Reciprocal Interactions Between Human GV-Oocytes and Cumulus Cells: Effects on GVBD, ROS Production, and AMPK Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Standards
2.2. Sample Collection After Follicle Puncture
- The cortical layer was separated from the medulla.
- Visible follicles within the cortical layer were mechanically disrupted.
- The large ovarian fragment was dissected into smaller pieces prior to freezing.
2.3. Isolation of Cumulus Cells and GV-Oocytes
2.4. In Vitro Maturation of GV-Oocytes
2.5. In Vitro Culture and Morphological Observation of Cumulus Cells
2.6. Measurement of Intracellular ROS Levels
2.7. Immunocytochemical Staining of Cumulus Cells
3. Results
3.1. GVBD Occurrence Under Different Culture Conditions
3.2. Morphology and Growth Status of Cumulus Cells Cultured Under Different Culture Conditions
3.3. Intracellular ROS Levels in Cumulus Cells Cultured Under Different Culture Conditions
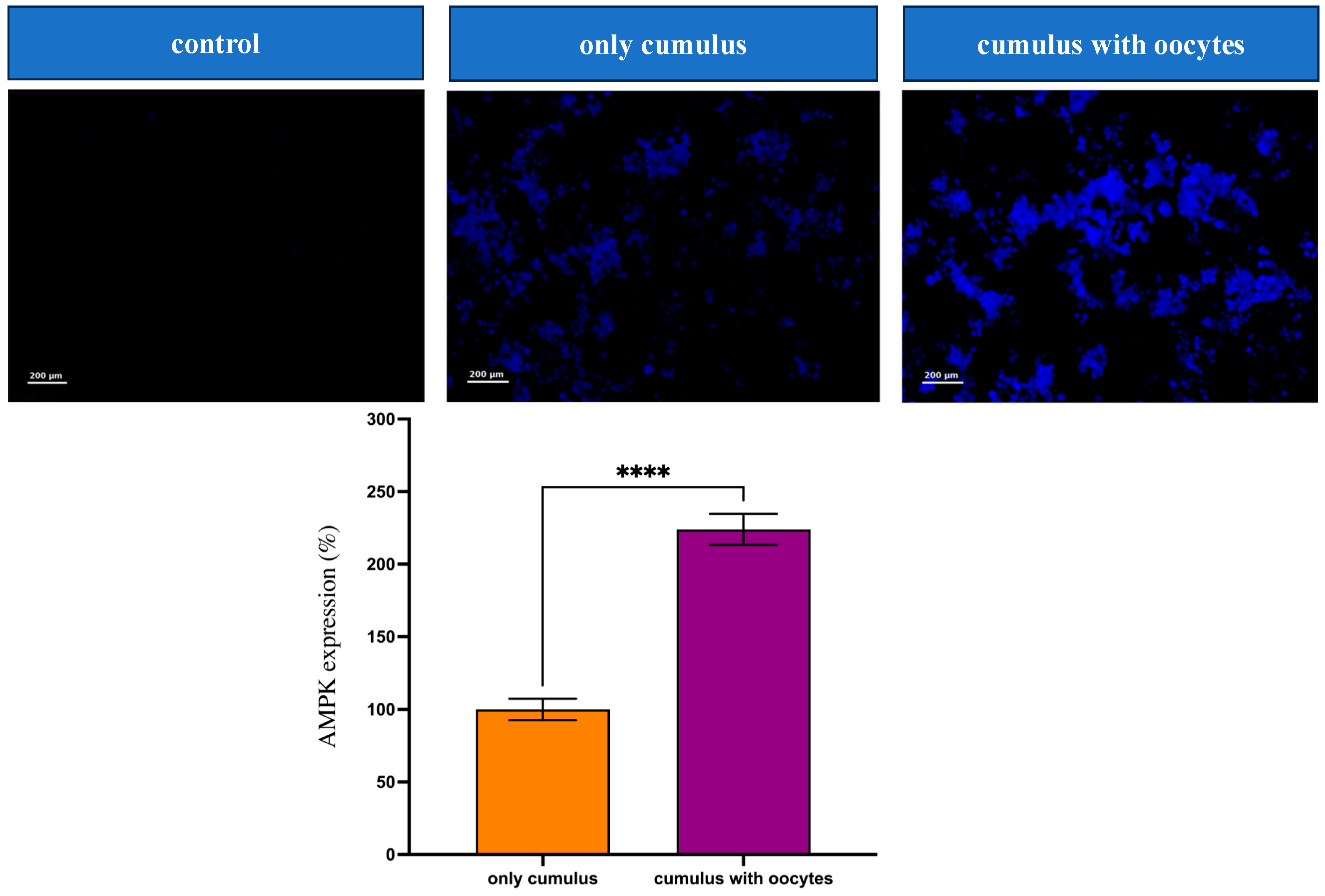
3.4. Effect of Oocytes on AMPK Protein Expression in Cumulus Cells
4. Discussion
4.1. Key Findings and Mechanistic Insights
4.2. Clinical Implications
4.3. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, M.; Son, W.Y. In vitro maturation (IVM) of human immature oocytes: Is it still relevant? Reprod. Biol. Endocrinol. 2023, 21, 110. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. Maturation of oocytes in vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Kotlyar, A.M.; Seifer, D.B. Women with PCOS who undergo IVF: A comprehensive review of therapeutic strategies for successful outcomes. Reprod. Biol. Endocrinol. 2023, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Collée, J.; Mawet, M.; Tebache, L.; Nisolle, M.; Brichant, G. Polycystic ovarian syndrome and infertility: Overview and insights of the putative treatments. Gynecol. Endocrinol. 2021, 37, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Torres-de la Roche, L.A.; Kahlert, U.D.; Isachenko, V.; Huang, H.; Hennefründ, J.; Yan, X.; Chen, Q.; Shi, W.; Li, Y. Artificial ovary for young female breast cancer patients. Front. Med. 2022, 9, 837022. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Todorov, P.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Isachenko, V. Construction and cryopreservation of an artificial ovary in cancer patients as an element of cancer therapy and a promising approach to fertility restoration. Hum. Fertil. 2022, 25, 651–661. [Google Scholar] [CrossRef]
