Cellular and Molecular Mechanisms of Oxidative DNA Damage and Repair
Abstract
1. Introduction
2. The Molecular Mechanisms of DNA Damage
2.1. Oxidation of Guanine to 8-Oxo-7,8-dihydroguanine (8-OxoG) and 8-Oxo-dG
2.1.1. Clinical Implications of 8-oxoG and 8-oxodG and Their Diagnostic and Prognostic Potential
2.1.2. Challenges and Limitations in the Clinical Application of 8-oxoG as a Biomarker of Oxidative DNA Damage
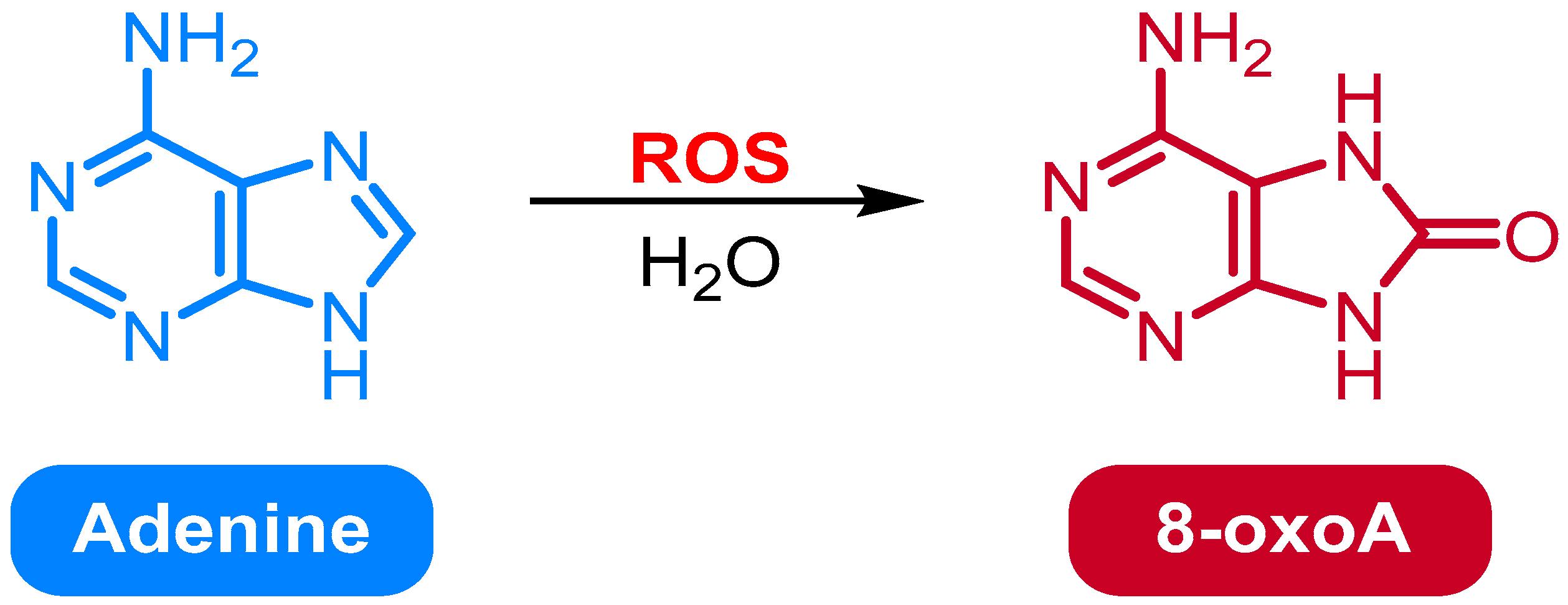
2.2. Oxidation of Adenine to 8-Oxo-7,8-dihydroadenine
2.3. Oxidation of Cytosine to 5-Hydroxycytosine
2.3.1. Oxidation of Cytosine to 5-hC in DNA by the Alkb Family of Enzymes
2.3.2. The Role of 5-hC in DNA Methylation and Epigenetic Regulation
2.4. Oxidation of Thymine to 5-Hydroxyuracil (5-hU)
2.4.1. The Role of Oxidative Stress in the Oxidation of Thymine to 5-hU
2.4.2. The Role of Iron in the Oxidation of Thymine to 5-hU
2.4.3. The Kinetics and Mechanism of the Oxidation of Thymine to 5-hU
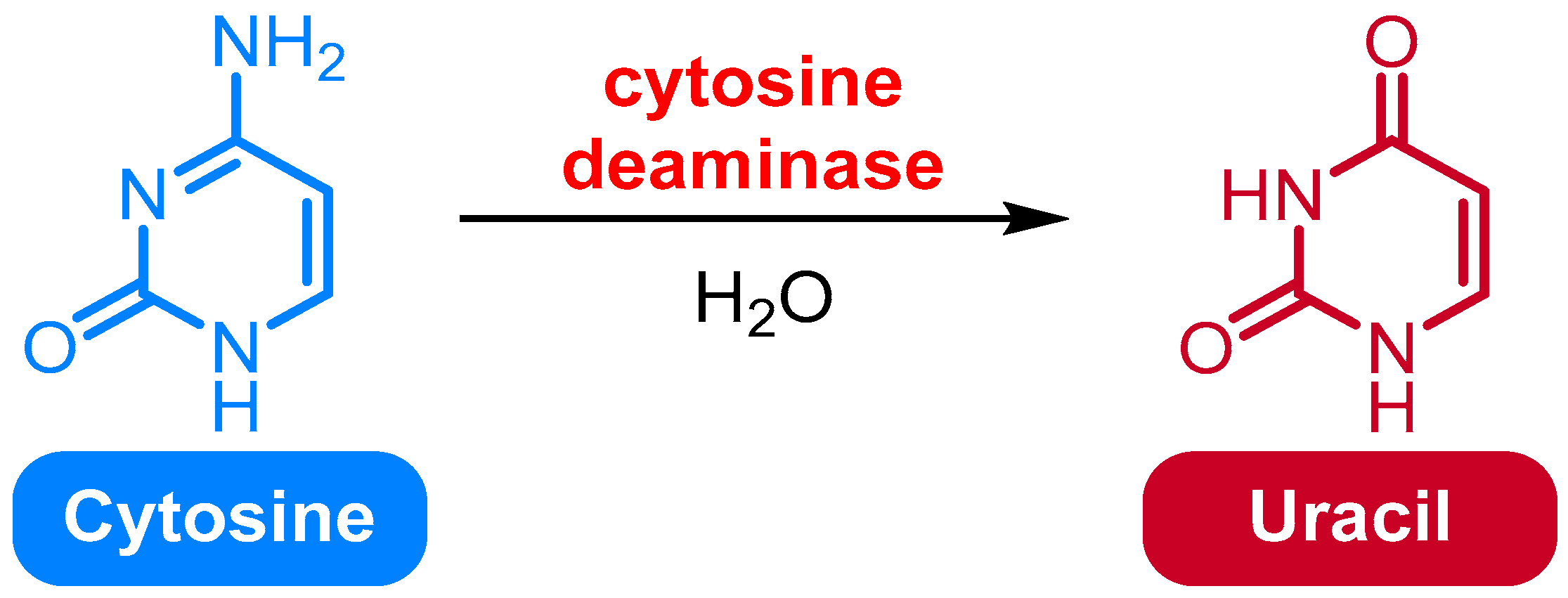
2.5. Deamination of Cytosine to Uracil
2.6. Deamination of Adenine to Hypoxanthine
2.7. Formation of DNA–Protein Crosslinks
2.8. Formation of DNA–DNA Crosslinks
2.9. Formation of DNA–RNA Crosslinks
2.10. Formation of DNA Single-Strand Breaks
2.11. Formation of DNA Double-Strand Breaks
3. DNA Repair Mechanisms
3.1. Base Excision Repair
3.1.1. Structural Basis for Human Base Excision Repair
3.1.2. Role of the Base Excision Repair Pathway in Cancer
3.1.3. Regulation of Base Excision Repair in Response to DNA Damage
3.2. Nucleotide Excision Repair
3.3. Mismatch Repair
3.4. Non-Homologous End Joining
3.4.1. The Role of DNA-Dependent Protein Kinase Catalytic Subunit in NHEJ
3.4.2. The Role of Ku70/80 in NHEJ
3.4.3. The Role of XRCC4 in NHEJ
3.4.4. The Role of DNA Ligase IV in NHEJ
3.4.5. The Role of Artemis in NHEJ
3.4.6. The Role of DNA Polymerase in NHEJ
3.4.7. The Role of Microhomology in NHEJ
3.4.8. The Role of Chromatin Structure in NHEJ
3.5. Homologous Recombination
3.5.1. The Role of Homologous Recombination in DNA Repair and Genome Stability
3.5.2. Homologous Recombination-Mediated DNA Repair Pathways in Bacteria
3.5.3. Homologous Recombination-Mediated DNA Repair in Eukaryotes
3.5.4. The Role of Homologous Recombination in Cancer Development
3.6. Translesion Synthesis
3.6.1. Structural Basis of Translesion Synthesis by DNA Polymerase H
3.6.2. Translesion Synthesis and the Role of DNA Polymerase H in Cancer
3.6.3. Translesion Synthesis and Its Role in the Development of Antibiotic Resistance
3.6.4. Translesion Synthesis and Its Role in the Development of Viral Pathogenesis
4. Pathophysiological and Therapeutic Implications of Oxidative DNA Damage and Repair
5. Targeting DNA Damage Response and Oxidative DNA Repair Pathways in Cancer Therapy
6. Emerging Technologies: CRISPR-Based Tools and Omics Approaches
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef]
- Aksoy, H.; Taysi, S.; Altinkaynak, K.; Bakan, E.; Bakan, N.; Kumtepe, Y. Antioxidant potential and transferrin, ceruloplasmin, and lipid peroxidation levels in women with preeclampsia. J. Investig. Med. 2003, 51, 284–287. [Google Scholar] [CrossRef]
- Baysal, E.; Taysi, S.; Aksoy, N.; Uyar, M.; Çelenk, F.; Karatas, Z.A.; Tarakcioglu, M.; Bilinç, H.; Mumbuç, S.; Kanlikama, M. Serum paraoxonase, arylesterase activity and oxidative status in patients with obstructive sleep apnea syndrome (OSAS). Eur. Rev. Med. Pharm. 2012, 16, 770–774. [Google Scholar]
- Uygun, H.; Çiçek, Z.N.; Ercan, K.; Taysi, S. A Novel Therapeutic Target for Pediatric Pneumonia: Sestrin2. Medicina 2025, 61, 1904. [Google Scholar] [CrossRef]
- Köçtürk, F.; Emekli, F.; Eği, K.; Taysi, S. Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine. Medicina 2025, 61, 1732. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Tascan, A.S.; Ugur, M.G.; Demir, M. Radicals, Oxidative/Nitrosative Stress and Preeclampsia. Mini-Rev. Med. Chem. 2019, 19, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Uslu, C.; Taysi, S.; Bakan, N. Lipid peroxidation and antioxidant enzyme activities in experimental maxillary sinusitis. Ann. Clin. Lab. Sci. 2003, 33, 18–22. [Google Scholar]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Cikman, O.; Ozkan, A.; Aras, A.B.; Soylemez, O.; Alkis, H.; Taysi, S.; Karaayvaz, M. Radioprotective effects of Nigella sativa oil against oxidative stress in liver tissue of rats exposed to total head irradiation. J. Investig. Surg. 2014, 27, 262–266. [Google Scholar] [CrossRef]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Al-Taie, A.; Sancar, M.; Izzettin, F.V. 8-Hydroxydeoxyguanosine: A valuable predictor of oxidative DNA damage in cancer and diabetes mellitus. In Cancer, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2021; Chapter 17; pp. 179–187. [Google Scholar]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jin, S.-G.; Meng, Y.; Johnson, J.; Szabó, P.E.; Pfeifer, G.P. Concordance of hydrogen peroxide–induced 8-oxo-guanine patterns with two cancer mutation signatures of upper GI tract tumors. Sci. Adv. 2022, 8, eabn3815. [Google Scholar] [CrossRef] [PubMed]
- Bellamri, M.; Walmsley, S.J.; Brown, C.; Brandt, K.; Konorev, D.; Day, A.; Wu, C.-F.; Wu, M.T.; Turesky, R.J. DNA Damage and Oxidative Stress of Tobacco Smoke Condensate in Human Bladder Epithelial Cells. Chem. Res. Toxicol. 2022, 35, 1863–1880. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E. Lipid Peroxidation in Biomembranes; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Slupska, M.M.; Baikalov, C.; Luther, W.M.; Chiang, J.-H.; Wei, Y.; Miller, J.H. Cloning and sequencing a human homolog (hMYH) of the Escherichia coli mutY gene whose function is required for the repair of oxidative DNA damage. J. Bacteriol. 1996, 178, 3885–3892. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Evans, M.D.; Saparbaev, M.; Cooke, M.S. DNA repair and the origins of urinary oxidized 2′-deoxyribonucleosides. Mutagenesis 2010, 25, 433–442. [Google Scholar] [CrossRef]
