Effects of Various Local Antibacterial Preparations on Bacterial Density in Pharyngeal and Tonsillar Mucosa of Patients with Acute Pharyngitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject and Materials
2.2. Methods
Statistical Methods and Data Analysis
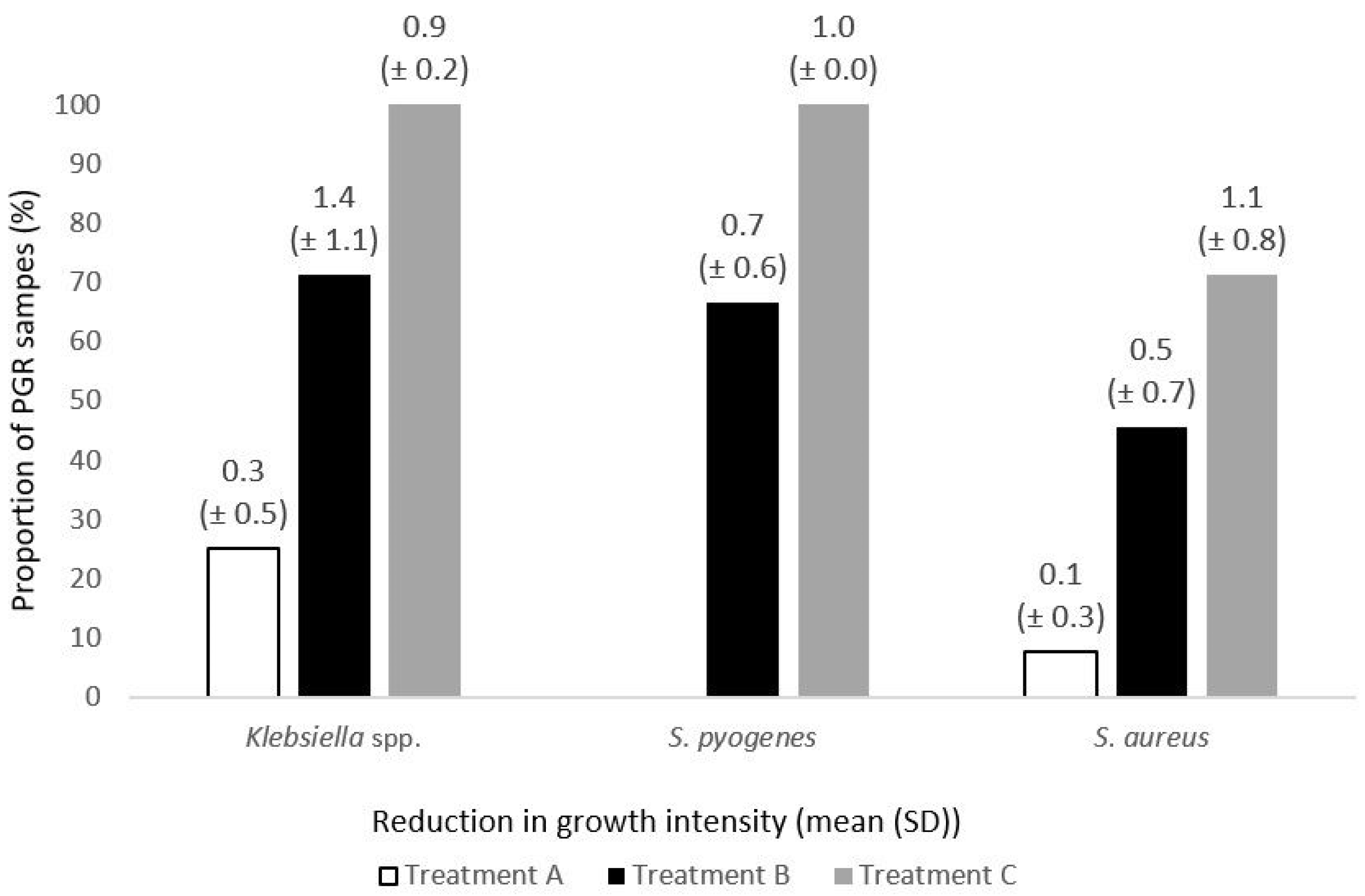
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finley, C.R.; Chan, D.S.; Garrison, S.; Korownyk, C.; Kolber, M.R.; Campbell, S.; Eurich, D.T.; Lindblad, A.J.; Vandermeer, B.; Allan, G.M. What are the most common conditions in primary care? Can. Fam. Physician 2018, 64, 832–840. [Google Scholar]
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. E Clin. Med. 2021, 37, 100986. [Google Scholar] [CrossRef]
- Pfoh, E.; Wessels, M.R.; Goldmann, D.; Lee, G.M. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics 2008, 121, 229–234. [Google Scholar] [CrossRef]
- Calderaro, A.; Buttrini, M.; Farina, B.; Montecchini, S.; De Conto, F.; Chezzi, C. Respiratory tract infections and laboratory diagnostic methods: A review with a focus on syndromic panel-based assays. Microorganisms 2022, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious diseases society of America. Clin. Infect. Diseases 2012, 55, e86–e102. [Google Scholar] [CrossRef] [PubMed]
- NICE Guidance. Rapid Tests for Group a Streptococcal Infections in People with a Sore Throat; NICE: Hoboken, NJ, USA, 2019. [Google Scholar]
- Reinholdt, K.B.; Rusan, M.; Hansen, P.R.; Klug, T.E. Management of sore throat in Danish general practices. BMC Fam. Pract. 2019, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Hendaus, M.A.; Jomha, F.A.; Alhammadi, A.H. Virus-induced secondary bacterial infection: A concise review. Ther. Clin. Risk Manag. 2015, 11, 1265–1271. [Google Scholar] [CrossRef]
- Alcaide, M.L.; Bisko, A.L. Pharyngitis and epiglottitis. Infect. Dis. Clin. N. Am. 2007, 21, 449–469. [Google Scholar] [CrossRef]
- Sykes, E.A.; Wu, V.; Beyea, M.M.; Simpson, M.T.W.; Beyea, J.A. Pharyngitis: Approach to diagnosis and treatment. Can. Fam. Physician 2020, 66, 251–257. [Google Scholar]
- Pichichero, M.E. Treatment and Prevention of Streptococcal Pharyngitis in Adults and Children; UpToDate: Waltham, MA, USA, 2025. [Google Scholar]
- Ray, P.; Singh, S.; Gupta, S. Topical antimicrobial therapy: Current status and challenges. Indian J. Med. Microbiol. 2019, 37, 299–308. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Huang, J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2021, 93, 4370–4373. [Google Scholar] [CrossRef] [PubMed]
- Palm, J.; Fuchs, K.; Stammer, H.; Schumacher-Stimpfl, A.; Milde, J.; DoriPha Investigators. Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: Results of a multi-centre, randomized, placebo-controlled, double-blind, parallel-group trial (DoriPha). Int. J. Clin. Pract. 2018, 72, e13272. [Google Scholar] [CrossRef] [PubMed]
