Application of Artificial Intelligence in Vulnerable Carotid Atherosclerotic Plaque Assessment—A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Definitions
2.5. Data Analysis
2.6. Ethics
3. Results
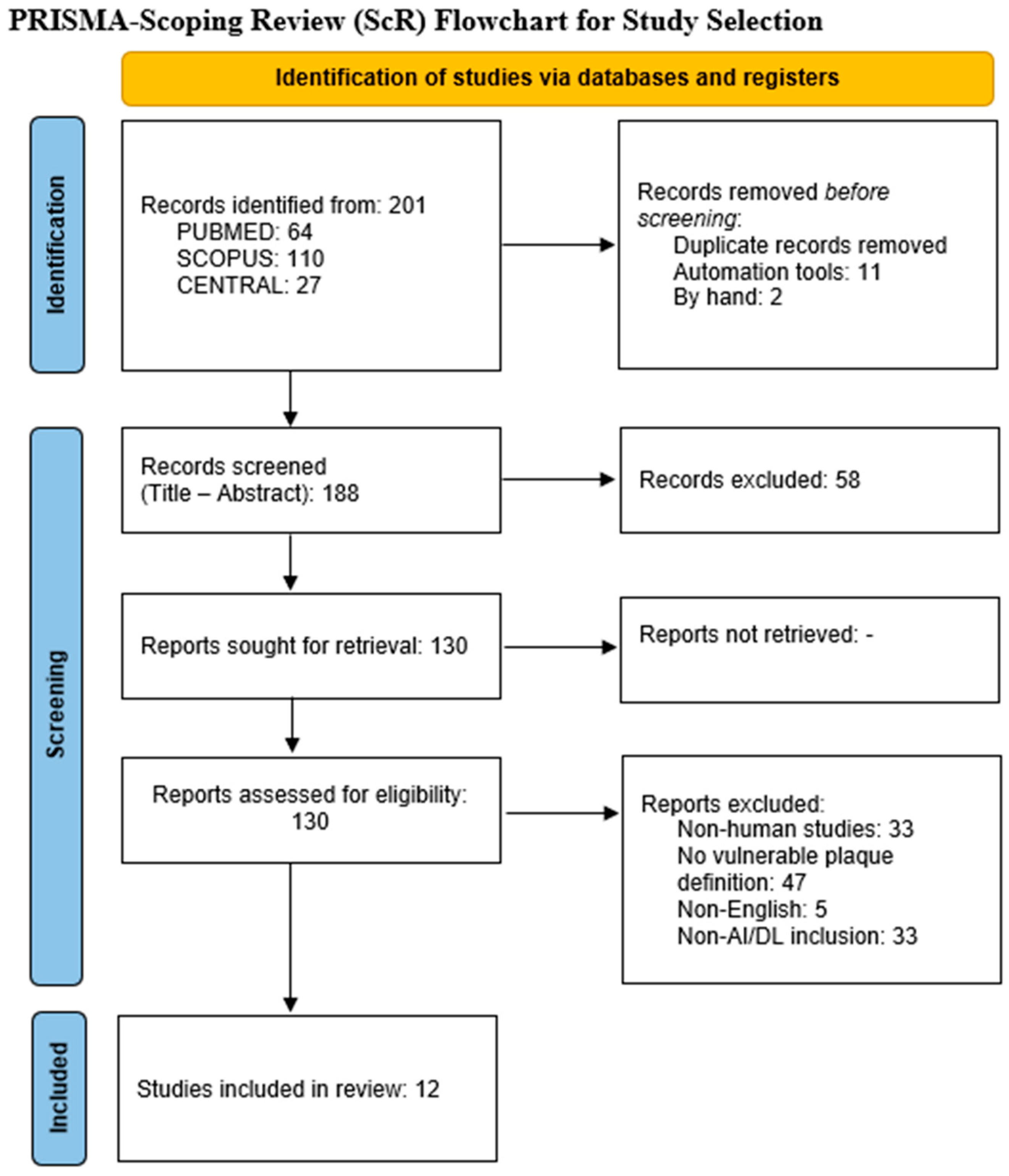
3.1. Study Selection
3.2. Study Population and Modality Breakdown
3.3. Reference Standards and Endpoints
3.4. Modality-Specific Outcomes
3.4.1. Computed Tomography Angiography (CTA)
3.4.2. High-Resolution MRI
3.4.3. Ultrasound and CEUS
3.4.4. Cross-Modality Synthesis and Methodological Signals
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naylor, A.R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C.; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75 (Suppl. 1), 4S–22S. [Google Scholar] [CrossRef]
- Zeebregts, C.J.; Paraskevas, K.I. The new 2023 European Society for Vascular Surgery (ESVS) Carotid Guidelines: Scope and highlights. Eur. J. Vasc. Endovasc. Surg. 2023, 65, e1–e3. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Bosch, J.G.; Staub, D.; Adam, D.; Feinstein, S.B. Contrast-enhanced ultrasound to assess carotid intraplaque neovascularization. Ultrasound Med. Biol. 2020, 46, 466–478. [Google Scholar] [CrossRef]
- Saito, K.; Nagatsuka, K.; Ishibashi-Ueda, H.; Watanabe, A.; Kannki, H.; Iihara, K. Contrast-Enchanced Ultrasound of the Evaluation of Neovascularization in AtheroscleroticCarotid Artery Plaques. Stroke 2014, 45, 3073–3075. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.C.; Saba, L.; Bathla, G.; Brinjikji, W.; Nardi, V.; Lanzino, G. MR imaging of carotid artery atherosclerosis: Updated evidence on High-risk plaque features and emerging trends. AJNR Am. J. Neuroradiol. 2023, 44, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Mu, C.; Yang, L.; Li, H.; Xiang, X.; Li, K.; Yang, Q. Carotid plaque magnetic resonance imaging and recurrent stroke risk: A systematic review and meta-analysis. Medicine 2020, 99, e19377. [Google Scholar] [CrossRef]
- Hou, C.; Xuan, J.Q.; Zhao, L.; Li, M.X.; He, W.; Liu, H. Comparison of the diagnostic performance of Contrast-enanced ultrasound and high-resolution magnetic resonance imaging in the evaluation of histologically defined vulnerable carotid plaque: A systematic review and meta-analysis. Quant. Imaging Med. Surg. 2024, 14, 5814–5830. [Google Scholar] [CrossRef]
- Yuan, J.; Usman, A.; Das, T.; Patterson, A.J.; Gillard, J.H.; Graves, M.J. Imaging carotid atherosclerosis plaque ulceration: Comparison of advanced imaging modalities and recent developmnents. AJNR Am. J. Neuroradiol. 2017, 38, 664–671. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Marincola, B.C.; Piga, M.; Raz, E.; Bassareo, P.P. CT vs ultrasound for ulcerated carotid plaque: MDCTA shows higher sensitivity/specificity. AJNR Am. J. Neuroradiol. 