- Hahm, J.Y.; Park, J.; Jang, E.-S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef]
- Sato, T.; Takeda, H.; Otake, S.; Yokozawa, J.; Nishise, S.; Fujishima, S.; Orii, T.; Fukui, T.; Takano, J.; Sasaki, Y. Increased plasma levels of 8-hydroxydeoxyguanosine are associated with development of colorectal tumors. J. Clin. Biochem. Nutr. 2010, 47, 59–63. [Google Scholar] [CrossRef]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Oikawa, S.; Murata, M. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Genes Environ. 2016, 38, 26. [Google Scholar] [CrossRef]
- Kuo, H.-W.; Chou, S.-Y.; Hu, T.-W.; Wu, F.-Y.; Chen, D.-J. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutat. Res. 2007, 631, 62–68. [Google Scholar] [CrossRef]
- Filaire, E.; Dupuis, C.; Galvaing, G.; Aubreton, S.; Laurent, H.; Richard, R.; Filaire, M. Lung cancer: What are the links with oxidative stress, physical activity and nutrition. Lung Cancer 2013, 82, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pylväs, M.; Puistola, U.; Laatio, L.; Kauppila, S.; Karihtala, P. Elevated serum 8-OHdG is associated with poor prognosis in epithelial ovarian cancer. Anticancer Res. 2011, 31, 1411–1415. [Google Scholar] [PubMed]
- Lovell, M.A.; Gabbita, S.P.; Markesbery, W.R. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J. Neurochem. 1999, 72, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.H.; Greenamyre, J.T. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 2013, 62, 111–120. [Google Scholar] [CrossRef]
- Abe, T.; Isobe, C.; Murata, T.; Sato, C.; Tohgi, H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci. Lett. 2003, 336, 105–108. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Jelinek, H.F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 2011, 164, 899–904. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.; Gluud, C. Systematic review: Primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment. Pharmacol. Ther. 2008, 28, 689–703. [Google Scholar] [CrossRef]
- Song, M.-F.; Li, Y.-S.; Ootsuyama, Y.; Kasai, H.; Kawai, K.; Ohta, M.; Eguchi, Y.; Yamato, H.; Matsumoto, Y.; Yoshida, R. Urea, the most abundant component in urine, cross-reacts with a commercial 8-OH-dG ELISA kit and contributes to overestimation of urinary 8-OH-dG. Free Radic. Biol. Med. 2009, 47, 41–46. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, W.-L.; Lin, C.-M.; Lai, C.-H.; Loh, C.-H.; Chen, H.-I.; Liou, S.-H. The relationship between plasma and urinary 8-hydroxy-2-deoxyguanosine biomarkers measured by liquid chromatography tandem mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 17496–17502. [Google Scholar] [CrossRef]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: A meta-analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef]
- Kumar, K.; Fornace, A.J., Jr.; Suman, S. 8-OxodG: A potential biomarker for chronic oxidative stress induced by high-LET radiation. DNA 2024, 4, 221–238. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chang, S.J.; Gibala, K.S.; Resendiz, M.J.E. 8-Oxo-7,8-dihydroadenine and 8-Oxo-7,8-dihydroadenosine—Chemistry, Structure, and Function in RNA and Their Presence in Natural Products and Potential Drug Derivatives. Chem.-A Eur. J. 2017, 23, 6706–6716. [Google Scholar] [CrossRef]
- Mello Filho, A.C.; Meneghini, R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim. Biophys. Acta 1984, 781, 56–63. [Google Scholar] [CrossRef]
- Henle, E.S.; Linn, S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J. Biol. Chem. 1997, 272, 19095–19098. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA Damage by Hydrogen Peroxide Through the Fenton Reaction in Vivo and in Vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Nabel, C.S.; Manning, S.A.; Kohli, R.M. The Curious Chemical Biology of Cytosine: Deamination, Methylation, and Oxidation as Modulators of Genomic Potential. ACS Chem. Biol. 2012, 7, 20–30. [Google Scholar] [CrossRef]
- Klungland, A.; Robertson, A.B. Oxidized C5-methyl cytosine bases in DNA: 5-Hydroxymethylcytosine; 5-formylcytosine; and 5-carboxycytosine. Free Radic. Biol. Med. 2017, 107, 62–68. [Google Scholar] [CrossRef]
- Chen, F.; Bian, K.; Tang, Q.; Fedeles, B.I.; Singh, V.; Humulock, Z.T.; Essigmann, J.M.; Li, D. Oncometabolites d- and l-2-Hydroxyglutarate Inhibit the AlkB Family DNA Repair Enzymes under Physiological Conditions. Chem. Res. Toxicol. 2017, 30, 1102–1110. [Google Scholar] [CrossRef]
- Bjørnstad, L.G.; Meza, T.J.; Otterlei, M.; Olafsrud, S.M.; Meza-Zepeda, L.A.; Falnes, P.Ø. Human ALKBH4 Interacts with Proteins Associated with Transcription. PLoS ONE 2012, 7, e49045. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Q.; Xie, L. The AlkB Family of Fe (II)/Alpha-Ketoglutarate-Dependent Dioxyg enases Modulates Embryogenesis through Epigenetic Regulation. Curr. Stem Cell Res. Ther. 2018, 13, 136–143. [Google Scholar] [CrossRef]
- Onabote, O.; Hassan, H.M.; Isovic, M.; Torchia, J. The Role of Thymine DNA Glycosylase in Transcription, Active DNA Demethylation, and Cancer. Cancers 2022, 14, 765. [Google Scholar] [CrossRef]
- Lotsof, E.R.; Krajewski, A.E.; Anderson-Steele, B.; Rogers, J.; Zhang, L.; Yeo, J.; Conlon, S.G.; Manlove, A.H.; Lee, J.K.; David, S.S. NEIL1 Recoding due to RNA Editing Impacts Lesion-Specific Recognition and Excision. J. Am. Chem. Soc. 2022, 144, 14578–14589. [Google Scholar] [CrossRef]
