Clinical Pathways in Managing High-Caries Children with Adverse Dental Histories
Abstract
1. Introduction
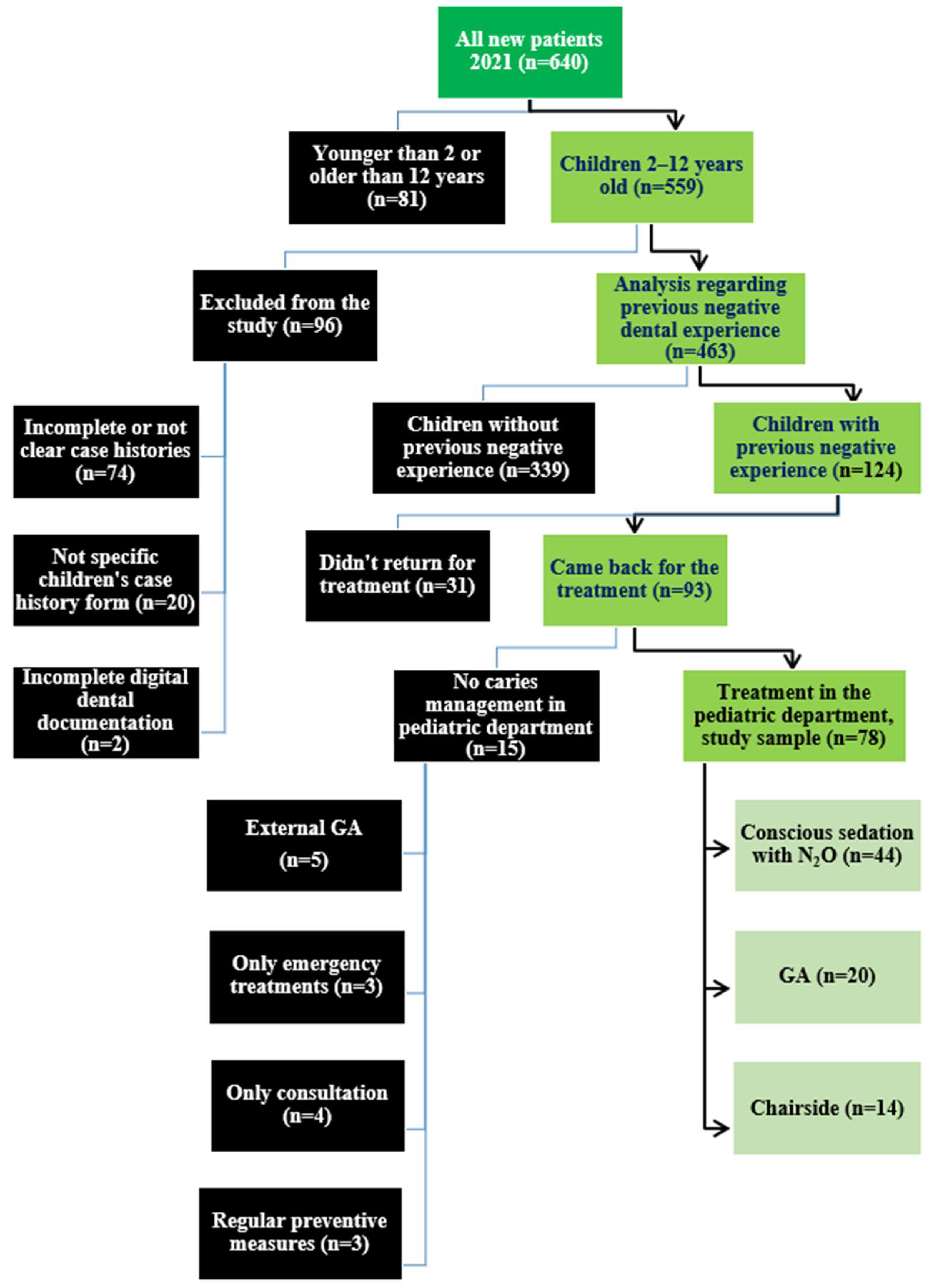
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Sample Size
2.3. Patient Selection
2.3.1. Inclusion Criteria
- First visit to the department of Pediatric Dentistry in 2021 (January–December);
- Child-specific case history fully completed and signed by parents/legal guardians;
- Age between 2 and 12 years (as dental GA is covered by German health insurance until the age of 12 years);
- Reported history of negative dental experience;
- Dental treatment needs (dt/DT > 0);
- Completion of dental treatment within a maximum of 18 months.