2007, 28, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, X.Y.; Du, Y.; Cheng, L.G.; Liu, L.P.; Nie, F.; Zhang, W.; He, W. Radiomics in carotid plaque: A systematic review and Radiomics Quality Score assessment. Ultrasound Med. Biol. 2023, 49, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized radiomics feature definitions. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image Biomarker Standardisation Initiative (IBSI) reference manual. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine (introducing RQS). Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Pisu, F.; Williamson, B.J.; Nardi, V.; Paraskevas, K.I.; Puig, J.; Vagal, A.; de Rubeis, G.; Porcu, M.; Cau, R.; Benson, J.C.; et al. Machine learning detects symptomatic plaques in patients with carotid atherosclerosis on CT angiography. Circ. Cardiovasc. Imaging 2024, 17, e016274. [Google Scholar] [CrossRef] [PubMed]
- Buckler, A.J.; Gotto, A.M., Jr.; Rajeev, A.; Nicolaou, A.; Sakamoto, A.; St Pierre, S.; Phillips, M.; Virmani, R.; Villines, T.C. Atherosclerosis risk classification with CT angiography: A radiologic–pathologic validation study. Atherosclerosis 2023, 366, 42–48. [Google Scholar] [CrossRef]
- Zhao, T.; Lin, G.; Chen, W.; Wu, J.; Hu, W.; Xu, L.; Chen, Y.; Jing, Y.; Shen, L.; Xia, S.; et al. Predicting symptomatic carotid artery plaques with radiomics-based carotid perivascular adipose tissue characteristics: A multicentre, multiclassifier study. BMC Med. Imaging 2025, 25, 337. [Google Scholar] [CrossRef]
- Zhang, X.; Hua, Z.; Chen, R.; Jiao, Z.; Shan, J.; Li, C.; Li, Z. Identifying vulnerable plaques: A 3D carotid plaque radiomics model based on HRMRI. Front. Neurol. 2023, 14, 1050899. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Ji, A.; Lv, P.; Zhang, J.; Fu, C.; Lin, J. Identification of high-risk carotid plaque with MRI-based radiomics and machine learning. Eur. Radiol. 2021, 31, 3116–3126. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, T.; Wang, Y.N.; Schoepf, U.J.; Bidwell, S.L.; Qiao, H.; Feng, Y.; Xu, C.; Xu, H.; Xie, G.; et al. A coronary CT angiography radiomics model to identify vulnerable plaque and predict cardiovascular events. Radiology 2023, 307, e221693. [Google Scholar] [CrossRef]
- Charalambous, S.; Klontzas, M.E.; Kontopodis, N.; Ioannou, C.V.; Perisinakis, K.; Maris, T.G.; Damilakis, J.; Karantanas, A.; Tsetis, D. Radiomics and machine learning to predict aggressive type II endoleaks after EVAR: A proof of concept. Acta Radiol. 2022, 63, 1282–1291. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, J.; Luo, Y.; Jia, D.; Chen, N.; Yao, C.; Wu, R. Detection of endoleak after EVAR using deep learning on non-contrast CT: Development and validation. Cardiovasc. Interv. Radiol. 2024, 47, 1270–1279. [Google Scholar] [CrossRef]