- Seeberg, E.; Eide, L.; Bjørås, M. The base excision repair pathway. Trends Biochem. Sci. 1995, 20, 391–397. [Google Scholar] [CrossRef]
- Zuo, S.; Boorstein, R.J.; Teebor, G.W. Oxidative damage to 5-methylcytosine in DNA. Nucleic Acids Res. 1995, 23, 3239–3243. [Google Scholar] [CrossRef]
- Cortázar, D.; Kunz, C.; Saito, Y.; Steinacher, R.; Schär, P. The enigmatic thymine DNA glycosylase. DNA Repair 2007, 6, 489–504. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ahmad, A.; Aatif, M.; Alam, M.W.; Hadi, S.M. Structure of Some Green Tea Catechins and the Availability of Intracellular Copper Influence Their Ability to Cause Selective Oxidative DNA Damage in Malignant Cells. Biomedicines 2022, 10, 664. [Google Scholar] [CrossRef]
- Thornburg, L.D.; Lai, M.T.; Wishnok, J.S.; Stubbe, J. A non-heme iron protein with heme tendencies: An investigation of the substrate specificity of thymine hydroxylase. Biochemistry 1993, 32, 14023–14033. [Google Scholar] [CrossRef]
- Yi, C.; Jia, G.; Hou, G.; Dai, Q.; Zhang, W.; Zheng, G.; Jian, X.; Yang, C.-G.; Cui, Q.; He, C. Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature 2010, 468, 330–333. [Google Scholar] [CrossRef]
- Hildenbrand, K.; Behrens, G.; Schulte-Frohlinde, D.; Herak, J.N. Comparison of the reaction of •OH and of SO4−• radicals with pyrimidine nucleosides. An electron spin resonance study in aqueous solution. J. Chem. Soc. Perkin Trans. 2 1989, 3, 283–289. [Google Scholar] [CrossRef]
- Adhikary, A.; Kumar, A.; Heizer, A.N.; Palmer, B.J.; Pottiboyina, V.; Liang, Y.; Wnuk, S.F.; Sevilla, M.D. Hydroxyl Ion Addition to One-Electron Oxidized Thymine: Unimolecular Interconversion of C5 to C6 OH-Adducts. J. Am. Chem. Soc. 2013, 135, 3121–3135. [Google Scholar] [CrossRef]
- Schumacher April, J.; Haché, G.; MacDuff Donna, A.; Brown William, L.; Harris Reuben, S. The DNA Deaminase Activity of Human APOBEC3G Is Required for Ty1, MusD, and Human Immunodeficiency Virus Type 1 Restriction. J. Virol. 2008, 82, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Chelico, L.; Pham, P.; Calabrese, P.; Goodman, M.F. APOBEC3G DNA deaminase acts processively 3′ → 5′ on single-stranded DNA. Nat. Struct. Mol. Biol. 2006, 13, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Zhulai, G.; Oleinik, E.; Shibaev, M.; Ignatev, K. Adenosine-Metabolizing Enzymes, Adenosine Kinase and Adenosine Deaminase, in Cancer. Biomolecules 2022, 12, 418. [Google Scholar] [CrossRef]
- Bagheri, S.; Saboury, A.A.; Haertlé, T. Adenosine deaminase inhibition. Int. J. Biol. Macromol. 2019, 141, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Gillerman, I.; Fischer, B. Investigations into the Origin of the Molecular Recognition of Several Adenosine Deaminase Inhibitors. J. Med. Chem. 2011, 54, 107–121. [Google Scholar] [CrossRef]
- Chválová, K.; Brabec, V.; Kašpárková, J. Mechanism of the formation of DNA–protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007, 35, 1812–1821. [Google Scholar] [CrossRef]
- Weickert, P.; Stingele, J. DNA–protein crosslinks and their resolution. Annu. Rev. Biochem. 2022, 91, 157–181. [Google Scholar] [CrossRef]
- Johnson, K.M.; Price, N.E.; Wang, J.; Fekry, M.I.; Dutta, S.; Seiner, D.R.; Wang, Y.; Gates, K.S. On the Formation and Properties of Interstrand DNA–DNA Cross-Links Forged by Reaction of an Abasic Site with the Opposing Guanine Residue of 5′-CAp Sequences in Duplex DNA. J. Am. Chem. Soc. 2013, 135, 1015–1025. [Google Scholar] [CrossRef]
- Dexheimer, T.S.; Kozekova, A.; Rizzo, C.J.; Stone, M.P.; Pommier, Y. The modulation of topoisomerase I-mediated DNA cleavage and the induction of DNA–topoisomerase I crosslinks by crotonaldehyde-derived DNA adducts. Nucleic Acids Res. 2008, 36, 4128–4136. [Google Scholar] [CrossRef] [PubMed]
- Dator, R.P.; Murray, K.J.; Luedtke, M.W.; Jacobs, F.C.; Kassie, F.; Nguyen, H.D.; Villalta, P.W.; Balbo, S. Identification of Formaldehyde-Induced DNA–RNA Cross-Links in the A/J Mouse Lung Tumorigenesis Model. Chem. Res. Toxicol. 2022, 35, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.W.; O’Neill, K.L. The effect of electromagnetic field exposure on the formation of DNA single strand breaks in human cells. Cell Mol. Biol. 1994, 40, 561–567. [Google Scholar] [PubMed]
- Abbotts, R.; Wilson, D.M. Coordination of DNA single strand break repair. Free Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef]
- Osipov, A.N.; Smetanina, N.M.; Pustovalova, M.V.; Arkhangelskaya, E.; Klokov, D. The formation of DNA single-strand breaks and alkali-labile sites in human blood lymphocytes exposed to 365-nm UVA radiation. Free Radic. Biol. Med. 2014, 73, 34–40. [Google Scholar] [CrossRef]
- Yadav, V.K.; Claeys Bouuaert, C. Mechanism and Control of Meiotic DNA Double-Strand Break Formation in S. cerevisiae. Front. Cell Dev. Biol. 2021, 9, 642737. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E.; Costes, S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef]
- Cristini, A.; Ricci, G.; Britton, S.; Salimbeni, S.; Huang, S.-Y.N.; Marinello, J.; Calsou, P.; Pommier, Y.; Favre, G.; Capranico, G.; et al. Dual Processing of R-Loops and Topoisomerase I Induces Transcription-Dependent DNA Double-Strand Breaks. Cell Rep. 2019, 28, 3167–3181.e3166. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Gao, H.; Kuang, X.; Xiao, H.; Yang, C.; Cheng, Y.; Zhang, L.; Guo, X.; Zhong, Y.; et al. APE1 promotes non-homologous end joining by initiating DNA double-strand break formation and decreasing ubiquitination of artemis following oxidative genotoxic stress. J. Transl. Med. 2023, 21, 183. [Google Scholar] [CrossRef]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue light induces DNA damage in normal human skin keratinocytes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 69–75. [Google Scholar] [CrossRef]