2.3.2. Exclusion Criteria
- Incomplete or unsigned case histories;
- Absence of complete documentation in patient’s digital records;
- Absence of child-specific case histories;
- No further appointments after the first session;
- Emergency treatment needs only.
2.4. Initial Visit Protocol in the Department of Pediatric Dentistry of the Dental Clinic of the University Medicine Greifswald
2.5. Data Collection
2.6. Data Analysis
3. Results
3.1. Characteristics of the Study Sample
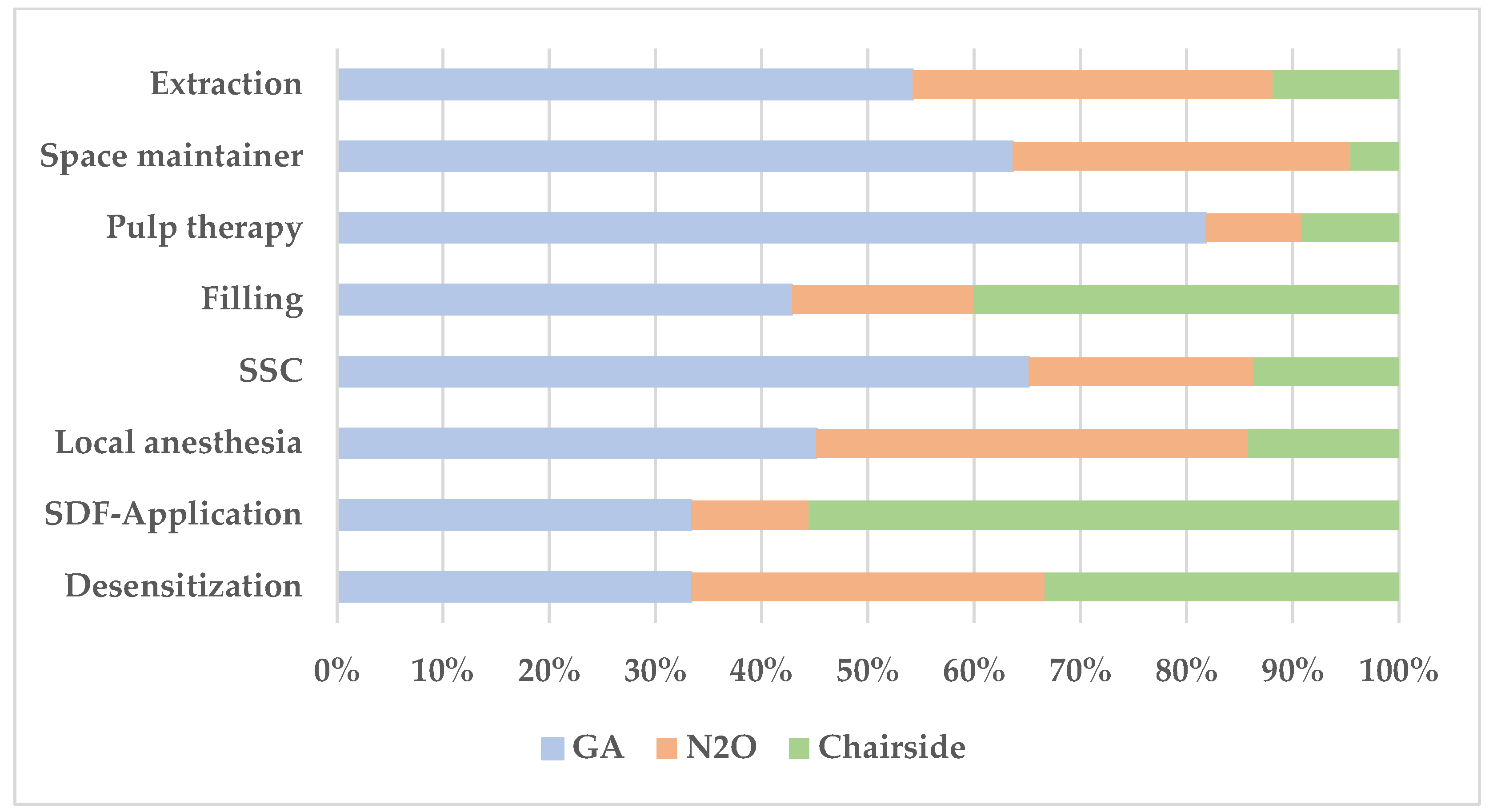
3.2. Performed Dental Treatment for the Study Sample
3.3. Predictors for Dental Treatment Under General Anesthesia
4. Discussion
- The results encourage clinicians to exhaust non-pharmacological and behavioral interventions as well as minimally invasive caries management before proceeding to GA.
