± 4.58 a | 139.0 ± 2.0 e | 178.33 ± 2.1 b | 173.0 ± 2.0 b | 157.33 ± 2.52 c | 148.6 ± 2.52 d |
| Hematological Parameters | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
|
WBC
(103/μL) | 11.65 ± 0.19 a | 7.13 ± 0.12 g | 10.68 ± 0.36 c | 9.910 ± 0.21 d | 9.35 ± 0.2 e | 8.51 ± 0.31 f |
|
Lymphocytes
(103/μL) | 7.40 ± 0.1 a | 5.06 ± 0.15 f | 7.13 ± 0.12 b | 6.83 ± 0.06 c | 6.43 ± 0.08 d | 6.10 ± 0.10 e |
|
Neutrophils
(103/μL) | 2.34 ± 0.2 a | 1.34 ± 0.08 d | 2.08 ± 0.10 b | 1.88 ± 0.1 bc | 1.71 ± 0.04 c | 1.48 ± 0.17 d |
|
MXD
(103/μL) | 1.91 ± 0.15 a | 0.92 ± 0.06 d | 1.46 ± 0.16 b | 1.19 ± 0.07 c | 1.23 ± 0.12 c | 0.92 ± 0.05 de |
|
Platelets
(103/μL) | 698.3 ± 5.86 g | 1062.0 ± 6.08 a | 782.3 ± 12.7 e | 809.0 ± 10.39 d | 878.0 ± 2.0 c | 960.7 ± 4.16 b |
| Parameters | G0 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
| RBC (106/µL) | 7.103 ± 0.1 g | 10.89 ± 0.21 a | 8.55 ± 0.21 f | 8.98 ± 0.11 e | 9.45 ± 0.21 d | 10.08 ± 0.23 c |
| Hb (g/dL) | 12.97 ± 0.12 g | 14.96 ± 0.12 a | 13.53 ± 0.15 e | 13.86 ± 0.16 d | 14.27 ± 0.15 c | 14.63 ± 0.15 b |
| HCT (%) | 33.93 ± 0.69 f | 46.73 ± 0.26 a | 41.63 ± 0.06 d | 41.83 ± 0.12 d | 43.33 ± 0.55 c | 44.62 ± 0.56 b |
| MCV (fl) | 49.38 ± 0.08 e | 50.92 ± 0.53 ab | 49.76 ± 0.09 de | 49.89 ± 0.04 cde | 49.99 ± 0.06 cd | 50.34 ± 0.29 bc |
| MCH (pg) | 12.64 ± 0.1 e | 17.13 ± 0.74 a | 13.44 ± 0.08 d | 14.20 ± 0.01 c | 14.81 ± 0.04 c | 15.58 ± 0.52 b |
| MCHC (g/dL) | 31.70 ± 0.53 f | 35.01 ± 0.25 a | 32.86 ± 0.32 d | 33.36 ± 0.20 cd | 33.80 ± 0.17 bc | 34.18 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, R.; Afzal, K.; Abbas, A.; Sultan, M.T.; Iqbal, T.B.; Malik, A.; Siddiqi, N.J.; Ola, M.S.; Alamri, A.A.; Abiodum, A.O.; et al. Unlocking the Therapeutic Potential of Trigonella foenum-graecum and Trigonella corniculata Against High-Fat-Diet-Induced Hyperlipidemia: Antioxidant and Histopathological Evidence. Medicina 2025, 61, 2130. https://doi.org/10.3390/medicina61122130
Shamim R, Afzal K, Abbas A, Sultan MT, Iqbal TB, Malik A, Siddiqi NJ, Ola MS, Alamri AA, Abiodum AO, et al. Unlocking the Therapeutic Potential of Trigonella foenum-graecum and Trigonella corniculata Against High-Fat-Diet-Induced Hyperlipidemia: Antioxidant and Histopathological Evidence. Medicina. 2025; 61(12):2130. https://doi.org/10.3390/medicina61122130
Chicago/Turabian StyleShamim, Rabiya, Khurram Afzal, Asad Abbas, Muhammad Tauseef Sultan, Talha Bin Iqbal, Abdul Malik, Nikhat J. Siddiqi, Mohammad Shamsul Ola, Abdul Aziz Alamri, Abeeb Oyesiji Abiodum, and et al. 2025. "Unlocking the Therapeutic Potential of Trigonella foenum-graecum and Trigonella corniculata Against High-Fat-Diet-Induced Hyperlipidemia: Antioxidant and Histopathological Evidence" Medicina 61, no. 12: 2130. https://doi.org/10.3390/medicina61122130
APA StyleShamim, R., Afzal, K., Abbas, A., Sultan, M. T., Iqbal, T. B., Malik, A., Siddiqi, N. J., Ola, M. S., Alamri, A. A., Abiodum, A. O., & Pandey, B. (2025). Unlocking the Therapeutic Potential of Trigonella foenum-graecum and Trigonella corniculata Against High-Fat-Diet-Induced Hyperlipidemia: Antioxidant and Histopathological Evidence. Medicina, 61(12), 2130. https://doi.org/10.3390/medicina61122130







