Gastrointestinal Adverse Effects of Anti-Obesity Medications in Non-Diabetic Adults: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Databases and Search Strategies
2.2. Eligibility Criteria
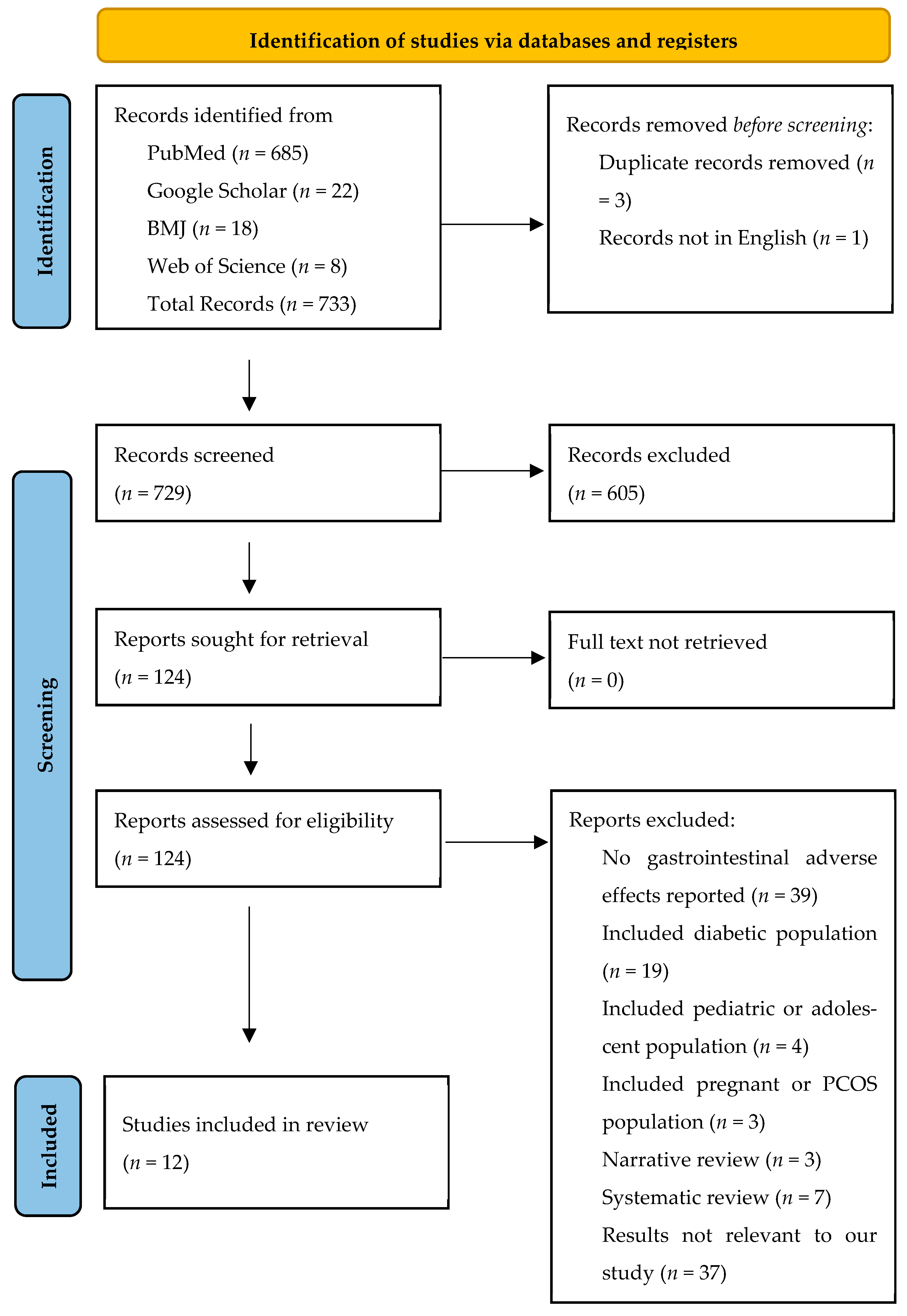
2.3. Data Collection and Screening Process
2.4. Data Extraction and Quality Assessment
2.5. Data Preparation
2.6. Tabulation and Visual Display
2.7. Synthesis Methods
2.8. Reporting Bias Assessment
2.9. Certainty Assessment
3. Results
3.1. Summary of Findings by Drug Class
3.2. Narrative Summary of Evidence Certainty (GRADE Approach)
3.3. Summary of Gastrointestinal Adverse Effects Across Studies
3.3.1. GLP-1 Receptor Agonists (Semaglutide, Liraglutide, Exenatide, Orforglipron)
3.3.2. Dual and Triple Incretin Agonists (Tirzepatide and Retatrutide)
3.3.3. Sympathomimetic and Combination Therapies
3.3.4. Lipase Inhibitors
3.3.5. Natural and Investigational Agents
3.3.6. Overall Summary
4. Discussion
4.1. Comparative Interpretation by Drug Class
4.1.1. Incretin-Based Therapies
4.1.2. Non-Incretin Pharmacotherapies
4.1.3. Lipase Inhibitors
4.1.4. Natural and Investigational Compounds
4.2. Cross-Class Patterns and Mechanistic Insights
4.3. Clinical Implications
4.4. Cross-Class Consderation for Mitigation
4.5. Mechanisms of Gastrointestinal Adverse Effects and Mitigation Strategies
4.6. Comparison with the Broader Literature
4.7. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Bays, H.E.; Fitch, A.; Christensen, S.; Burridge, K.; Tondt, J. Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes. Pillars 2022, 2, 100018. [Google Scholar] [CrossRef]
- Márquez-Cruz, M.; Kammar-García, A.; Huerta-Cruz, J.C.; Carrasco-Portugal, M.d.C.; Barranco-Garduño, L.M.; Rodríguez-Silverio, J.; González, H.I.R.; Reyes-García, J.G. Three- and six-month efficacy and safety of phentermine in a Mexican obese population. Int. J. Clin. Pharmacol. Ther. 2025, 59, 539–548. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Triple-Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]
- Jeon, E.; Lee, K.Y.; Kim, K.K. Approved Anti-Obesity Medications in 2022 KSSO Guidelines and the Promise of Phase 3 Clinical Trials: Anti-Obesity Drugs in the Sky and on the Horizon. J. Obes. Metab. Syndr. 2023, 32, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Blevins, T.; Connery, L.; Rosenstock, J.; Raha, S.; Liu, R.; Ma, X.; Mather, K.J.; Haupt, A.; Robins, D.; et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N. Engl. J. Med. 2023, 389, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.J.; Cartwright, B.M.G.; Gratzl, S.; Brar, R.; Baker, C.; Gluckman, T.J.; Stucky, N.L. Semaglutide vs Tirzepatide for Weight Loss in Adults with Overweight or Obesity. JAMA Intern. Med. 2024, 184, 1056–1064. [Google Scholar] [CrossRef]