- Referral to GA should be made more selectively by identifying behavioral, parental, or contextual factors that predict successful chairside treatment.
- Specialist care may be able to reduce unnecessary GA use in pediatric dentistry.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Atraumatic restorative treatment |
| BMT | Behavior management techniques |
| DFA | Dental fear and anxiety |
| DMFT | Decayed, missing, filled teeth (permanent dentition) |
| DT | Decayed teeth (permanent dentition) |
| dmft | Decayed, missing, filled teeth (primary dentition) |
| dt | Decayed teeth (primary dentition) |
| ECC | Early childhood caries |
| GA | General anesthesia |
| LA | Local anesthesia |
| N2O | Nitrous oxide (inhalation sedation) |
| NRCC | Non-restorative caries control |
| OHRQoL | Oral health-related quality of life |
| SaC index | Specific affected caries index |
| SiC index | Significant caries index |
| SDF | Silver diamine fluoride |
| SM | Space maintainer |
| SSC | Stainless steel crown |
| WHO | World Health Organization |
References
- Schmoeckel, J.; Santamaría, R.M.; Basner, R.; Schankath, E.; Splieth, C.H. Mundgesundheitstrends im Kindesalter: Ergebnisse aus den epidemiologischen Begleituntersuchungen zur Gruppenprophylaxe in Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2021, 64, 772–781. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moure-Leite, F.R.; Ramos-Jorge, J.; Ramos-Jorge, M.L.; Paiva, S.M.; Vale, M.P.; Pordeus, I.A. Impact of dental pain on daily living of five-year-old Brazilian preschool children: Prevalence and associated factors. Eur. Arch. Paediatr. Dent. 2011, 12, 293–297. [Google Scholar] [CrossRef]
- Leal, S.C.; Bronkhorst, E.M.; Fan, M.; Frencken, J.E. Untreated cavitated dentine lesions: Impact on children’s quality of life. Caries Res. 2012, 46, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jorge, J.; Pordeus, I.A.; Ramos-Jorge, M.L.; Marques, L.S.; Paiva, S.M. Impact of untreated dental caries on quality of life of preschool children: Different stages and activity. Community Dent. Oral Epidemiol. 2014, 42, 311–322. [Google Scholar] [CrossRef]
- Benzian, H.; Monse, B.; Heinrich-Weltzien, R.; Hobdell, M.; Mulder, J.; van Palenstein Helderman, W. Untreated severe dental decay: A neglected determinant of low Body Mass Index in 12-year-old Filipino children. BMC Public Health 2011, 11, 558. [Google Scholar] [CrossRef]
- Agaku, I.T.; Olutola, B.G.; Adisa, A.O.; Obadan, E.M.; Vardavas, C.I. Association between unmet dental needs and school absenteeism because of illness or injury among U.S. school children and adolescents aged 6-17 years, 2011-2012. Prev. Med. 2015, 72, 83–88. [Google Scholar] [CrossRef]
- Naidu, R.S.; Boodoo, D.; Percival, T.; Newton, J.T. Dental emergencies presenting to a university-based paediatric dentistry clinic in the West Indies. Int. J. Paediatr. Dent. 2005, 15, 177–184. [Google Scholar] [CrossRef]