- Koçak, B.; Durmaz, E.S.; Kaya, O.K.; Erdemir, A.G. Exploring radiomics research quality scoring tools: A comparative analysis of metrics and RQS. Diagn. Interv. Radiol. 2024, 30, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F. Deep learning-based carotid plaque ultrasound image detection and classification. Rev. Cardiovasc. Med. 2024, 25, 454. [Google Scholar] [CrossRef]
- Song, J.; Zou, L.; Li, Y.; Wang, X.; Qiu, J.; Gong, K. Combining AI-assisted segmentation and ultrasound-based radiomics for prediction of carotid plaque stability. BMC Med. Imaging 2025, 25, 89. [Google Scholar] [CrossRef]
- Guang, Y.; He, W.; Ning, B.; Zhang, H.; Yin, C.; Zhao, M.; Nie, F.; Huang, P.; Zhang, R.-F.; Yong, Q.; et al. Deep learning-based carotid plaque vulnerability classification with multicentre CEUS video: A comparative diagnostic study. BMJ Open 2021, 11, e047528. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, Q.; Ding, Y.; Hu, C.; Hui, P. Texture analysis based on vascular ultrasound to identify the vulnerable carotid plaques. Front. Neurol. 2022, 16, 885209. [Google Scholar] [CrossRef]
- Saba, L.; Sanagala, S.S.; Gupta, S.K.; Koppula, V.K.; Johri, A.M.; Sharma, A.M.; Kolluri, R.; Bhatt, D.L.; Nicolaides, A.; Suri, J.S. Ultrasound-based internal carotid artery plaque characterization using deep learning paradigm on a supercomputer. Int. J. Cardiovasc. Imaging 2021, 37, 1511–1528. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Adu, J.; Xie, S.; Li, Z.; Meng, Q.; Zhang, Q.; Yin, L.; Peng, B. Automatic segmentation of ultrasound images of carotid atherosclerotic plaque based on Dense-UNet. Technol. Health Care 2023, 31, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Cilla, S.; Macchia, G.; Lenkowicz, J.; Tran, E.H.; Pierro, A.; Petrella, L.; Fanelli, M.; Sardu, C.; Re, A.; Boldrini, L.; et al. CT angiography-based radiomics as a tool for carotid plaque characterization: A pilot study. Radiol. Med. 2022, 127, 743–753. [Google Scholar] [CrossRef]
- Wang, D.-d.; Lin, S.; Chen, G.-r. Advances in the application of artificial intelligence in the ultrasound diagnosis of vulnerable carotid atherosclerotic plaque. Ultrasound Med. Biol. 2025, 51, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Vacca, S.; Scicolone, R.; Pisu, F.; Cau, R.; Yang, Q.; Annoni, A.; Pontone, G.; Costa, F.; Paraskevas, K.I.; Nicolaides, A.; et al. Radiomics-based machine learning in atherosclerotic carotid arteriosclerosis using ultrasound: Systematic review with meta-analysis. J. Ultrasound 2025, 28, 587–597. [Google Scholar] [CrossRef]
- Alajlouni, Y.; Alnatour, L.; Tarazi, A.; Musleh, A. Artificial intelligence in predicting asymptomatic carotid artery stenosis: A systematic review. In Proceedings of the 11th Congress of the European Academy of Neurology (EAN 2025), Helsinki, Finland, 21–24 June 2025. [Google Scholar]