- Salama, M.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Isachenko, V. Emergency fertility preservation in a young woman with non-Hodgkin lymphoma. Oncol. Williston Park. 2021, 35, 332–334. [Google Scholar]
- Shani, A.K.; Haham, L.M.; Balakier, H.; Kuznyetsova, I.; Bashar, S.; Day, E.N.; Librach, C.L. The developmental potential of mature oocytes derived from rescue in vitro maturation. Fertil. Steril. 2023, 120, 860–869. [Google Scholar] [CrossRef]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Wang, W.; Todorov, P.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Wang, M.; Isachenko, V. In vitro activation of cryopreserved ovarian tissue: A single-arm meta-analysis and systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 258–264. [Google Scholar] [CrossRef]
- Campen, K.A.; Abbott, C.R.; Rispoli, L.A.; Payton, R.R.; Saxton, A.M.; Edwards, J.L. Heat stress impairs gap junction communication and cumulus function of bovine oocytes. J. Reprod. Dev. 2018, 64, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Combelles, C.M.H.; Albertini, D.F. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol. Reprod. 2003, 68, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, B.F.; Braga, D.P.A.F.; Setti, A.S.; Iaconelli, A., Jr.; Borges, E.J. Effect of GnRH analogues for pituitary suppression on oocyte morphology in repeated ovarian stimulation cycles. JBRA Assist. Reprod. 2020, 24, 24–29. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, C.; Downs, S.M. Meiotic induction by heat stress in mouse oocytes: Involvement of AMP-activated protein kinase and MAPK family members. Biol. Reprod. 2007, 76, 476–485. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Ramé, C.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS ONE 2015, 10, e0119680. [Google Scholar] [CrossRef]
- LaRosa, C.; Downs, S.M. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol. Reprod. 2006, 74, 585–592. [Google Scholar] [CrossRef]
- Morimoto, A.; Rose, R.D.; Smith, K.M.; Dinh, D.T.; Umehara, T.; Winstanley, Y.E.; Shibahara, H.; Russell, D.L.; Robker, R.L. Granulosa cell metabolism at ovulation correlates with oocyte competence and is disrupted by obesity and aging. Hum. Reprod. 2024, 39, 2053–2066. [Google Scholar] [CrossRef]