- Nalliah, R.P.; Allareddy, V.; Elangovan, S.; Karimbux, N.; Allareddy, V. Hospital based emergency department visits attributed to dental caries in the United States in 2006. J. Evid. Based Dent. Pract. 2010, 10, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Alshoraim, M.A.; El-Housseiny, A.A.; Farsi, N.M.; Felemban, O.M.; Alamoudi, N.M.; Alandejani, A.A. Effects of child characteristics and dental history on dental fear: Cross-sectional study. BMC Oral Health 2018, 18, 33. [Google Scholar] [CrossRef]
- Majstorović, M.; Skrinjarić, I.; Glavina, D.; Szirovicza, L. Factors predicting a child’s dental fear. Coll. Antropol. 2001, 25, 493–500. [Google Scholar]
- Lee, C.-Y.; Chang, Y.-Y.; Huang, S.-T. The clinically related predictors of dental fear in Taiwanese children. Int. J. Paediatr. Dent. 2008, 18, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Dahlander, A.; Soares, F.; Grindefjord, M.; Dahllöf, G. Factors Associated with Dental Fear and Anxiety in Children Aged 7 to 9 Years. Dent. J. 2019, 7, 68. [Google Scholar] [CrossRef]
- Paryab, M.; Hosseinbor, M. Dental anxiety and behavioral problems: A study of prevalence and related factors among a group of Iranian children aged 6-12. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 82–86. [Google Scholar] [CrossRef]
- Shepherd, A.R.; Ali, H. A Care Pathway for Children Unable to Accept Dental Care Within the General Dental Services Involving the Use of Inhalation Sedation and General Anaesthesia. Prim. Dent. J. 2015, 4, 29–34. [Google Scholar] [CrossRef]
- Isong, I.; Dantas, L.; Gerard, M.; Kuhlthau, K. Oral Health Disparities and Unmet Dental Needs among Preschool Children in Chelsea, MA: Exploring Mechanisms, Defining Solutions. J. Oral Hyg. Health 2014, 2, 1000138. [Google Scholar] [CrossRef]
- Tickle, M.; Milsom, K.M.; Humphris, G.M.; Blinkhorn, A.S. Parental attitudes to the care of the carious primary dentition. Br. Dent. J. 2003, 195, 451–455, discussion 449. [Google Scholar] [CrossRef]
- Mueller, M.; Schorle, S.; Vach, K.; Hartmann, A.; Zeeck, A.; Schlueter, N. Relationship between dental experiences, oral hygiene education and self-reported oral hygiene behaviour. PLoS ONE 2022, 17, e0264306. [Google Scholar] [CrossRef] [PubMed]
- Bernabé, E.; Lie, S.A.; Mastrovito, B.; Sannevik, J.; Astrom, A.N. Childhood negative dental experiences and tooth loss in later life: A 25-year longitudinal study in Sweden. J. Dent. 2019, 89, 103198. [Google Scholar] [CrossRef] [PubMed]
- Kumar Verma, R.; Sindgi, R.; Gavarraju, D.N.; Lakshmi Manasa, P.; Bakkuri, P.K.; Dubey, A.; Ravula, S.R. Effectiveness of Different Behavior Management Techniques in Pediatric Dentistry. J. Pharm. Bioallied Sci. 2024, 16, S2434–S2436. [Google Scholar] [CrossRef]
- Hegde, D.; Suprabha, B.S.; Rao, A. Silver modified atraumatic restorative treatment: A paradigm shift in dental caries management. Prim. Dent. J. 2024, 13, 29–35. [Google Scholar] [CrossRef]