- Wolford, R.W.; Goyal, A.; Belgam Syed, S.Y.; Schaefer, T.J. Pharyngitis. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519550/ (accessed on 10 September 2025).
- Leber, A.L. Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Cox, N.A.; Richardson, L.J.; Cosby, D.E.; Berrang, M.E.; Wilson, J.L.; Harrison, M.A. A four-quadrant sequential streak technique to evaluate Campylobacter selective broths for suppressing background flora in broiler carcass rinses. J. Food Saf. 2017, 37, e12311. [Google Scholar] [CrossRef]
- Manohar, P.; Loh, B.; Athira, S.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary bacterial infections during pulmonary viral disease: Phage therapeutics as alternatives to antibiotics? Front. Microbiol. 2020, 11, 1434. [Google Scholar] [CrossRef]
- González-García, S.; Hamdan-Partida, A.; Bustos-Hamdan, A.; Bustos-Martínez, J. Factors of nasopharynx that favor the colonization and persistence of Staphyloccocus aureus. In Pharynx—Diagnosis and Treatment; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- An, N.; Hai, L.H.L.; Luong, V.H.; Vinh, N.T.H.; Hoa, P.Q.; Hung, L.; Son, N.T.; Hong, L.T.; Hung, D.V.; Kien, H.T.; et al. Antimicrobial resistance patterns of Staphylococcus aureus isolated at a General Hospital in Vietnam between 2014 and 2021. Infect. Drug Resist. 2024, 17, 259–273. [Google Scholar] [CrossRef]
- Gitau, W.; Masika, M.; Musyoki, M.; Museve, B.; Mutwiri, T. Antimicrobial susceptibility pattern of Staphyloccocus aureus isolates from clinical specimens at Kenyatta National Hospital. BMC Res. Notes 2018, 11, 226. [Google Scholar] [CrossRef]
- Li, G.; Zhao, S.; Wang, S.; Sun, Y.; Zhou, Y.; Pan, X. A 7-year surveillance of the drug resistance in Klebsiella pneumoniae from primary health care center. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 34. [Google Scholar] [CrossRef]
- Park, K.-S.; Kim, D.R.; Baek, J.Y.; Shin, A.; Kim, K.-R.; Park, H.; Son, S.; Cho, H.; Kim, Y.-J. Susceptibility to fosfomycin and nitrofurantoin of ESBL-positive Escherichia coli and Klebsiella pneumoniae isolated from urine of pediatric patients. J. Korean Med. Sci. 2023, 48, e361. [Google Scholar] [CrossRef]
- Asri, N.A.M.; Ahmad, S.; Mohamud, R.; Hanafi, N.M.; Zaidi, N.F.M.; Irekeola, A.A.; Shueb, R.H.; Yee, L.C.; Noor, N.M.; Mustafa, F.H.; et al. Global prevalence of nosocomial multidrug-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. Antibiotics 2021, 10, 1508. [Google Scholar] [CrossRef]
- Dequalinium. Available online: https://go.drugbank.com/drugs/DB04209 (accessed on 25 September 2024).