- Hanawalt, P. DNA Repair Mechanisms; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Helena, J.M.; Joubert, A.M.; Grobbelaar, S.; Nolte, E.M.; Nel, M.; Pepper, M.S.; Coetzee, M.; Mercier, A.E. Deoxyribonucleic Acid Damage and Repair: Capitalizing on Our Understanding of the Mechanisms of Maintaining Genomic Integrity for Therapeutic Purposes. Int. J. Mol. Sci. 2018, 19, 1148. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Mammalian DNA base excision repair: Dancing in the moonlight. DNA Repair 2020, 93, 102921. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Okabe, K.; Hirayama, S.; Chirifu, M.; Ikemizu, S.; Morioka, H.; Nakabeppu, Y.; Yamagata, Y. Structure of the mammalian adenine DNA glycosylase MUTYH: Insights into the base excision repair pathway and cancer. Nucleic Acids Res. 2021, 49, 7154–7163. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Dianov, G.L. Co-ordination of base excision repair and genome stability. DNA Repair 2013, 12, 326–333. [Google Scholar] [CrossRef]
- Demple, B.; Herman, T.; Chen, D.S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: Definition of a family of DNA repair enzymes. Proc. Natl. Acad. Sci. USA 1991, 88, 11450–11454. [Google Scholar] [CrossRef]
- Boiteux, S.; Radicella, J.P. The Human OGG1 Gene: Structure, Functions, and Its Implication in the Process of Carcinogenesis. Arch. Biochem. Biophys. 2000, 377, 1–8. [Google Scholar] [CrossRef]
- Hazra, T.K.; Izumi, T.; Boldogh, I.; Imhoff, B.; Kow, Y.W.; Jaruga, P.; Dizdaroglu, M.; Mitra, S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc. Natl. Acad. Sci. USA 2002, 99, 3523–3528. [Google Scholar] [CrossRef]
- Hazra, T.K.; Kow, Y.W.; Hatahet, Z.; Imhoff, B.; Boldogh, I.; Mokkapati, S.K.; Mitra, S.; Izumi, T. Identification and characterization of a novel human DNA glycosylase for repair of cytosine-derived lesions. J. Biol. Chem. 2002, 277, 30417–30420. [Google Scholar] [CrossRef]
- Takao, M.; Kanno, S.-I.; Kobayashi, K.; Zhang, Q.-M.; Yonei, S.; Van Der Horst, G.T.; Yasui, A. A back-up glycosylase in Nth1 knock-out mice is a functional Nei (endonuclease VIII) homologue. J. Biol. Chem. 2002, 277, 42205–42213. [Google Scholar] [CrossRef]
- Liu, M.; Bandaru, V.; Bond, J.P.; Jaruga, P.; Zhao, X.; Christov, P.P.; Burrows, C.J.; Rizzo, C.J.; Dizdaroglu, M.; Wallace, S.S. The mouse ortholog of NEIL3 is a functional DNA glycosylase in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 4925–4930. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kim, K. Excision of Deoxyribose Phosphate Residues by DNA Polymerase β During DNA Repair. Science 1995, 269, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Sobol, R.W.; Horton, J.K.; Kühn, R.; Gu, H.; Singhal, R.K.; Prasad, R.; Rajewsky, K.; Wilson, S.H. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 1996, 379, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, L.; Leppard, J.B.; Kedar, P.; Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M.; Tomkinson, A.E.; Izumi, T.; Prasad, R.; Wilson, S.H.; et al. AP Endonuclease-Independent DNA Base Excision Repair in Human Cells. Mol. Cell 2004, 15, 209–220. [Google Scholar] [CrossRef]
- Vodicka, P.; Urbanova, M.; Makovicky, P.; Tomasova, K.; Kroupa, M.; Stetina, R.; Opattova, A.; Kostovcikova, K.; Siskova, A.; Schneiderova, M.; et al. Oxidative Damage in Sporadic Colorectal Cancer: Molecular Mapping of Base Excision Repair Glycosylases in Colorectal Cancer Patients. Int. J. Mol. Sci. 2020, 21, 2473. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Selvakumaran, M.; Pisarcik, D.A.; Bao, R.; Yeung, A.T.; Hamilton, T.C. Enhanced Cisplatin Cytotoxicity by Disturbing the Nucleotide Excision Repair Pathway in Ovarian Cancer Cell Lines. Cancer Res. 2003, 63, 1311–1316. [Google Scholar]
- Saville, K.M.; Clark, J.; Wilk, A.; Rogers, G.D.; Andrews, J.F.; Koczor, C.A.; Sobol, R.W. NAD+-mediated regulation of mammalian base excision repair. DNA Repair 2020, 93, 102930. [Google Scholar] [CrossRef]
- Adamowicz, M.; Hailstone, R.; Demin, A.A.; Komulainen, E.; Hanzlikova, H.; Brazina, J.; Gautam, A.; Wells, S.E.; Caldecott, K.W. XRCC1 protects transcription from toxic PARP1 activity during DNA base excision repair. Nat. Cell Biol. 2021, 23, 1287–1298. [Google Scholar] [CrossRef]
- Maynard, S.; Keijzers, G.; Akbari, M.; Ezra, M.B.; Hall, A.; Morevati, M.; Scheibye-Knudsen, M.; Gonzalo, S.; Bartek, J.; Bohr, V.A. Lamin A/C promotes DNA base excision repair. Nucleic Acids Res. 2019, 47, 11709–11728. [Google Scholar] [CrossRef]
- Moore, S.P.G.; Toomire, K.J.; Strauss, P.R. DNA modifications repaired by base excision repair are epigenetic. DNA Repair 2013, 12, 1152–1158. [Google Scholar] [CrossRef]
- D’Souza, A.; Blee, A.M.; Chazin, W.J. Mechanism of action of nucleotide excision repair machinery. Biochem. Soc. Trans. 2022, 50, 375–386. [Google Scholar] [CrossRef]
- Pietrasik, S.; Zajac, G.; Morawiec, J.; Soszynski, M.; Fila, M.; Blasiak, J. Interplay between BRCA1 and GADD45A and Its Potential for Nucleotide Excision Repair in Breast Cancer Pathogenesis. Int. J. Mol. Sci. 2020, 21, 870. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Silva, C.; Sabatella, M.; Helfricht, A.; Marteijn, J.A.; Theil, A.F.; Vermeulen, W.; Lans, H. Ubiquitin and TFIIH-stimulated DDB2 dissociation drives DNA damage handover in nucleotide excision repair. Nat. Commun. 2020, 11, 4868. [Google Scholar] [CrossRef] [PubMed]