- Shahin, M.; Schmoeckel, J.; Splieth, C.; Mourad, M.S. Cross-sectional Analysis of Emergency Dental Pediatric Patients and Proposal of Guidance for Clinical Management. J. Int. Soc. Prev. Community Dent. 2025, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-154864-9. [Google Scholar]
- Klingberg, G.; Andersson-Wenckert, I.; Grindefjord, M.; Lundin, S.-A.; Ridell, K.; Tsilingaridis, G.; Ullbro, C. Specialist paediatric dentistry in Sweden 2008—A 25-year perspective. Int. J. Paediatr. Dent. 2010, 20, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.A.; Newton, T.; Milgrom, P. Who is referred for sedation for dentistry and why? Br. Dent. J. 2009, 206, E12, discussion 322–323. [Google Scholar] [CrossRef]
- Carson, P.; Freeman, R. Dental caries, age and anxiety: Factors influencing sedation choice for children attending for emergency dental care. Community Dent. Oral Epidemiol. 2001, 29, 30–36. [Google Scholar] [CrossRef]
- Mourad, M.S.; Splieth, C.H.; Al Masri, A.; Schmoeckel, J. Potential for nitrous oxide sedation in pedodontics practice to reduce the need for dental general anesthesia. Quintessence Int. 2022, 53, 598–606. [Google Scholar] [CrossRef]
- Xia, B.; Qin, M.; Ma, W.-L.; Liu, H.; Wang, J.-H.; Liu, K.-Y.; Liu, R.-C.; Yang, X.-D.; Ge, L.-H. A retrospective study of 693 children’s dental treatment under general anesthesia. Beijing Da Xue Xue Bao Yi Xue Ban 2013, 45, 984–988. [Google Scholar] [PubMed]
- Patel, S.; Fantauzzi, A.J.; Patel, R.; Buscemi, J.; Lee, H.H. Childhood caries and dental surgery under general anesthesia: An overview of a global disease and its impact on anesthesiology. Int. Anesthesiol. Clin. 2023, 61, 21–25. [Google Scholar] [CrossRef]
- König, T.; Reicherts, P.; Leha, A.; Hrasky, V.; Wiegand, A. Retrospective study on risk factors for repeated dental treatment of children under general anaesthesia. Eur. J. Paediatr. Dent. 2020, 21, 183–186. [Google Scholar] [CrossRef]
- Abolfotouh, M.; Alhumaidan, G.; Almalki, B.; Alhasson, A.; Bushnak, I.; Adlan, A. Predictors of dental general anesthesia receipt among children attending a tertiary hospital in Saudi Arabia. Ibnosina J. Med. Biomed. Sci. 2020, 12, 288–294. [Google Scholar] [CrossRef]
- Shahnavaz, S.; Hedman, E.; Grindefjord, M.; Reuterskiöld, L.; Dahllöf, G. Cognitive Behavioral Therapy for Children with Dental Anxiety: A Randomized Controlled Trial. JDR Clin. Trans. Res. 2016, 1, 234–243. [Google Scholar] [CrossRef]
- Armfield, J.M.; Heaton, L.J. Management of fear and anxiety in the dental clinic: A review. Aust. Dent. J. 2013, 58, 390–407, quiz 531. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A. Dental Behaviour Management Problems Among Children and Adolescents—A Matter of Understanding? Ph.D Dissertation, University of Gothenburg, Gothenburg, Sweden, 2010. [Google Scholar]
- ten Berge, M.; Veerkamp, J.S.; Hoogstraten, J.; Prins, P.J. Parental beliefs on the origins of child dental fear in The Netherlands. ASDC J. Dent. Child. 2001, 68, 51–54+12. [Google Scholar] [PubMed]