- Raba, G.; Ďurkech, A.; Malík, T.; Bassfeld, D.; Grob, P.; Hurtado-Chong, A.; Botta, S.; Sach, A.; Golańska-Wróblewska, M.; Paškala, M.; et al. Efficacy of dequalinium chloride vs. metronidazole for the treatment of bacterial vaginosis: A randomized clinical trial. JAMA Netw. Open 2024, 7, e248661. [Google Scholar] [CrossRef]
- Schwarz, S.R.; Hirsch, S.; Hiergeist, A.; Kirschneck, C.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Al-Ahmad, A.; Gessner, A.; Buchalla, W.; et al. Limited antimicrobial efficacy of oral care antiseptics in microcosm biofilms and phenotypic adaptation of bacteria upon repeated exposure. Clin. Oral. Investig. 2021, 25, 2939–2950. [Google Scholar] [CrossRef]
- Radkova, E.; Burova, N.; Bychkova, V.; DeVito, R. Efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection: A randomized, non-inferiority trial in the Russian Federation. J. Pain. Res. 2017, 10, 1591–1600. [Google Scholar] [CrossRef]
- Satomura, K.; Kitamura, T.; Kawamura, T.; Shimbo, T.; Watanabe, M.; Kamei, M.; Takano, Y.; Tamakoshi, A. Prevention of upper respiratory tract infections by gargling: A randomized trial. Am. J. Prev. Med. 2005, 29, 302–307. [Google Scholar] [CrossRef]
- Ramalingam, S.; Graham, C.; Dove, J.; Morrice, L.; Sheikh, A. A pilot, open labelled, randomized controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci. Rep. 2019, 9, 1015. [Google Scholar] [CrossRef]
| Growth Intensity | Description |
|---|---|
| (½+) scant growth | 1–5 colonies |
| (1+) small growth | growth only in quadrant 1 * |
| (2+) moderate growth | growth in quadrants 1 and 2 * |
| (3+) heavy growth | growth in quadrants 1, 2, and 3 * |
| (4+) very heavy growth | growth in all 4 quadrants |
| Species | Treatment A | Treatment B | Treatment C | p < 0.05 | |||
|---|---|---|---|---|---|---|---|
| Paired Samples n | PGR n (%) | Paired Samples n | PGR n (%) | Paired Samples n | PGR n (%) | ||
| S. aureus | 13 | 1 (7.7) | 11 | 5 (45.5) | 14 | 10 (71.4) | A/B, A/C |
| Klebsiella spp. | 4 | 1 (25.0) | 7 | 5 (71.4) | 9 | 9 (100.0) | A/C |
| S. pyogenes | 4 | 0 (0.0) | 3 | 2 (66.7) | 3 | 3 (100.0) | - |
| H. influenzae | 0 | - | 1 | 0 (0.0) | 0 | - | - |
| Total | 20 | 2 (10) | 21 | 12 (57.1) | 26 | 22 (84.6) | A/B/C |
| Treatment A | Treatment B | Treatment C | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | (a) | (b) | Species | (a) | (b) | Species | (a) | (b) |
| Klebsiella spp. | 2+ | 2+ | Klebsiella spp. | 4+ | 1+ | Klebsiella spp. | 3+ | 2+ |
| Klebsiella spp. | 2+ | 2+ | Klebsiella spp. | 3+ | 1+ | Klebsiella spp. | 3+ | 2+ |
| Klebsiella spp. | 2+ | 2+ | Klebsiella spp. | 3+ | 1+ | Klebsiella spp. | 3+ | 2+ |
| Klebsiella spp. | 2+ | 1+ | Klebsiella spp. | 1+ | 1+ | Klebsiella spp. | 2+ | 1+ |
| S. pyogenes | 3+ | 3+ | Klebsiella spp. | 2+ | 1+ | Klebsiella spp. | 2+ | 1+ |
