Beyond the Score Study: Retrospective Analysis of Single-Graft Kidney Transplant with Karpinski Score 4 Versus Score 5 Grafts
Abstract
1. Introduction
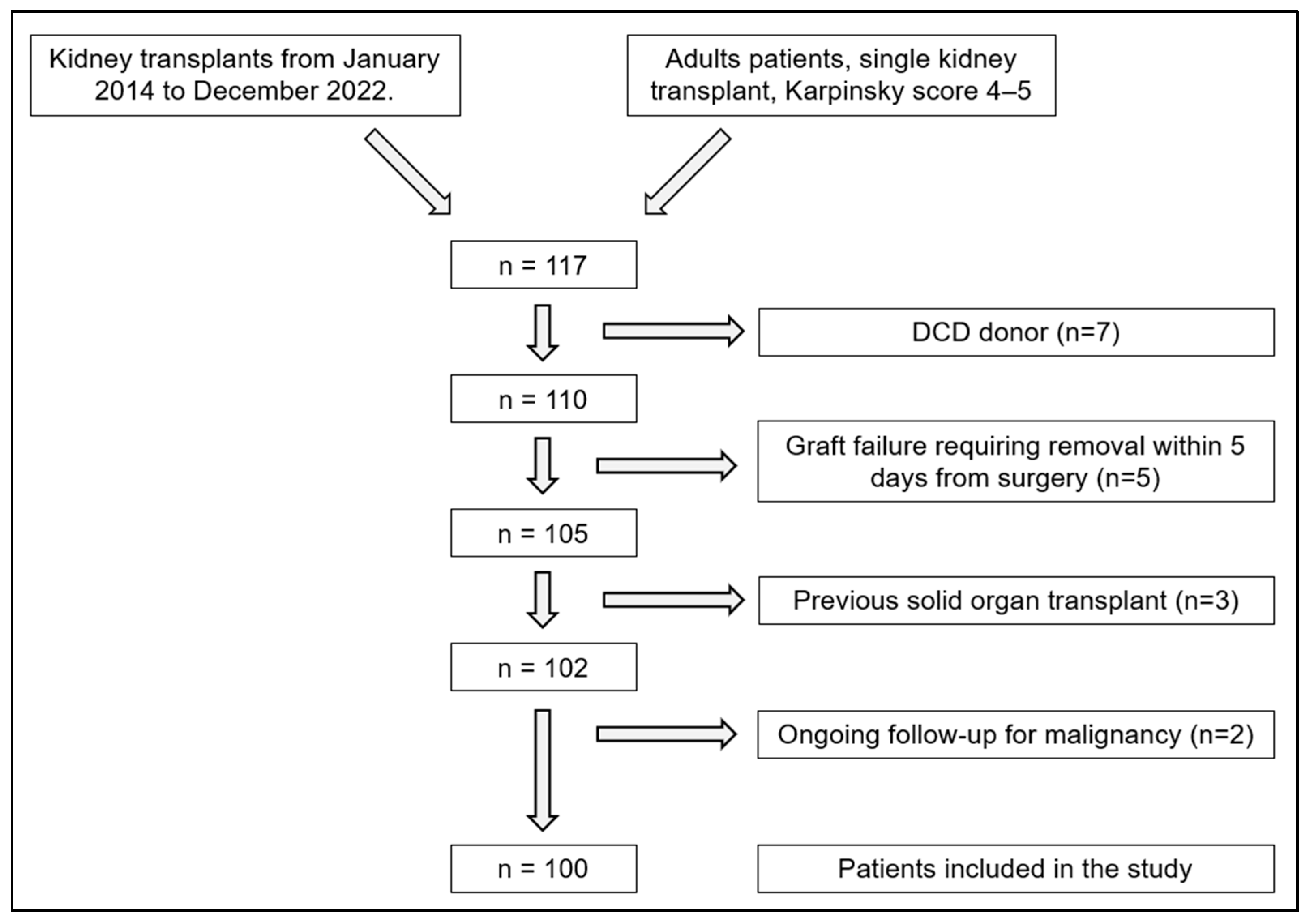
2. Materials and Methods
2.1. Donors