- Rodgers, M.; Migdal, A.L.; Rodríguez, T.G.; Chen, Z.-Z.; Nath, A.K.; Gerszten, R.E.; Kasid, N.; Toschi, E.; Tripaldi, J.; Heineman, B.; et al. Weight Loss Outcomes Among Early High Responders to Exenatide Treatment: A Randomized, Placebo Controlled Study in Overweight and Obese Women. Front. Endocrinol. 2021, 12, 742873. [Google Scholar] [CrossRef]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.K.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Møller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Z.; Lu, Y.; Liu, M.; Chen, H.; Zhang, M.; Wang, R.; Yuan, Y.; Li, X. Tirzepatide for Weight Reduction in Chinese Adults with Obesity: The SURMOUNT-CN Randomized Clinical Trial. JAMA 2024, 332, 551–560. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment with Tirzepatide for Maintenance of Weight Reduction in Adults with Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Kushner, R.F.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Bandala, C.; Carro-Rodríguez, J.; Cárdenas-Rodríguez, N.; Peña-Montero, I.; Gómez-López, M.; Hernández-Roldán, A.P.; Huerta-Cruz, J.C.; Muñoz-González, F.; Ignacio-Mejía, I.; Domínguez, B.; et al. Comparative Effects of Gymnema sylvestre and Berberine on Adipokines, Body Composition, and Metabolic Parameters in Obese Patients: A Randomized Study. Nutrients 2024, 16, 2284. [Google Scholar] [CrossRef]
- Won, H.; Yoon, D.Y.; Lee, S.; Cho, J.; Oh, J.; Jang, I.; Yoo, S.; Yu, K. Effects of meal type on the bioavailability of vutiglabridin, a novel anti-obesity agent, in healthy subjects. Clin. Transl. Sci. 2024, 17, e13744. [Google Scholar] [CrossRef]
- Ishibashi, T.; Tanioka, H.; Ikehara, T.; Kezbor, S.; Sonoyama, T. Safety, Tolerability, and Pharmacokinetics of a Novel Anti-obesity Agent, S-309309, in Healthy Adults with or Without Obesity. Clin. Drug Investig. 2025, 45, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Valero-Pérez, M.; Bermejo, L.M.; López-Plaza, B.; García, M.A.; Palma-Milla, S.; Gómez-Candela, C. Regular Consumption of Lipigo® Promotes the Reduction of Body Weight and Improves the Rebound Effect of Obese People Undergo a Comprehensive Weight Loss Program. Nutrients 2020, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cabrera-Rode, E.; Cubas-Dueñas, I.; Acosta, J.R.; Hernández, J.C.; González, A.I.C.; Calero, T.M.G.; Domínguez, Y.A.; Rodríguez, J.H.; Rodríguez, A.D.R.; Álvarez Álvarez, A.; et al. Efficacy and safety of Obex® in overweight and obese subjects: A randomised, double-blind, placebo-controlled clinical trial. BMC Complement. Med. Ther. 2023, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.K.; Jensen, M.E.; Møller, M.; Le Dous, N.; Jensen, A.Ø.; Zeeman, V.A.; Johannsen, C.F.; Lee, A.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 2022, 7, e159863. [Google Scholar] [CrossRef]