- von Mengden, L.; Klamt, F.; Smitz, J. Redox biology of human cumulus cells: Basic concepts, impact on oocyte quality, and potential clinical use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef]
- Buratini, J.; Dellaqua, T.T.; Dal Canto, M.; La Marca, A.; Carone, D.; Mignini Renzini, M.; Webb, R. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: From fertility prognosis to mechanisms underlying age-related subfertility. Hum. Reprod. Update 2022, 28, 232–254. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Jiang, B.; Liang, P.; Liu, M.; Deng, G.; Xiao, X. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones 2010, 15, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Strand, K.R.; Sun, C.; Li, T.; Jenney, F.E., Jr.; Schut, G.J.; Adams, M.W.W. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch. Microbiol. 2010, 192, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jiao, X.; Simpson, J.L.; Chen, Z.J. Genetics of primary ovarian insufficiency: New developments and opportunities. Hum. Reprod. Update 2015, 21, 787–808. [Google Scholar] [CrossRef] [PubMed]
- Kedem, A.; Fisch, B.; Garor, R.; Ben-Zaken, A.; Gizunterman, T.; Felz, C.; Ben-Haroush, A.; Kravarusic, D.; Abir, R. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J. Clin. Endocrinol. Metab. 2011, 96, E1246–E1254. [Google Scholar] [CrossRef]
- Belli, M.; Shimasaki, S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar]
- Liu, M.N.; Zhang, K.; Xu, T.M. The role of BMP15 and GDF9 in the pathogenesis of primary ovarian insufficiency. Hum. Fertil. 2021, 24, 325–332. [Google Scholar] [CrossRef]
- Khan, D.R.; Guillemette, C.; Sirard, M.A.; Richard, F.J. Transcriptomic analysis of cyclic AMP response in bovine cumulus cells. Physiol. Genom. 2015, 47, 432–442. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Yang, C.Y.; Swelum, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Abdo, M.; Shang, J.-H.; Lu, Y.-Q. Molecular, functional, and cellular alterations of oocytes and cumulus cells induced by heat stress and shock in animals. Environ. Sci. Pollut. Res. Int. 2020, 27, 38472–38490. [Google Scholar] [CrossRef]
- Babayev, E.; Duncan, F.E. Age-associated changes in cumulus cells and follicular fluid: The local oocyte microenvironment as a determinant of gamete quality. Biol. Reprod. 2022, 106, 351–365. [Google Scholar] [CrossRef]