- Fu, I.; Mu, H.; Geacintov, N.E.; Broyde, S. Mechanism of lesion verification by the human XPD helicase in nucleotide excision repair. Nucleic Acids Res. 2022, 50, 6837–6853. [Google Scholar] [CrossRef] [PubMed]
- Spitz, M.R.; Wu, X.; Wang, Y.; Wang, L.-E.; Shete, S.; Amos, C.I.; Guo, Z.; Lei, L.; Mohrenweiser, H.; Wei, Q. Modulation of Nucleotide Excision Repair Capacity by XPD Polymorphisms in Lung Cancer Patients. Cancer Res. 2001, 61, 1354–1357. [Google Scholar]
- Tomescu, D.; Kavanagh, G.; Ha, T.; Campbell, H.; Melton, D.W. Nucleotide excision repair gene XPD polymorphisms and genetic predisposition to melanoma. Carcinogenesis 2001, 22, 403–408. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; Xing, C.; Yuan, Y. Nucleotide excision repair related gene polymorphisms and genetic susceptibility, chemotherapeutic sensitivity and prognosis of gastric cancer. Mutat. Res. 2014, 765, 11–21. [Google Scholar] [CrossRef]
- Kinsella, T.J. Coordination of DNA Mismatch Repair and Base Excision Repair Processing of Chemotherapy and Radiation Damage for Targeting Resistant Cancers. Clin. Cancer Res. 2009, 15, 1853–1859. [Google Scholar] [CrossRef]
- Barckhausen, C.; Roos, W.P.; Naumann, S.C.; Kaina, B. Malignant melanoma cells acquire resistance to DNA interstrand cross-linking chemotherapeutics by p53-triggered upregulation of DDB2/XPC-mediated DNA repair. Oncogene 2014, 33, 1964–1974. [Google Scholar] [CrossRef]
- Park, J.-M.; Kang, T.-H. Transcriptional and Posttranslational Regulation of Nucleotide Excision Repair: The Guardian of the Genome against Ultraviolet Radiation. Int. J. Mol. Sci. 2016, 17, 1840. [Google Scholar] [CrossRef]
- Coin, F.; Oksenych, V.; Egly, J.-M. Distinct Roles for the XPB/p52 and XPD/p44 Subcomplexes of TFIIH in Damaged DNA Opening during Nucleotide Excision Repair. Mol. Cell 2007, 26, 245–256. [Google Scholar] [CrossRef]
- Kang, T.-H.; Reardon, J.T.; Sancar, A. Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic Acids Res. 2011, 39, 3176–3187. [Google Scholar] [CrossRef]
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132. [Google Scholar] [CrossRef]
- Subba Rao, K. Mechanisms of Disease: DNA repair defects and neurological disease. Nat. Clin. Pract. Neurol. 2007, 3, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar Geri, F.; Moorman, C.; Goosen, N. Role of the Escherichia coli Nucleotide Excision Repair Proteins in DNA Replication. J. Bacteriol. 2000, 182, 5706–5714. [Google Scholar] [CrossRef] [PubMed]
- Ogi, T.; Lehmann, A.R. The Y-family DNA polymerase κ (pol κ) functions in mammalian nucleotide-excision repair. Nat. Cell Biol. 2006, 8, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef]
- Borsellini, A.; Kunetsky, V.; Friedhoff, P.; Lamers, M.H. Cryogenic electron microscopy structures reveal how ATP and DNA binding in MutS coordinates sequential steps of DNA mismatch repair. Nat. Struct. Mol. Biol. 2022, 29, 59–66. [Google Scholar] [CrossRef]
- Mardenborough, Y.S.N.; Nitsenko, K.; Laffeber, C.; Duboc, C.; Sahin, E.; Quessada-Vial, A.; Winterwerp, H.H.K.; Sixma, T.K.; Kanaar, R.; Friedhoff, P.; et al. The unstructured linker arms of MutL enable GATC site incision beyond roadblocks during initiation of DNA mismatch repair. Nucleic Acids Res. 2019, 47, 11667–11680. [Google Scholar] [CrossRef]
- Junop, M.S.; Yang, W.; Funchain, P.; Clendenin, W.; Miller, J.H. In vitro and in vivo studies of MutS, MutL and MutH mutants: Correlation of mismatch repair and DNA recombination. DNA Repair 2003, 2, 387–405. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Shaukat, F.; Isaacsson Velho, P.; Kaur, H.; Shenderov, E.; Pardoll, D.M.; Lotan, T.L. Clinical Features and Therapeutic Outcomes in Men with Advanced Prostate Cancer and DNA Mismatch Repair Gene Mutations. Eur. Urol. 2019, 75, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Y.; Eisfeld, A.-K.; Zhen, F.; Jin, S.; Gao, W.; Yu, T.; Chen, L.; Wang, W.; Chen, W.; et al. Identification of Germline Mismatch Repair Gene Mutations in Lung Cancer Patients with Paired Tumor-Normal Next Generation Sequencing: A Retrospective Study. Front. Oncol. 2019, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Ijsselsteijn, R.; Jansen, J.G.; de Wind, N. DNA mismatch repair-dependent DNA damage responses and cancer. DNA Repair 2020, 93, 102923. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Heinen, C.D. The mismatch repair-dependent DNA damage response: Mechanisms and implications. DNA Repair 2019, 78, 60–69. [Google Scholar] [CrossRef]
- Tan, E.; Sahin, I.H. Defining the current role of immune checkpoint inhibitors in the treatment of mismatch repair-deficient/microsatellite stability-high colorectal cancer and shedding light on future approaches. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 735–742. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.-M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Lewinger, J.P.; Song, C.; Campbell, P.T.; Conti, D.V.; Edlund, C.K.; Duggan, D.J.; Rangrej, J.; Lemire, M.; Hudson, T.; et al. Genotype–Environment Interactions in Microsatellite Stable/Microsatellite Instability-Low Colorectal Cancer: Results from a Genome-Wide Association Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 758–766. [Google Scholar] [CrossRef]
- Watkins, J.C.; Yang, E.J.; Muto, M.G.; Feltmate, C.M.; Berkowitz, R.S.; Horowitz, N.S.; Syngal, S.; Yurgelun, M.B.; Chittenden, A.; Hornick, J.L.; et al. Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients with Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol. 2017, 36, 115–127. [Google Scholar] [CrossRef]