| S. pyogenes | 3+ | 3+ | Klebsiella spp. | 2+ | 2+ | Klebsiella spp. | 2+ | 1+ |
| S. pyogenes | 3+ | 3+ | Klebsiella spp. | 3+ | 1+ | Klebsiella spp. | 2+ | 1+ |
| S. pyogenes | 2+ | 2+ | S. pyogenes | 3+ | 2+ | Klebsiella spp. | 2+ | 1+ |
| S. aureus | 3+ | 3+ | S. pyogenes | 2+ | 1+ | Klebsiella spp. | 1+ | 0.5+ |
| S. aureus | 3+ | 3+ | S. pyogenes | 2+ | 2+ | S. pyogenes | 3+ | 2+ |
| S. aureus | 3+ | 3+ | S. aureus | 3+ | 2+ | S. pyogenes | 3+ | 2+ |
| S. aureus | 3+ | 3+ | S. aureus | 3+ | 2+ | S. pyogenes | 2+ | 1+ |
| S. aureus | 3+ | 3+ | S. aureus | 3+ | 2+ | S. aureus | 4+ | 3+ |
| S. aureus | 3+ | 3+ | S. aureus | 3+ | 1+ | S. aureus | 3+ | 2+ |
| S. aureus | 3+ | 3+ | S. aureus | 2+ | 1+ | S. aureus | 3+ | 2+ |
| S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ | S. aureus | 3+ | 1+ |
| S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ | S. aureus | 3+ | 1+ |
| S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ | S. aureus | 3+ | 1+ |
| S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ | S. aureus | 3+ | 1+ |
| S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ |
| S. aureus | 2+ | 1+ | S. aureus | 2+ | 2+ | S. aureus | 2+ | 2+ |
| H. influenzae | 3+ | 3+ | S. aureus | 2+ | 2+ | |||
| S. aureus | 2+ | 1+ | ||||||
| S. aureus | 2+ | 1+ | ||||||
| (a)—pre-exposure growth | S. aureus | 2+ | 1+ | |||||
| (b)—post-exposure growth | S. aureus | 1+ | 1+ | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinis, A.; Dansone, G.; Balode, L.; Gintere, S.; Tolstiks, A.; Verbovenko, K.; Zašibajevs, O.; Safina, T. Effects of Various Local Antibacterial Preparations on Bacterial Density in Pharyngeal and Tonsillar Mucosa of Patients with Acute Pharyngitis. Medicina 2025, 61, 2100. https://doi.org/10.3390/medicina61122100
Reinis A, Dansone G, Balode L, Gintere S, Tolstiks A, Verbovenko K, Zašibajevs O, Safina T. Effects of Various Local Antibacterial Preparations on Bacterial Density in Pharyngeal and Tonsillar Mucosa of Patients with Acute Pharyngitis. Medicina. 2025; 61(12):2100. https://doi.org/10.3390/medicina61122100
Chicago/Turabian StyleReinis, Aigars, Guna Dansone, Līga Balode, Sandra Gintere, Andrejs Tolstiks, Katrīna Verbovenko, Oļegs Zašibajevs, and Taira Safina. 2025. "Effects of Various Local Antibacterial Preparations on Bacterial Density in Pharyngeal and Tonsillar Mucosa of Patients with Acute Pharyngitis" Medicina 61, no. 12: 2100. https://doi.org/10.3390/medicina61122100
APA StyleReinis, A., Dansone, G., Balode, L., Gintere, S., Tolstiks, A., Verbovenko, K., Zašibajevs, O., & Safina, T. (2025). Effects of Various Local Antibacterial Preparations on Bacterial Density in Pharyngeal and Tonsillar Mucosa of Patients with Acute Pharyngitis. Medicina, 61(12), 2100. https://doi.org/10.3390/medicina61122100