2.2. Graft
2.3. Imaging
2.4. Recipients
2.5. Statistical Analysis
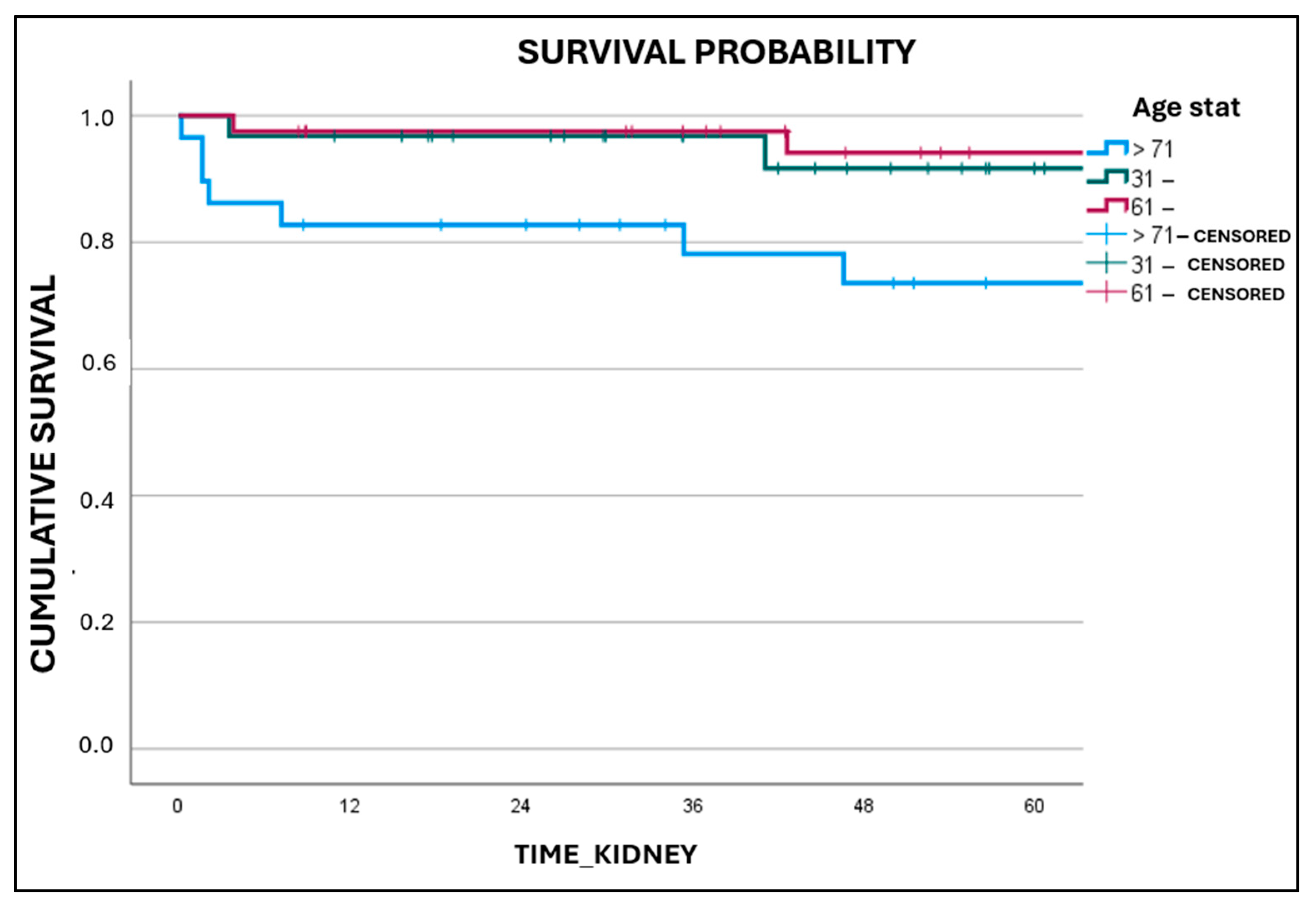
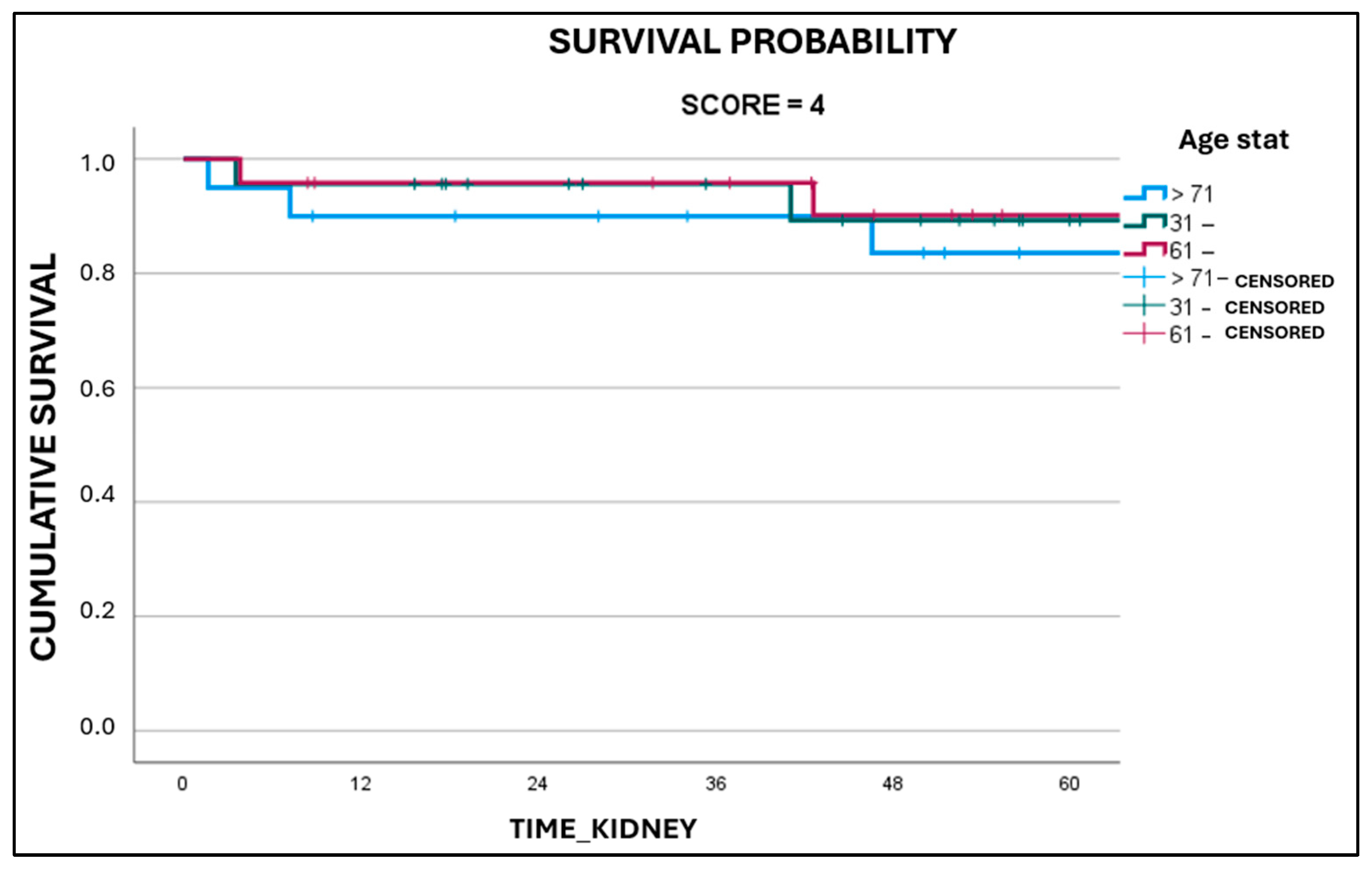
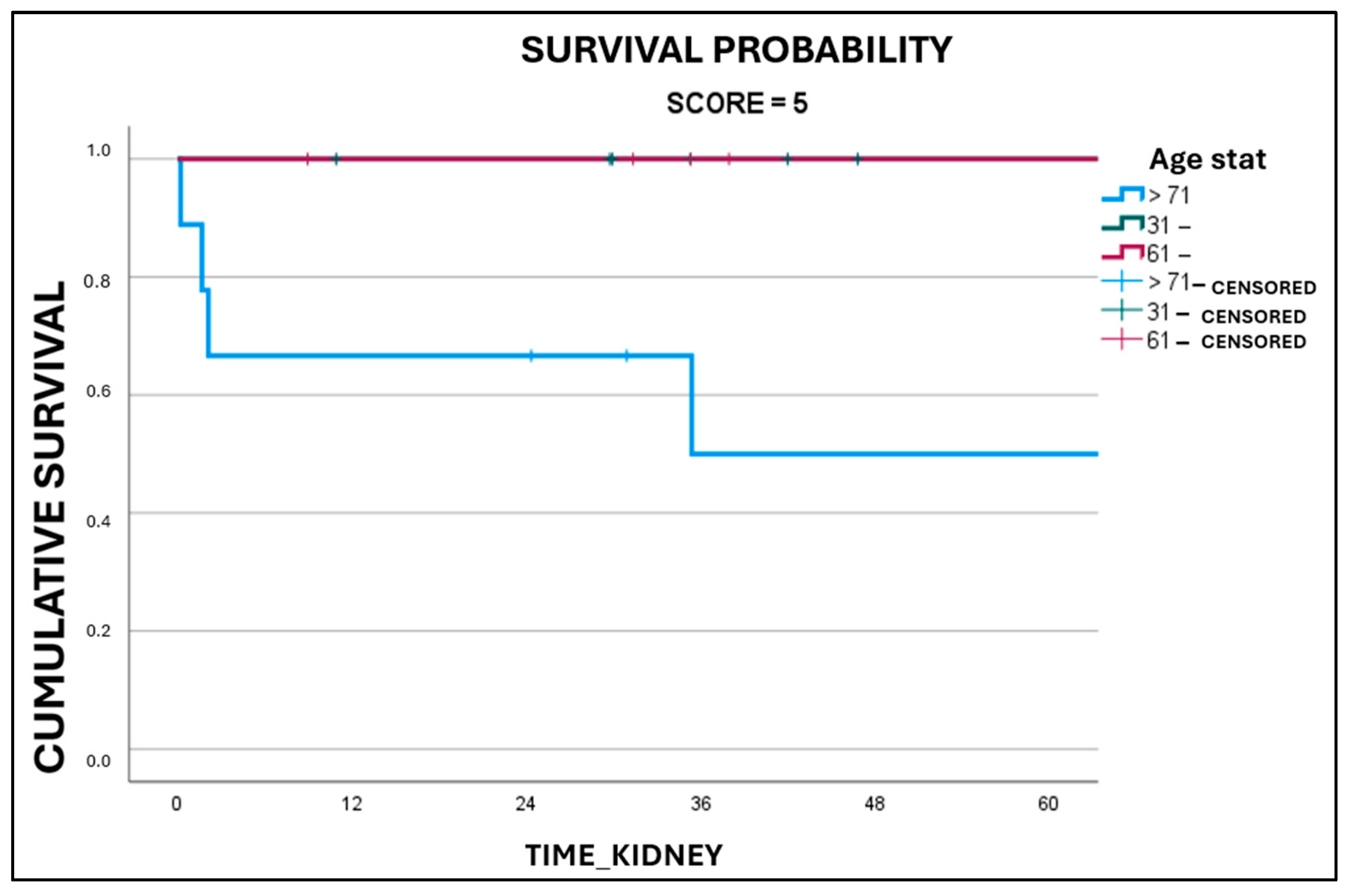
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voora, S.; Adey, D.B. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 866–879. [Google Scholar] [CrossRef] [PubMed]
- USRDS. Annual Data Report. 2017. Available online: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/prior-data-reports/2017#:~:text=Incidence%2C%20Preva-lence%2C%20Patient%20Characteristics%2C%20and%20Treatment%20Modalities.%20United,Epidemiology%20of%20kidney%20disease%20in%20the%20United%20States (accessed on 31 August 2018).