- Post, C.C.B.; Stelloo, E.; Smit, V.T.H.B.M.; Ruano, D.; Tops, C.M.; Vermij, L.; Rutten, T.A.; Jürgenliemk-Schulz, I.M.; Lutgens, L.C.H.W.; Jobsen, J.J.; et al. Prevalence and Prognosis of Lynch Syndrome and Sporadic Mismatch Repair Deficiency in Endometrial Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 1212–1220. [Google Scholar] [CrossRef]
- Peltomäki, P.; Olkinuora, A.; Nieminen, T.T. Updates in the field of hereditary nonpolyposis colorectal cancer. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 707–720. [Google Scholar] [CrossRef]
- Weterings, E.; Chen, D.J. The endless tale of non-homologous end-joining. Cell Res. 2008, 18, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, T.A.; Tainer, J.A.; Lees-Miller, S.P. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair 2010, 9, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, M.; Miller, C.A.; De Haro, L.P.; Durant, S.T.; Chen, B.P.C.; Chen, D.J.; Nickoloff, J.A. DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair 2009, 8, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Kragelund, B.B.; Weterings, E.; Hartmann-Petersen, R.; Keijzers, G. The Ku70/80 ring in non-homologous end-joining: Easy to slip on, hard to remove. Front. Biosci.-Landmark 2016, 21, 514–527. [Google Scholar]
- Hanakahi, L.A.; West, S.C. Specific interaction of IP6 with human Ku70/80, the DNA-binding subunit of DNA-PK. EMBO J. 2002, 21, 2038–2044. [Google Scholar] [CrossRef]
- Boboila, C.; Yan, C.; Wesemann, D.R.; Jankovic, M.; Wang, J.H.; Manis, J.; Nussenzweig, A.; Nussenzweig, M.; Alt, F.W. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J. Exp. Med. 2010, 207, 417–427. [Google Scholar] [CrossRef]
- Mari, P.-O.; Florea, B.I.; Persengiev, S.P.; Verkaik, N.S.; Brüggenwirth, H.T.; Modesti, M.; Giglia-Mari, G.; Bezstarosti, K.; Demmers, J.A.A.; Luider, T.M.; et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc. Natl. Acad. Sci. USA 2006, 103, 18597–18602. [Google Scholar] [CrossRef]
- Grawunder, U.; Zimmer, D.; Fugmann, S.; Schwarz, K.; Lieber, M.R. DNA Ligase IV Is Essential for V(D)J Recombination and DNA Double-Strand Break Repair in Human Precursor Lymphocytes. Mol. Cell 1998, 2, 477–484. [Google Scholar] [CrossRef]
- Rouhani, M. Modeling the interplay between DNA-PK, Artemis, and ATM in non-homologous end-joining repair in G1 phase of the cell cycle. J. Biol. Phys. 2019, 45, 127–146. [Google Scholar] [CrossRef]
- Pospiech, H.; Rytkönen, A.K.; Syväoja, J.E. The role of DNA polymerase activity in human non-homologous end joining. Nucleic Acids Res. 2001, 29, 3277–3288. [Google Scholar] [CrossRef]
- Pannunzio, N.R.; Li, S.; Watanabe, G.; Lieber, M.R. Non-homologous end joining often uses microhomology: Implications for alternative end joining. DNA Repair 2014, 17, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.-H.; Shim, E.Y.; Lee, S.E. Microhomology-mediated end joining: Good, bad and ugly. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2018, 809, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, M.S.; de Krijger, I.; Wiegant, W.W.; Shah, R.G.; Smeenk, G.; de Groot, A.J.L.; Pines, A.; Vertegaal, A.C.O.; Jacobs, J.J.L.; Shah, G.M.; et al. PARP1 Links CHD2-Mediated Chromatin Expansion and H3.3 Deposition to DNA Repair by Non-homologous End-Joining. Mol. Cell 2016, 61, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Manova, V.; Singh, S.K.; Iliakis, G. Processing of DNA double strand breaks by alternative non-homologous end-joining in hyperacetylated chromatin. Genome Integr. 2012, 3, 4. [Google Scholar] [CrossRef]
- Sun, Y.; McCorvie, T.J.; Yates, L.A.; Zhang, X. Structural basis of homologous recombination. Cell. Mol. Life Sci. 2020, 77, 3–18. [Google Scholar] [CrossRef]
- Piazza, A.; Heyer, W.-D. Homologous Recombination and the Formation of Complex Genomic Rearrangements. Trends Cell Biol. 2019, 29, 135–149. [Google Scholar] [CrossRef]
- Ali, J.Y.; Fitieh, A.M.; Ismail, I.H. The Role of DNA Repair in Genomic Instability of Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 5688. [Google Scholar] [CrossRef]
- Nogueira, A.; Fernandes, M.; Catarino, R.; Medeiros, R. RAD52 Functions in Homologous Recombination and Its Importance on Genomic Integrity Maintenance and Cancer Therapy. Cancers 2019, 11, 1622. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Peng, D.; Jiang, A.; He, Y.; Zeng, Y.; Xie, C.; Zhou, H.; Luo, X.; Liu, H.; et al. METTL3 and N6-Methyladenosine Promote Homologous Recombination-Mediated Repair of DSBs by Modulating DNA-RNA Hybrid Accumulation. Mol. Cell 2020, 79, 425–442.e427. [Google Scholar] [CrossRef]
- Gupta, R.; Unciuleac, M.-C.; Shuman, S.; Glickman, M.S. Homologous recombination mediated by the mycobacterial AdnAB helicase without end resection by the AdnAB nucleases. Nucleic Acids Res. 2017, 45, 762–774. [Google Scholar] [CrossRef]
- Lowrey, L.C.; Kent, L.A.; Rios, B.M.; Ocasio, A.B.; Cotter, P.A. An IS-mediated, RecA-dependent, bet-hedging strategy in Burkholderia thailandensis. eLife 2023, 12, e84327. [Google Scholar] [CrossRef]
- Goswami, S.; Gowrishankar, J. Role for DNA double strand end-resection activity of RecBCD in control of aberrant chromosomal replication initiation in Escherichia coli. Nucleic Acids Res. 2022, 50, 8643–8657. [Google Scholar] [CrossRef]
- Seigneur, M.; Ehrlich, S.D.; Michel, B. RuvABC-dependent double-strand breaks in dnaBts mutants require RecA. Mol. Microbiol. 2000, 38, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cook, D.E. The contribution of DNA repair pathways to genome editing and evolution in filamentous pathogens. FEMS Microbiol. Rev. 2022, 46, fuac035. [Google Scholar] [CrossRef] [PubMed]