- Schmoeckel, J.; Gorseta, K.; Splieth, C.H.; Juric, H. How to Intervene in the Caries Process: Early Childhood Caries—A Systematic Review. Caries Res. 2020, 54, 102–112. [Google Scholar] [CrossRef]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef]
- Splieth, C.H.; Banerjee, A.; Bottenberg, P.; Breschi, L.; Campus, G.; Ekstrand, K.R.; Giacaman, R.A.; Haak, R.; Hannig, M.; Hickel, R.; et al. How to Intervene in the Caries Process in Children: A Joint ORCA and EFCD Expert Delphi Consensus Statement. Caries Res. 2020, 54, 297–305. [Google Scholar] [CrossRef]
- Elamin, F.; Abdelazeem, N.; Salah, I.; Mirghani, Y.; Wong, F. A randomized clinical trial comparing Hall vs conventional technique in placing preformed metal crowns from Sudan. PLoS ONE 2019, 14, e0217740. [Google Scholar] [CrossRef]
- Innes, N.P.T.; Ricketts, D.; Chong, L.Y.; Keightley, A.J.; Lamont, T.; Santamaria, R.M. Preformed crowns for decayed primary molar teeth. Cochrane Database Syst. Rev. 2015, 2015, CD005512. [Google Scholar] [CrossRef]
- Taani, D.Q. Dental attendance and anxiety among public and private school children in Jordan. Int. Dent. J. 2002, 52, 25–29. [Google Scholar] [CrossRef]
- Akbay Oba, A.; Dülgergil, C.T.; Sönmez, I.S. Prevalence of dental anxiety in 7- to 11-year-old children and its relationship to dental caries. Med. Princ. Pract. 2009, 18, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, A.; Makanz, V.M.; Abergel, C. Dental Treatment in Children under General Anesthesia: A 24-year Retrospective Study. Int. J. Clin. Pediatr. Dent. 2025, 18, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Bin Hussain, N.A.; Galadari, H.; Bin Hussain, A.A.; Alnuaimi, R.S.; Ahmed, M.M. Dental General Anesthesia in Pediatric Dentistry Departments of All Dubai Health Authority Sectors: Solutions to Meet with The Rising Demands. EC Dent. Sci. 2018, 17, 913–920. [Google Scholar]
|
Treatment Setting
n (%) |
Total 78 (100%) |
N2O 44 (56.4%) |
GA 20 (25.6%) |
Chairside 14 (18%) | p Value * | |
|---|---|---|---|---|---|---|
| Age (y) (mean ± SD) | 6.7 ± 2.2 | 7.3 ± 1.7 | 5.13 ± 1.9 | 6.9 ± 2.8 | 0.001 ** | |
|
Gender n (%) | Female | 37 (47.4%) | 20 (45%) | 11 (55%) | 6 (42.9%) | 0.724 |
| Male | 41 (52.6%) | 24 (55%) | 9 (45%) | 8 (57.1%) | ||
|
Known diseases n (%) | No | 69 (88.5%) | 39 (88.6%) | 16 (80%) | 14 (100%) | 0.199 |
| Yes | 9 (11.5%) | 5 (11.4%) | 4 (20%) | 0 | ||
|
Chief complaint n (%) | Caries | 69 (88.5%) | 40 (90.9%) | 18 (90%) | 11 (78.6%) | 0.097 |
| Trauma | 2 (2.6%) | 1 (2.3%) | 0 | 1 (7.1%) | ||
| Orthodontic | 1 (1.3%) | 1 (2.3%) | 0 | 0 | ||
| Checkup | 2 (2.6%) | 0 | 0 | 2 (14.3%) | ||
| Others | 4 (5.1%) | 2 (4.5%) | 2 (10%) | 0 | ||
|
Reason of referral n (%) | No | 30 (38.5%) | 18 (40.9%) | 5 (25%) | 7 (50%) | 0.795 |
| Lack of cooperation | 28 (35.9%) | 14 (31.8%) | 10 (50%) | 4 (28.6%) | ||
| N2O | 10 (12.8%) | 7 (15.9%) | 2 (10%) | 1 (7.1%) | ||
| GA | 7 (8.9%) | 4 (9.1%) | 2 (10%) | 1 (7.1%) | ||
| Others | 3 (3.8%) | 1 (2.3%) | 1 (5%) | 1 (7.1%) | ||
|
Pain history n (%) | No | 25 (32.1%) | 16 (36.4%) | 5 (25%) | 4 (28.6%) | 0.634 |