- Bastani, B. The present and future of transplant organ shortage: Some potential remedies. J. Nephrol. 2020, 33, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, M.; Adani, G.L.; Della Penna, A.; Guthoff, M.; Cherchi, V.; Nadalin, S. Kidney Transplantation in Case of Renal Graft with Multiple Arteries: Challenges and Long-Term Results of Microsurgical Anastomosis Between Lower Polar Renal Artery and Inferior Epigastric Artery. Medicina 2025, 61, 1645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosengard, B.R.; Feng, S.; Alfrey, E.J.; Zaroff, J.G.; Emond, J.C.; Henry, M.L.; Garrity, E.R.; Roberts, J.P.; Wynn, J.J.; Metzger, R.A.; et al. Report of the Crystal City Meeting to Maximize the Use of Organs Recovered from the Cadaver Donor. Am. J. Transplant. 2002, 2, 701–711. [Google Scholar] [CrossRef]
- Echterdiek, F.; Schwenger, V.; Döhler, B.; Latus, J.; Kitterer, D.; Heemann, U.; Süsal, C. Kidneys From Elderly Deceased Donors—Is 70 the New 60? Front. Immunol. 2019, 10, 2701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Remuzzi, G.; Cravedi, P.; Perna, A.; Dimitrov, B.D.; Turturro, M.; Locatelli, G.; Rigotti, P.; Baldan, N.; Beatini, M.; Valente, U.; et al. Long-Term Outcome of Renal Transplantation from Older Donors. N. Engl. J. Med. 2006, 354, 343–352. [Google Scholar] [CrossRef]
- Karpinski, J.; Lajoie, G.; Cattran, D.; Fenton, S.; Zaltzman, J.; Cardella, C.; Cole, E. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation 1999, 67, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, P.I.; Cecka, J.M.; Gjertson, D.W.; Takemoto, S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 1995, 333, 333–336. [Google Scholar] [CrossRef]
- Johnson, L.B.; Kuo, P.C.; Dafoe, D.C.; Schweitzer, E.J.; Alfrey, E.J.; Klassen, D.K.; Hoehn-Sarie, E.W.; Weir, M.R.; Bartlett, S.T. Double adult renal allografts: A technique for expansion of the cadaveric kidney donor pool. Surgery 1996, 120, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Diena, D.; Fop, F.; Biancone, L. Aging e rene—Il trapianto renale con donatore deceduto anziano. In Giornale Italiano di Nefrologia; Società Italiana di Nefrologia: Rome, Italy, 2019. [Google Scholar]
- Rigotti, P.; Capovilla, G.; Di Bella, C.; Silvestre, C.; Donato, P.; Baldan, N.; Furian, L. A single-center experience with 200 dual kidney transplantations. Clin. Transplant. 2014, 28, 1433–1440. [Google Scholar] [CrossRef]
- Lee, K.W.; Park, J.B.; Cha, S.R.; Lee, S.H.; Chung, Y.J.; Yoo, H.; Kim, K.; Kim, S.J. Dual kidney transplantation offers a safe and effective way to use kidneys from deceased donors older than 70 years. BMC Nephrol. 2020, 21, 3. [Google Scholar] [CrossRef]