- Arzani, S.; Soltani, P.; Karimi, A.; Yazdi, M.; Ayoub, A.; Khurshid, Z.; Galderisi, D.; Devlin, H. Detection of carotid artery calcifications using artificial intelligence in dental radiographs: A systematic review and meta-analysis. BMC Med. Imaging 2025, 25, 174. [Google Scholar] [CrossRef]
- Miceli, G.; Rizzo, G.; Basso, M.G.; Cocciola, E.; Pennacchio, A.R.; Pintus, C.; Tuttolomondo, A. Artificial intelligence in symptomatic carotid plaque detection: A narrative review. Appl. Sci. 2023, 13, 4321. [Google Scholar] [CrossRef]
- Biswas, M.; Saba, L.; Omerzu, T.; Johri, A.M. A review on joint carotid intima-media thickness and plaque area measurement in ultrasound for cardiovascular/stroke risk monitoring: Artificial intelligence framework. J. Digit. Imaging 2021, 34, 581–604. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. A simple, step-by-step guide to interpreting decision curve analysis. Diagn. Progn. Res. 2019, 3, 18. [Google Scholar] [CrossRef]
- Heus, P.; Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 16, e078378. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Damen, J.A.A.; Kaul, T.; Hooft, L.; Andaur Navarro, C.L.; Dhiman, P.; van Smeden, M. PROBAST+AI: An updated quality, risk of bias, and applicability assessment tool for prediction models using regression or artificial intelligence methods. BMJ 2025, 388, e082505. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Liu, X.; Denniston, A.K.; Calvert, M.J. Reporting guidelines for clinical trial reportsfor interventions involving artificial intelligence:the CONSORT-AIextension. BMJ 2020, 370, m3164. [Google Scholar] [CrossRef]
| Author | Journal | Publication Year | Study Period | Type of Analysis | External Validation | Number of Participating Centers |
|---|---|---|---|---|---|---|
| Zhang, X. et al. [18] | Front. Neurol. | 2023 | 2021−2022 | Retrospective | No | 1 |
| Zhang, H. et al. [26] | Rev. Cardiovasc. Med. | 2024 | 2020−2022 | Retrospective | No | 4 |
| Pisu, F. et al. [15] | Circ. Cardiovasc. Imaging | 2024 | 2019−2022 | Retrospective | No | 1 |
| Song, J. et al. [27] | BMC Med. Imaging | 2025 | 2018−2023 | Retrospective | No | 3 |
| Guang et al. [28] | BMJ Open | 2021 | 2017−2018 | Prospective | No | 10 |
| Zhang, L. et al. [29] | Front. Neurosci. | 2022 | 2016−2020 | Retrospective | No | 1 |
| Buckler, A.J. et al. [16] | Atherosclerosis | 2023 | NR | Prospective | Yes | 2 |
| Zhao, T. et al. [17] | BMC Med. Imaging | 2025 | 2018−2023 | Retrospective | Yes | 3 |
| Saba, L. et al. [30] | Int. J. Cardiovasc. Imaging | 2021 | NR | Retrospective | No | 1 |
| Zhang, R. et al. [19] | Eur. Radiol. | 2021 | 2015−2019 | Retrospective | No | 1 |
| Deng, C. et al. [31] | Technol. Health Care | 2023 | NR | Retrospective | No | 1 |
| Cilla, S. et al. [32] | Radiol. Med. | 2022 | 2015−2019 | Retrospective | No | 1 |
| Number of Patients | Sex Number of Males (%) | Age | Symptomatic Patients (%) | Imaging Modality | AI Type | |