- Hanke, A.; Compton, R.P.; Ziraldo, R.; Levene, S.D. Kinetics of topological transitions in DNA mediated by topoisomerases and recombinases. Biophys. J. 2022, 121, 447a. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hirasawa, A. Homologous Recombination Deficiencies and Hereditary Tumors. Int. J. Mol. Sci. 2022, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef]
- Gelot, C.; Le-Guen, T.; Ragu, S.; Lopez, B.S. Double-Strand Break Repair: Homologous Recombination in Mammalian Cells. In Genome Stability; Kovalchuk, I., Kovalchuk, O., Eds.; Academic Press: Boston, MA, USA, 2016; Chapter 20; pp. 337–351. [Google Scholar]
- Patel, S.M.; Dash, R.C.; Hadden, M.K. Translesion synthesis inhibitors as a new class of cancer chemotherapeutics. Expert Opin. Investig. Drugs 2021, 30, 13–24. [Google Scholar] [CrossRef]
- Bainbridge, L.J.; Teague, R.; Doherty, A.J. Repriming DNA synthesis: An intrinsic restart pathway that maintains efficient genome replication. Nucleic Acids Res. 2021, 49, 4831–4847. [Google Scholar] [CrossRef]
- Ouzon-Shubeita, H.; Baker, M.; Koag, M.-C.; Lee, S. Structural basis for the bypass of the major oxaliplatin–DNA adducts by human DNA polymerase η. Biochem. J. 2019, 476, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, P.P.; Mali, J.R.; Patra, A.; Rizzo, C.J.; Guengerich, F.P.; Egli, M. Enzymatic bypass and the structural basis of miscoding opposite the DNA adduct 1, N2-ethenodeoxyguanosine by human DNA translesion polymerase η. J. Biol. Chem. 2021, 296, 100642. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, T.D.; Jain, R.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. Structural Basis for Error-free Replication of Oxidatively Damaged DNA by Yeast DNA Polymerase η. Structure 2010, 18, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Rogozin, I.B.; Goncearenco, A.; Lada, A.G.; De, S.; Yurchenko, V.; Nudelman, G.; Panchenko, A.R.; Cooper, D.N.; Pavlov, Y.I. DNA polymerase η mutational signatures are found in a variety of different types of cancer. Cell Cycle 2018, 17, 348–355. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef]
- Matsuda, T.; Bebenek, K.; Masutani, C.; Hanaoka, F.; Kunkel, T.A. Low fidelity DNA synthesis by human DNA polymerase-η. Nature 2000, 404, 1011–1013. [Google Scholar] [CrossRef]
- Buisson, R.; Niraj, J.; Pauty, J.; Maity, R.; Zhao, W.; Coulombe, Y.; Sung, P.; Masson, J.-Y. Breast Cancer Proteins PALB2 and BRCA2 Stimulate Polymerase η in Recombination-Associated DNA Synthesis at Blocked Replication Forks. Cell Rep. 2014, 6, 553–564. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, S.; Motea, E.; Berdis, A. Inhibiting translesion DNA synthesis as an approach to combat drug resistance to DNA damaging agents. Oncotarget 2017, 8, 40804–40816. [Google Scholar] [CrossRef]
- Dupuy, P.; Ghosh, S.; Adefisayo, O.; Buglino, J.; Shuman, S.; Glickman, M.S. Distinctive roles of translesion polymerases DinB1 and DnaE2 in diversification of the mycobacterial genome through substitution and frameshift mutagenesis. Nat. Commun. 2022, 13, 4493. [Google Scholar] [CrossRef]
- Zeltzer, S.; Longmire, P.; Svoboda, M.; Bosco, G.; Goodrum, F. Host translesion polymerases are required for viral genome integrity. Proc. Natl. Acad. Sci. USA 2022, 119, e2203203119. [Google Scholar] [CrossRef]
- Rakibuzzaman, A.; Piñeyro, P.; Pillatzki, A.; Ramamoorthy, S. Harnessing the Genetic Plasticity of Porcine Circovirus Type 2 to Target Suicidal Replication. Viruses 2021, 13, 1676. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, X.; Feng, R.; Fan, Y.; Zhang, Z.; Zhang, Q.W.; Wan, J.B.; Wang, Y.; Yu, H.; Li, G. OGG1: An emerging multifunctional therapeutic target for the treatment of diseases caused by oxidative DNA damage. Med. Res. Rev. 2024, 44, 2825–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L. The significance of 8-oxoGsn in aging-related diseases. Aging Dis. 2020, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. The NAD+/PARP1/SIRT1 axis in aging. Rejuvenation Res. 2017, 20, 244–247. [Google Scholar] [CrossRef]
- Luna, A.; Aladjem, M.I.; Kohn, K.W. SIRT1/PARP1 crosstalk: Connecting DNA damage and metabolism. Genome Integr. 2013, 4, 6. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. NAD+ as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity, and health span. Rejuvenation Res. 2016, 19, 406–413. [Google Scholar] [CrossRef]
- Cherbuin, N.; Patel, H.; Walsh, E.I.; Ambikairajah, A.; Burns, R.; Brüstle, A.; Rasmussen, L.J. Cognitive function is associated with the genetically determined efficiency of DNA repair mechanisms. Genes 2024, 15, 153. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial dysfunction and chronic inflammation: The cornerstones of vascular alterations in age-related diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Williamson, J.; Davison, G. Targeted antioxidants in exercise-induced mitochondrial oxidative stress: Emphasis on DNA damage. Antioxidants 2020, 9, 1142. [Google Scholar] [CrossRef]
- Visnes, T.; Grube, M.; Hanna, B.M.F.; Benitez-Buelga, C.; Cázares-Körner, A.; Helleday, T. Targeting BER enzymes in cancer therapy. DNA Repair 2018, 71, 118–126. [Google Scholar] [CrossRef]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 2020, 5, e000706. [Google Scholar] [CrossRef]
- Grundy, G.J.; Parsons, J.L. Base excision repair and its implications to cancer therapy. Essays Biochem. 2020, 64, 831–843. [Google Scholar]
- Li, X.; Song, Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Fei, X.; Ding, Z.; Peng, X.; Su, Z.; Pan, W.; Chen, J. Recent progress in DNA damage response-targeting PROTAC degraders. J. Med. Chem. 2024, 67, 6906–6921. [Google Scholar] [CrossRef] [PubMed]