- Collini, A.; Kalmar, P.; Dhamo, A.; Ruggieri, G.; Carmellini, M. Renal transplant from very old donors: How far can we go? Transplantation 2009, 87, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sáez, M.J.; Arcos, E.; Comas, J.; Crespo, M.; Lloveras, J.; Pascual, J.; the Catalan Renal Registry Committee. Survival Benefit From Kidney Transplantation Using Kidneys From Deceased Donors Aged ≥75 Years: A Time-Dependent Analysis. Am. J. Transplant. 2016, 16, 2724–2733. [Google Scholar] [CrossRef]
- Marconi, L.; Figueiredo, A.; Campos, L.; Nunes, P.; Roseiro, A.; Parada, B.; Mota, A. Renal Transplantation With Donors Older Than 70 Years: Does Age Matter? Transplant. Proc. 2013, 45, 1251–1254. [Google Scholar] [CrossRef]
- Gallinat, A.; Feldkamp, T.; Schaffer, R.; Radünz, S.; Treckmann, J.W.; Minor, T.; Witzke, O.; Paul, A.; Sotiropoulos, G.C. Single-Center Experience With Kidney Transplantation Using Deceased Donors Older Than 75 Years. Transplantation 2011, 92, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Englum, B.R.; Schechter, M.A.; Irish, W.D.; Ravindra, K.V.; Vikraman, D.S.; Sanoff, S.L.; Ellis, M.J.; Sudan, D.L.; Patel, U.D. Outcomes in kidney transplant recipients from older living donors. Transplantation 2015, 99, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Merion, R.M.; Ashby, V.B.; Wolfe, R.A.; Distant, D.A.; Hulbert-Shearon, T.E.; Metzger, R.A.; Ojo, A.O.; Port, F.K. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA J. Am. Med. Assoc. 2005, 294, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- van Ittersum, F.J.; Hemke, A.C.; Dekker, F.W.; Hilbrands, L.B.; Christiaans, M.H.L.; Roodnat, J.I.; Hoitsma, A.J.; van Diepen, M. Increased risk of graft failure and mortality in Dutch recipients receiving an expanded criteria donor kidney transplant. Transpl. Int. 2016, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Carta, P.; Zanazzi, M.; Caroti, L.; Buti, E.; Mjeshtri, A.; Di Maria, L.; Raspollini, M.R.; Minetti, E.E. Impact of the pre-transplant histological score on 3-year graft outcomes of kidneys from marginal donors: A single-centre study. Nephrol. Dial. Transplant. 2013, 28, 2637–2644. [Google Scholar] [CrossRef]
- Azancot, M.A.; Moreso, F.; Salcedo, M.; Cantarell, C.; Perello, M.; Torres, I.B.; Montero, A.; Trilla, E.; Sellarés, J.; Morote, J.; et al. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 2014, 85, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Escofet, X.; Osman, H.; Griffiths, D.F.R.; Woydag, S.; Jurewicz, W.A. The presence of glomerular sclerosis at time zero has a significant impact on function after cadaveric renal transplantation. Transplantation 2003, 75, 344–346. [Google Scholar] [CrossRef]
- Cockfield, S.M.; Moore, R.B.; Todd, G.; Solez, K.; Gourishankar, S. The Prognostic Utility of Deceased Donor Implantation Biopsy in Determining Function and Graft Survival After Kidney Transplantation. Transplantation 2010, 89, 559–566. [Google Scholar] [CrossRef]