- Di Giacomo, M.; Camaioni, A.; Klinger, F.G.; Bonfiglio, R.; Salustri, A. Cyclic AMP-elevating agents promote cumulus cell survival and hyaluronan matrix stability, thereby prolonging the time of mouse oocyte fertilizability. J. Biol. Chem. 2016, 291, 3821–3836. [Google Scholar] [CrossRef]
- Zhao, Y.; Namei, E.; Yang, B.; Bao, X.; Sun, W.; Subudeng, G.; Cao, G.; Li, H.; Wang, G. Cyclic AMP mediates ovine cumulus–oocyte gap junctional function via balancing connexin 43 expression and phosphorylation. Endocr. Connect. 2023, 12, e230337. [Google Scholar] [CrossRef]
- Del Bianco, D.; Gentile, R.; Sallicandro, L.; Biagini, A.; Quellari, P.T.; Gliozheni, E.; Sabbatini, P.; Ragonese, F.; Malvasi, A.; D’amato, A.; et al. Electro-metabolic coupling of cumulus–oocyte complex. Int. J. Mol. Sci. 2024, 25, 5349. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
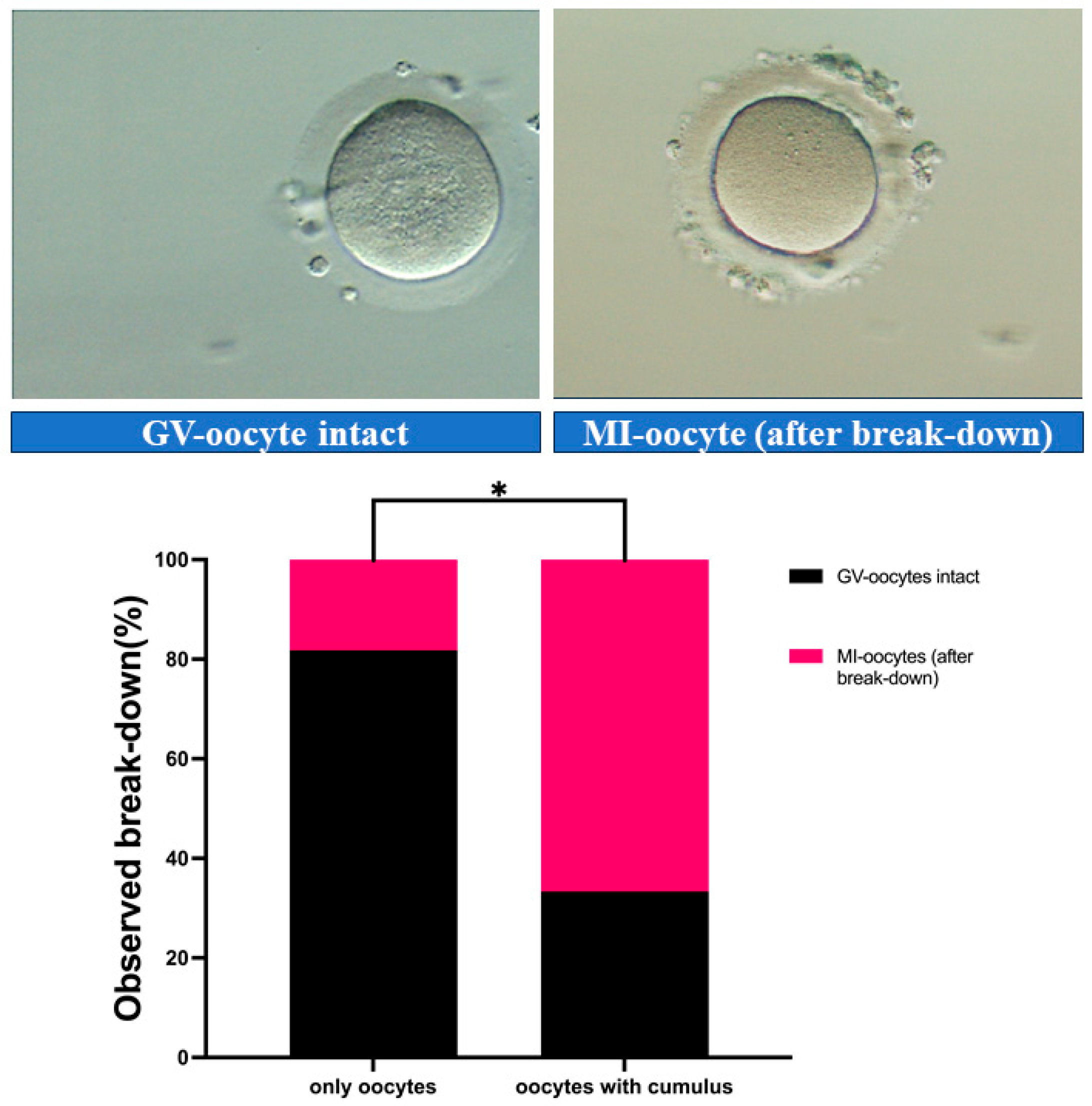


| Group | GV-Intact (n, %) | GVBD (n, %) | Total (n) | p-Value |
|---|---|---|---|---|
| Oocytes-CCs | 9 (81.82%) | 2 (18.18%) | 11 | |
| Oocytes + CCs | 4 (33.33%) | 8 (66.67%) | 12 | 0.036 (*) |
| Total | 13 | 10 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, Z.; Todorov, P.; Rahimi, G.; Skala, C.; Isachenko, V. Reciprocal Interactions Between Human GV-Oocytes and Cumulus Cells: Effects on GVBD, ROS Production, and AMPK Expression. Medicina 2025, 61, 2107. https://doi.org/10.3390/medicina61122107
Ban Z, Todorov P, Rahimi G, Skala C, Isachenko V. Reciprocal Interactions Between Human GV-Oocytes and Cumulus Cells: Effects on GVBD, ROS Production, and AMPK Expression. Medicina. 2025; 61(12):2107. https://doi.org/10.3390/medicina61122107
Chicago/Turabian StyleBan, Zhaoqiao, Plamen Todorov, Gohar Rahimi, Christine Skala, and Volodimir Isachenko. 2025. "Reciprocal Interactions Between Human GV-Oocytes and Cumulus Cells: Effects on GVBD, ROS Production, and AMPK Expression" Medicina 61, no. 12: 2107. https://doi.org/10.3390/medicina61122107
APA StyleBan, Z., Todorov, P., Rahimi, G., Skala, C., & Isachenko, V. (2025). Reciprocal Interactions Between Human GV-Oocytes and Cumulus Cells: Effects on GVBD, ROS Production, and AMPK Expression. Medicina, 61(12), 2107. https://doi.org/10.3390/medicina61122107