|---|---|---|---|---|---|---|
| Zhang, X. et al. [18] | 90 | 58 (64.4) | 58.85 ± 10.89 | 48 (53.3) | HR-MRI | radiomics |
| Zhang, H. et al. [26] | 3683 | NR | NR | 0 (0 | US | CNNs |
| Pisu, F. et al. [15] | 268 | 122 (45) | 76 | 12 (44) | CTA | ML |
| Song, J. et al. [27] | 202 | 99 (49) | 56.6 ± 1.9 | 202 (100) | US | ML |
| Guang et al. [28] | 205 | 166 (80.9) | 61.6 ± 8.4 | 67 (32.7) | CEUS video | DL |
| Zhang, L. et al. [29] | 150 | 120 (80) | 61.95 ± 9.9 | 150 (100) | US | ML |
| Buckler, A.J. et al. [16] | 53 | 30 (57) | 59.7 | NR | CTA | DL |
| Zhao, T. et al. [17] | 645 | 311 (48.2) | 70.2 | 232 (35.9) | CTA | radiomics |
| Saba, L. et al. [30] | 346 | 246 (71) | 69.9 ± 7.8 | 196 (56.6) | US | DL + ML |
| Zhang, R. et al. [19] | 162 | 148 (91.4) | 66.8 ± 7.4 | 108 (66.7) | HR-MRI | radiomics |
| Deng, C. et al. [31] | - | - | - | - | US | Dense—Unet |
| Cilla, S. et al. [32] | 30 | 19 (63.3) | 72.96 | 0 (0) | CTA | radiomics |
| AUC% | Accuracy% | Sensitivity% | Specificity% | |
|---|---|---|---|---|
| Zhang, X. et al. [18] | 83.5 | NR | 93.8 | 63.6 |
| Zhang, H. et al. [26] | 91 | 88 | 94 | 71 |
| Pisu, F. et al. [15] | 89 | NR | NR | NR |
| Song, J. et al. [27] | 82.73 | NR | 81.82 | 80 |
| Guang et al. [28] | 87 | NR | 79.2 | 84.4 |
| Zhang, L. et al. [29] | 87 | 79.05 | 85.94 | 68.29 |
| Buckler, A.J. et al. [16] | 95-99 | NR | NR | NR |
| Zhao, T. et al. [17] | NR | NR | NR | NR |
| Saba, L. et al. [30] | 91 | 89.7 | NR | NR |
| Zhang, R. et al. [19] | 98.4 | 90.49 | 81.48 | 100 |
| Deng, C. et al. [31] | - | - | - | - |
| Cilla, S. et al. [32] | 98.7 | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbatis, A.; Dakis, K.; Nana, P.; Kouvelos, G.; Matsagkas, M.; Giannoukas, A.; Spanos, K. Application of Artificial Intelligence in Vulnerable Carotid Atherosclerotic Plaque Assessment—A Scoping Review. Medicina 2025, 61, 2082. https://doi.org/10.3390/medicina61122082
Barbatis A, Dakis K, Nana P, Kouvelos G, Matsagkas M, Giannoukas A, Spanos K. Application of Artificial Intelligence in Vulnerable Carotid Atherosclerotic Plaque Assessment—A Scoping Review. Medicina. 2025; 61(12):2082. https://doi.org/10.3390/medicina61122082
Chicago/Turabian StyleBarbatis, Alexandros, Konstantinos Dakis, Petroula Nana, George Kouvelos, Miltiadis Matsagkas, Athanasios Giannoukas, and Konstantinos Spanos. 2025. "Application of Artificial Intelligence in Vulnerable Carotid Atherosclerotic Plaque Assessment—A Scoping Review" Medicina 61, no. 12: 2082. https://doi.org/10.3390/medicina61122082
APA StyleBarbatis, A., Dakis, K., Nana, P., Kouvelos, G., Matsagkas, M., Giannoukas, A., & Spanos, K. (2025). Application of Artificial Intelligence in Vulnerable Carotid Atherosclerotic Plaque Assessment—A Scoping Review. Medicina, 61(12), 2082. https://doi.org/10.3390/medicina61122082