- Losappio, V.; Stallone, G.; Infante, B.; Schena, A.; Rossini, M.; Maiorano, A.; Fiorentino, M.; Ditonno, P.; Lucarelli, G.; Battaglia, M.; et al. A Single-Center Cohort Study to Define the Role of Pretransplant Biopsy Score in the Long-term Outcome of Kidney Transplantation. Transplantation 2014, 97, 934–939. [Google Scholar] [CrossRef]
- Woestenburg, A.; Sennesael, J.; Bosmans, J.-L.; Verbeelen, D. Vasculopathy in the Kidney Allograft at Time of Transplantation: Impact on Later Function of the Graft. Transplantation 2008, 85, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes With the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transplant. 2020, 34, e13814. [Google Scholar] [CrossRef]
| Average age (years) | 65 ± 12 (31–80) | |
| Average BMI (kg/m2) | 25.9 ± 5.0 (17.1–51.1) | |
| ICU length of stay (days) | 2 ± 4 (1–19) | |
| Present | Absent | |
| Use of inotropes | 72 | 28 |
| Hypertension | 45 | 55 |
| Cardiomyopathy | 16 | 84 |
| Vasculopathy | 20 | 80 |
| Dyslipidemia | 26 | 74 |
| Diabetes mellitus | 8 | 92 |
| Hepatopathy | 0 | 100 |
| Pneumopathy | 10 | 90 |
| Psychiatric pathology | 21 | 79 |
| Karpinski score 4 | 67 | |||
| Karpinski score 5 | 33 | |||
| Size (mm) | 110.0 ± 15 (80–130) | |||
| Cortical thickness (mm) | 15.0 ± 3.4 (9.0–25.0) | |||
| 0 | 1 | 2 | 3 | |
| Vascular score | 0 | 57 | 42 | 1 |
| Interstitial score | 3 | 93 | 4 | 0 |
| Tubular score | 2 | 97 | 1 | 0 |
| Glomerular score | 15 | 82 | 3 | 0 |
| Average age at KT (years) | 57 ± 11 (25–71) | |
| Average CIT (hours) | 15 ± 4.9 (6–26.5) | |
| Average WIT (minutes) | 30.0 ± 5 (14–43) | |
| Average BMI (kg/m2) | 25.2 ± 5 (16–34) | |
| Hemodyalisis (#) | 75 | |
| Peritoneal dyalisis (#) | 19 | |
| Present | Absent | |
| Diabetes mellitus | 19 | 81 |
| Hypertension | 66 | 34 |
| Cardiomyopathy | 21 | 79 |
| DGF | 52 | 48 |
| DONORS | K SCORE 4 N = 67 | K SCORE 5 N = 33 | p |
|---|---|---|---|
| Age | 0.435 | ||
| 31–60 | 23 | 8 | |
| 61–70 | 24 | 16 | |
| ≥71 | 20 | 9 | |
| BMI | 0.092 | ||
| <18.5 | 1 | 0 | |
| 18.5–24.9 | 30 | 10 | |
| 25–29.9 | 26 | 17 | |
| >30 | 10 | 6 | |
| ICU length of stay (days) | 0.066 | ||
| 1 | 26 | 9 | |
| 2–3 | 21 | 9 | |
| 4–7 | 16 | 7 | |
| >7 | 4 | 8 | |
| Inotropes use in ICU | 0.404 | ||
| Yes | 50 | 22 | |
| No | 17 | 11 | |
| Arterial hypertension | 0.076 | ||
| Yes | 26 | 19 | |
| No | 41 | 14 | |
| Cardiopathy | 0.115 | ||
| Yes | 8 | 8 | |
| No | 59 | 25 | |
| Vasculopathy | 0.395 | ||
| Yes | 15 | 5 | |
| No | 52 | 28 | |
| Dyslipidemia | 0.211 | ||
| Yes | 20 | 6 | |
| No | 47 | 27 | |
| Diabetes mellitus | 0.064 | ||
| Yes | 3 | 5 | |
| No | 64 | 28 | |
| Hepatopathy | / | ||
| Yes | 0 | 0 | |
| No | 67 | 33 | |
| Pneumopathy | 0.357 | ||
| Yes | 8 | 2 | |
| No | 59 | 31 | |
| Psychiatric pathology | 0.971 | ||
| Yes | 14 | 7 | |
| No | 53 | 26 |
| GRAFT | K SCORE 4 n = 67 | K SCORE 5 n = 33 | p |
|---|---|---|---|
| Size (mm) | 109.5 ± 9.4 | 112.6 ± 10.8 | 0.149 |