| Yes | 53 (67.9%) | 28 (63.6%) | 15 (75%) | 10 (71.4%) | ||
|
Parents ready to leave the treatment room n (%) | No | 16 (20.5%) | 8 (18.2%) | 7 (35%) | 1 (7.1%) | 0.304 |
| Yes | 55 (70.5%) | 31 (70.4%) | 12 (60%) | 12 (85.8%) | ||
| No Information | 7 (9%) | 5 (11.4%) | 1 (5%) | 1 (7.1%) | ||
|
Parents ready to come and pay for extra desensitization visits n (%) | No | 2 (2.6%) | 2 (4.6%) | 0 | 0 | 0.246 |
| Yes | 66 (84.6%) | 39 (88.6%) | 15 (75%) | 12 (85.7%) | ||
| No Information | 10 (12.8%) | 3 (6.8%) | 5 (25%) | 2 (14.3%) | ||
|
Treatment Setting
n (%) |
Total 78 (100%) |
N2O 44 (56.4%) |
GA 20 (25.6%) |
Chairside 14 (18%) | p Value * | |
|---|---|---|---|---|---|---|
|
Emergency visit n (%) | Yes | 4 (5.1%) | 3 (6.8%) | 1 (5%) | 0 | 0.602 |
| No | 74 (94.9%) | 41 (93.2%) | 19 (95%) | 14 (100%) | ||
|
Reason of referral n (%) | No | 30 (38.5%) | 18 (40.9%) | 5 (25%) | 7 (50%) | 0.795 |
| Lack of cooperation | 28 (35.9%) | 14 (31.8%) | 10 (50%) | 4 (28.6%) | ||
| N2O | 10 (12.8%) | 7 (15.9%) | 2 (10%) | 1 (7.1%) | ||
| GA | 7 (9%) | 4 (9.1%) | 2 (10%) | 1 (7.1%) | ||
| Others | 3 (3.8%) | 1 (2.3%) | 1 (5%) | 1 (7.1%) | ||
|
Cooperation in first visit: Frankl scale n (%) | 1 | 16 (20.5%) | 5 (11.4%) | 10 (50%) | 1 (7.1%) | 0.016 ** |
| 2 | 7 (8.9%) | 3 (6.8%) | 2 (10%) | 2 (14.3%) | ||
| 3 | 36 (46.2%) | 24 (54.6%) | 6 (30%) | 6 (42.9%) | ||
| 4 | 16 (20.5%) | 10 (22.7%) | 1 (5%) | 5 (35.7%) | ||
| No Information | 3 (3.9%) | 2 (4.5%) | 1 (5%) | 0 | ||
| Baseline dt (mean ± SD) | 3.4 ± 2.7 | 3.3 ± 2.4 | 5.6 ± 2.6 | 1.9 ± 2.4 | 0.000 ** | |
| Baseline dmft (mean ± SD) | 4.5 ± 3 | 4.2 ± 2.8 | 6.2 ± 2.7 | 2.8 ± 2.8 | 0.002 ** | |
| Baseline DT (mean ± SD) | 0.4 ± 1 | 0.4 ± 1.1 | 0.6 ± 1.1 | 0.4 ± 1 | 0.932 | |
| Baseline DMFT (mean ± SD) | 0.6 ± 1.2 | 0.6 ± 1.3 | 0.6 ± 1.1 | 0.6 ± 1 | 0.942 | |
| Pain at first visit, n (%) | No | 49 (62.8%) | 29 (65.9%) | 10 (50%) | 10 (71.4%) | 0.362 |
| Yes | 29 (37.2%) | 15 (34.1%) | 10 (50%) | 4 (28.6%) | ||
| Treatment Profile | N2O (n = 44) | GA (n = 20) | Chairside (n = 14) | |
|---|---|---|---|---|
|
Number of desensitization visits n (%) | 0 | 22 (50%) | 12 (60%) | 9 (64.3%) |
| 1 | 20 (45.5%) | 6 (30%) | 3 (21.4%) | |
| 2 | 2 (4.5%) | 2 (10%) | 2 (14.3%) | |
|
Number of N2O-sessions n (%) | 0 | - | 13 (65%) | 14 (0%) |
| 1 | 20 (45.5%) | 5 (25%) | - | |
| 2 | 15 (34.1%) | 2 (10%) | - | |
| 3 | 6 (13.6%) | - | - | |
| 4 | 2 (4.5%) | - | - | |
| 5 | 1 (2.3%) | - | - | |
|
Number of SDF applications n (%) | 0 | 38 (86.4%) | 16 (80%) | 8 (57.1%) |
| 1 | 6 (13.6%) | 3 (15%) | 5 (35.7%) | |
| 2 | - | - | 1 (7.1%) | |
| 3 | - | 1 (5%) | - | |
|
Number of teeth extracted n (%) | 0 | 5 (11.4%) | - | 11 (78.6%) |
| 1 | 16 (36.4%) | 4 (20%) | 2 (14.3%) | |
| 2 | 9 (20.4%) | 4 (20%) | - | |
| 3 | 8 (18.1%) | 2 (10%) | - | |
| 4 or more | 6 (13.6%) | 10 (50%) | 1 (7.1%) | |
|
Number of injected local anesthesia n (%) | 0 | 4 (9.1%) | - | 10 (71.4%) |
| 1 | 13 (29.5%) | 3 (15%) | 3 (21.4%) | |
| 2 | 11 (25%) | 8 (40%) | - | |