- Mossakowska, B.J.; Shahmoradi Ghahe, S.; Cysewski, D.; Fabisiewicz, A.; Tudek, B.; Siedlecki, J.A. Mechanisms of resistance to photodynamic therapy (PDT) in vulvar cancer. Int. J. Mol. Sci. 2022, 23, 4117. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Van Houten, B. The multiple cellular roles of SMUG1 in genome maintenance and cancer. Int. J. Mol. Sci. 2021, 22, 1981. [Google Scholar] [CrossRef]
- Lirussi, L.; Ayyildiz, D.; Liu, Y.; Montaldo, N.P.; Carracedo, S.; Aure, M.R.; Jobert, L.; Tekpli, X.; Touma, J.; Sauer, T. A regulatory network comprising let-7 miRNA and SMUG1 is associated with good prognosis in ER+ breast tumours. Nucleic Acids Res. 2022, 50, 10449–10468. [Google Scholar] [CrossRef]
- Khanna, A. DNA damage in cancer therapeutics: A boon or a curse? Cancer Res. 2015, 75, 2133–2138. [Google Scholar] [CrossRef]
- Quan, C.; Xiao, J.; Liu, L.; Duan, Q.; Yuan, P.; Zhu, F. Protein kinases as tumor biomarkers and therapeutic targets. Curr. Pharm. Des. 2017, 23, 4209–4225. [Google Scholar] [CrossRef]
- Weber, A.M.; Ryan, A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015, 149, 124–138. [Google Scholar] [CrossRef]
- Pospisilova, M.; Seifrtova, M.; Rezacova, M. Small molecule inhibitors of DNA-PK for tumor sensitization to anticancer therapy. J. Physiol. Pharmacol. 2017, 68, 337–344. [Google Scholar] [PubMed]
- Sundar, R.; Brown, J.; Russo, A.I.; Yap, T.A. Targeting ATR in cancer medicine. Curr. Probl. Cancer 2017, 41, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Cseh, A.M.; Fábián, Z.; Sümegi, B.; Scorrano, L. Poly (adenosine diphosphate-ribose) polymerase as therapeutic target: Lessons learned from its inhibitors. Oncotarget 2017, 8, 50221. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.G.; Matuo, R.; Soares, D.G.; Escargueil, A.E.; Henriques, J.A.; Larsen, A.K.; Saffi, J. PARPs and the DNA damage response. Carcinogenesis 2012, 33, 1433–1440. [Google Scholar] [CrossRef]
- Hocsak, E.; Szabo, V.; Kalman, N.; Antus, C.; Cseh, A.; Sumegi, K.; Eros, K.; Hegedus, Z.; Gallyas, F., Jr.; Sumegi, B. PARP inhibition protects mitochondria and reduces ROS production via PARP-1-ATF4-MKP-1-MAPK retrograde pathway. Free Radic. Biol. Med. 2017, 108, 770–784. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Li, L.; Aparicio, A.M.; Corn, P.G.; Kim, J.; Thompson, T.C. Combination platinum-based and DNA damage response-targeting cancer therapy: Evolution and future directions. Curr. Med. Chem. 2017, 24, 1586–1606. [Google Scholar] [CrossRef]
- Pecháčková, S.; Burdová, K.; Macurek, L. WIP1 phosphatase as pharmacological target in cancer therapy. J. Mol. Med. 2017, 95, 589–599. [Google Scholar] [CrossRef]
- Doudna, J.A. The promise and challenge of therapeutic genome editing. Nature 2020, 578, 229–236. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Huang, H.Y.; Lin, Z.; Ranieri, M.; Li, S.; Sahu, S.; Liu, Y.; Ban, Y.; Guidry, K.; Hu, H.; et al. Genome-Wide CRISPR Screens Identify Multiple Synthetic Lethal Targets That Enhance KRASG12C Inhibitor Efficacy. Cancer Res. 2023, 83, 4095–4111. [Google Scholar] [CrossRef]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C: G-to-T: A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-based therapeutic genome editing: Strategies and in vivo delivery by AAV vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef]
- Muralidharan, M.; Krogan, N.J.; Bouhaddou, M.; Kim, M. Current proteomics methods applicable to dissecting the DNA damage response. NAR Cancer 2023, 5, zcad020. [Google Scholar] [CrossRef]
- Li, C.G.; Mahon, C.; Sweeney, N.M.; Verschueren, E.; Kantamani, V.; Li, D.; Hennigs, J.K.; Marciano, D.P.; Diebold, I.; Abu-Halawa, O. PPARγ interaction with UBR5/ATMIN promotes DNA repair to maintain endothelial homeostasis. Cell Rep. 2019, 26, 1333–1343.e1337. [Google Scholar] [CrossRef]
- Lecona, E.; Rodriguez-Acebes, S.; Specks, J.; Lopez-Contreras, A.J.; Ruppen, I.; Murga, M.; Muñoz, J.; Mendez, J.; Fernandez-Capetillo, O. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat. Struct. Mol. Biol. 2016, 23, 270–277. [Google Scholar] [CrossRef] [PubMed]
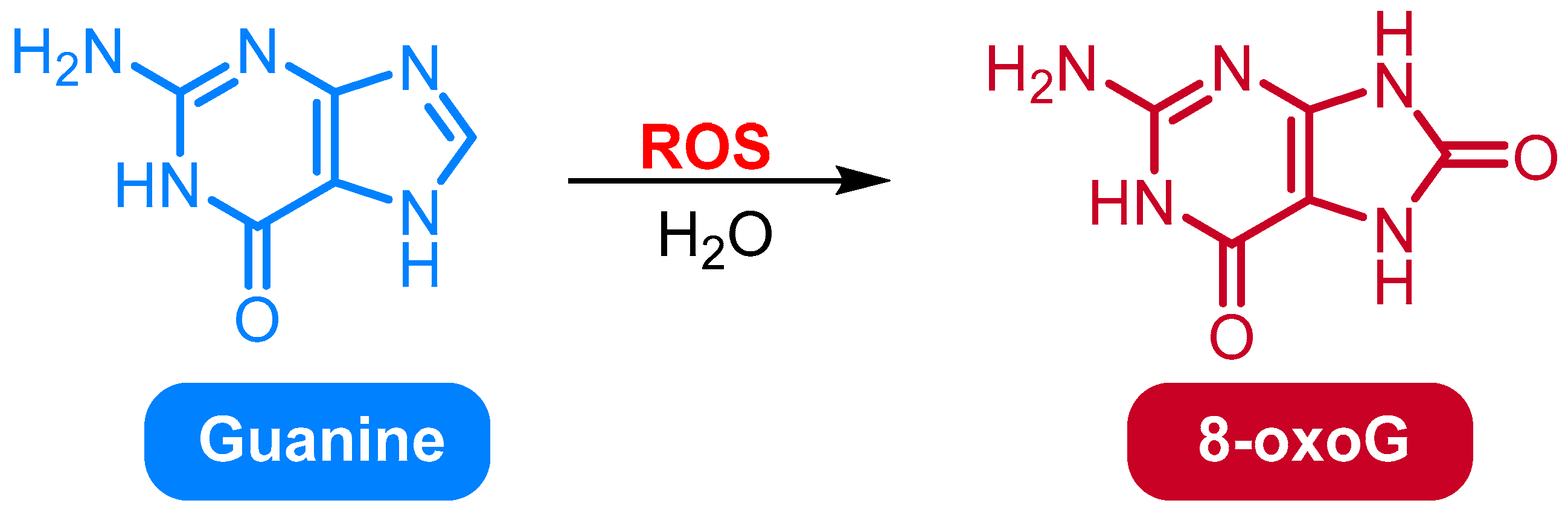


| Lesion Type | Primary Repair Pathway | Key Enzymes | Associated Diseases/Biological Consequences |
|---|---|---|---|
| 8-oxoGuanine (8-oxoG) | BER | OGG1, MUTYH, APE1 | MUTYH-associated polyposis, colorectal cancer, carcinogenesis |
| Oxidation of thymine to 5-hydroxyuracil (5-hU) | BER/NER (minor contribution) | NTHL1, NEIL1, APE1 | Aging-related genomic instability, neurodegeneration |
| AP site (apurinic/apyrimidinic site) | BER | APE1, XRCC1, DNA polymerase β | Genomic instability, increased mutation frequency |
| Single-strand break (ssBreak) | SSBR/PARP-mediated repair | PARP1, XRCC1, DNA ligase III | Ataxia, neurodegenerative disorders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayna, A.; Caglayan, C.; Taysi, S. Cellular and Molecular Mechanisms of Oxidative DNA Damage and Repair. Medicina 2025, 61, 2013. https://doi.org/10.3390/medicina61112013
Ayna A, Caglayan C, Taysi S. Cellular and Molecular Mechanisms of Oxidative DNA Damage and Repair. Medicina. 2025; 61(11):2013. https://doi.org/10.3390/medicina61112013
Chicago/Turabian StyleAyna, Adnan, Cuneyt Caglayan, and Seyithan Taysi. 2025. "Cellular and Molecular Mechanisms of Oxidative DNA Damage and Repair" Medicina 61, no. 11: 2013. https://doi.org/10.3390/medicina61112013
APA StyleAyna, A., Caglayan, C., & Taysi, S. (2025). Cellular and Molecular Mechanisms of Oxidative DNA Damage and Repair. Medicina, 61(11), 2013. https://doi.org/10.3390/medicina61112013