| Cortical thickness (mm) | 14.9 ± 3.3 | 15.1 ± 2.7 | 0.827 |
| Side of harvesting | 0.026 | ||
| Right kidney | 34 | 9 | |
| Left kidney | 33 | 24 | |
| Vascular score | 0.001 | ||
| 1 | 53 | 4 | |
| 2 | 14 | 28 | |
| 3 | 0 | 1 | |
| Interstitial score | 0.189 | ||
| 0 | 2 | 1 | |
| 1 | 64 | 29 | |
| 2 | 1 | 3 | |
| 3 | 0 | 0 | |
| Tubular score | 0.221 | ||
| 0 | 2 | 0 | |
| 1 | 65 | 32 | |
| 2 | 0 | 1 | |
| 3 | 0 | 0 | |
| Glomerular score | 0.027 | ||
| 0 | 12 | 3 | |
| 1 | 55 | 27 | |
| 2 | 0 | 3 | |
| 3 | 0 | 0 |
| RECIPIENTS | K Score 4 N = 67 | K Score 5 N = 33 | p Value |
|---|---|---|---|
| Age at transplant | 0.321 | ||
| >30 | 1 | 0 | |
| 31–60 | 45 | 18 | |
| 61–70 | 21 | 15 | |
| BMI | 0.118 | ||
| 18.5–24.9 | 27 | 19 | |
| 25.0–29.9 | 33 | 10 | |
| >30 | 7 | 4 | |
| Cold ischemia | 15.2 ± 3.8 | 15.7 ± 3.2 | 0.503 |
| Warm ischemia | 32.8 ± 4.8 | 32.3 ± 5.1 | 0.651 |
| Dialysis | 0.557 | ||
| Hemodialysis | 50 | 25 | |
| Peritoneal dialysis | 14 | 5 | |
| Pre-emptive | 3 | 3 | |
| Diabetes mellitus | 0.139 | ||
| Yes | 10 | 9 | |
| No | 57 | 24 | |
| Arterial hypertension | 0.424 | ||
| Yes | 46 | 20 | |
| No | 21 | 13 | |
| Cardiopathy | 0.627 | ||
| Yes | 15 | 6 | |
| No | 52 | 27 | |
| DGF | 0.946 | ||
| Yes | 35 | 17 | |
| No | 32 | 16 | |
| 5-year graft survival | 0.979 | ||
| Yes | 8 | 4 | |
| No | 59 | 29 | |
| 5-year patient survival with functioning graft | 0.986 | ||
| Yes | 4 | 2 | |
| No | 63 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchetta, M.; Piccioni, S.A.; Micheletti, G.; Ietto, G.; Li Marzi, V.; Calomino, N.; Bagnacci, G.; Collini, A.; Garosi, G.; Adani, G.L. Beyond the Score Study: Retrospective Analysis of Single-Graft Kidney Transplant with Karpinski Score 4 Versus Score 5 Grafts. Medicina 2025, 61, 2074. https://doi.org/10.3390/medicina61122074
Zanchetta M, Piccioni SA, Micheletti G, Ietto G, Li Marzi V, Calomino N, Bagnacci G, Collini A, Garosi G, Adani GL. Beyond the Score Study: Retrospective Analysis of Single-Graft Kidney Transplant with Karpinski Score 4 Versus Score 5 Grafts. Medicina. 2025; 61(12):2074. https://doi.org/10.3390/medicina61122074
Chicago/Turabian StyleZanchetta, Matteo, Stefania Angela Piccioni, Giorgio Micheletti, Giuseppe Ietto, Vincenzo Li Marzi, Natale Calomino, Giulio Bagnacci, Andrea Collini, Guido Garosi, and Gian Luigi Adani. 2025. "Beyond the Score Study: Retrospective Analysis of Single-Graft Kidney Transplant with Karpinski Score 4 Versus Score 5 Grafts" Medicina 61, no. 12: 2074. https://doi.org/10.3390/medicina61122074
APA StyleZanchetta, M., Piccioni, S. A., Micheletti, G., Ietto, G., Li Marzi, V., Calomino, N., Bagnacci, G., Collini, A., Garosi, G., & Adani, G. L. (2025). Beyond the Score Study: Retrospective Analysis of Single-Graft Kidney Transplant with Karpinski Score 4 Versus Score 5 Grafts. Medicina, 61(12), 2074. https://doi.org/10.3390/medicina61122074