| 3 | 8 (18.2%) | 4 (20%) | - | |
| 4 or more | 8 (18.2%) | 5 (25%) | 1 (7.1%) | |
|
Number of SSC n (%) | 0 | 21 (47.7%) | 1 (5%) | 7 (50%) |
| 1 | 5 (11.4%) | 2 (10%) | 2 (14.3%) | |
| 2 | 8 (18.2%) | 2 (10%) | 5 (35.7%) | |
| 3 | 2 (4.5%) | 1 (5%) | - | |
| 4 or more | 8 (18.2%) | 14 (70%) | - | |
|
Number of pulp therapies n (%) | 0 | 40 (90.9%) | 13 (65%) | 13 (92.9%) |
| 1 | 3 (6.8%) | 1 (5%) | 1 (7.1%) | |
| 2 | 1 (2.3%) | 3 (15%) | - | |
| 3 | - | 1 (5%) | - | |
| 4 | - | 2 (10%) | - | |
|
Number of fillings n (%) | 0 | 27 (61.4%) | 10 (50%) | 6 (42.9%) |
| 1 | 10 (22.7%) | 2 (10%) | 3 (21.4%) | |
| 2 | 6 (13.6%) | 3 (15%) | 2 (14.3%) | |
| 3 | 1 (2.3%) | 2 (10%) | 1 (7.1%) | |
| 4 or more | - | 3 (15%) | 2 (14.3%) | |
| Type of space maintainer (%) | 0 | 23 (52.3%) | 5 (25%) | 13 (92.9%) |
| Removable | 13 (29.5%) | 2 (10%) | - | |
| fixed | 8 (18.2%) | 13 (65%) | 1 (7.1%) | |
| Average treatment time (days) | - | 129 ± 120 | 152 ± 147 | 237 ± 163 |
| Factor |
N2O (n = 44) |
GA
(n = 20) | Chairside (n = 14) | p Value * | |
|---|---|---|---|---|---|
| Age (y) | (mean ± SD) | 7.3 ± 1.7 | 5.1 ± 1.9 | 6.9 ± 2.8 | <0.001 ** |
| dmft | (mean ± SD) | 4.2 ± 2.8 | 6.2 ± 2.7 | 2.8 ± 2.8 | 0.034 ** |
| dt | (mean ± SD) | 3.3 ± 2.4 | 5.6 ± 2.6 | 1.9 ± 2.4 | 0.005 *** |
|
Frankl scale n (%) | 1 | 5 (11.4%) | 10 (50%) | 1 (7.1%) | 0.016 **** |
| 2 | 3 (6.8%) | 2 (10%) | 2 (14.3%) | ||
| 3 | 24 (54.6%) | 6 (30%) | 6 (42.9%) | ||
| 4 | 10 (22.7%) | 1 (5%) | 5 (35.7%) | ||
| No information | 2 (4.5%) | 1 (5%) | - | ||
|
Children with dmft > age n (%) | 12 (27.3%) | 14 (70%) | 2 (14.3%) | 0.007 **** | |
| Logistic Regression | p Value * | OR ** | CI (95%) *** | |
|---|---|---|---|---|
| Age (y) | 0.18 | 0.688 | 0.398–1.188 | |
| dmft | 0.531 | 0.815 | 0.430–1.545 | |
| dt | 0.415 | 1.318 | 0.679–2.557 | |
| Frankl scale | 1 | 0.244 | Ref. | |
| 2 | 0.421 | 0.348 | 0.027–4.538 | |
| 3 | 0.093 | 0.238 | 0.044–1.273 | |
| 4 | 0.080 | 0.102 | 0.008–1.308 | |
|
Children with dmft > age n (%) | 0.582 | 2.056 | 0.158–26.797 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, F.; Al Masri, A.; Splieth, C.H.; Schmoeckel, J. Clinical Pathways in Managing High-Caries Children with Adverse Dental Histories. Medicina 2025, 61, 2105. https://doi.org/10.3390/medicina61122105
Hashemi F, Al Masri A, Splieth CH, Schmoeckel J. Clinical Pathways in Managing High-Caries Children with Adverse Dental Histories. Medicina. 2025; 61(12):2105. https://doi.org/10.3390/medicina61122105
Chicago/Turabian StyleHashemi, Flora, Ahmad Al Masri, Christian H. Splieth, and Julian Schmoeckel. 2025. "Clinical Pathways in Managing High-Caries Children with Adverse Dental Histories" Medicina 61, no. 12: 2105. https://doi.org/10.3390/medicina61122105
APA StyleHashemi, F., Al Masri, A., Splieth, C. H., & Schmoeckel, J. (2025). Clinical Pathways in Managing High-Caries Children with Adverse Dental Histories. Medicina, 61(12), 2105. https://doi.org/10.3390/medicina61122105