- Ponce Martínez, C.; Murcia García, E.; Pérez Sánchez, H.; Milagro, F.I.; Riezu-Boj, J.I.; Ramos Molina, B.; Gómez Gallego, M.; Zamora, S.; Cañavate Cutillas, R.; Hernández Morante, J.J. Effect of Silibinin on Human Pancreatic Lipase Inhibition and Gut Microbiota in Healthy Volunteers: A Randomized Controlled Trial. Int. J. Mol. Sci. 2024, 25, 12853. [Google Scholar] [CrossRef]
- Sayed, U.F.S.M.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Scarlata, G.G.M.; Boitos, I.; Leucuta, D.-C.; Popa, S.-L.; Al Srouji, N.; Abenavoli, L.; Dumitrascu, D.L. Gastrointestinal adverse events associated with GLP-1 RA in non-diabetic patients with overweight or obesity: A systematic review and network meta-analysis. Int. J. Obes. 2025, 49, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Jaroenlapnopparat, A.; Colak, S.C.; Yu, C.-C.; Xanthavanij, N.; Wang, T.-H.; See, X.Y.; Lo, S.-W.; Ko, A.; Chang, Y.-C.; et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology 2025, 169, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, J.; Zhao, C.; Liu, H.; He, C. Comparative efficacy of incretin drugs on glycemic control, body weight, and blood pressure in adults with overweight or obesity and with/without type 2 diabetes: A systematic review and network meta-analysis. Front. Endocrinol. 2025, 16, 1513641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGowan, B.; Ciudin, A.; Baker, J.L.; Busetto, L.; Dicker, D.; Frühbeck, G.; Goossens, G.H.; Monami, M.; Sbraccia, P.; Martinez-Tellez, B.; et al. A systematic review and meta-analysis of the efficacy and safety of pharmacological treatments for obesity in adults. Nat. Med. 2025, 31, 3317–3329. [Google Scholar] [CrossRef]
- Fredrick, T.W.; Camilleri, M.; Acosta, A. Pharmacotherapy for Obesity: Recent Updates. Clin. Pharmacol. 2025, 17, 305–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Databases | Keywords | Search Strategy | Filters | Search Result | Last Date Searched |
|---|---|---|---|---|---|
| PubMed (Advanced search) | Anti-obesity agents, Adverse effects (“Anti-Obesity Agents/adverse effects” [Majr] OR “Anti-Obesity Agents/therapeutic use” [Majr] OR “Anti-Obesity Agents/toxicity” [Majr]) | Advanced filtered search and MeSH | 5-year publication date | 685 | July 2025 |
| Google Scholar (Advanced Search) | Anti-Obesity drugs | Advanced filtered search | 5-year publication date, sort by relevance, | 22 | July 2025 |
| BMJ | Anti-obesity agents, Adverse effects | Advances filtered search | 5-year publication | 18 | July 2025 |
| Web of Science | Anti-obesity agents, Adverse effects | Advanced filtered search | 5-year publication, English, Full text | 8 | July 2025 |
| Component | Inclusion | Exclusion |
|---|---|---|
| Population | Obese adults without diabetes | Young entity (<18 years), adults with diabetes |
| Intervention | Use of anti-obesity pharmacologic agents (e.g., Semaglutide, Orlistat, Setmelanotide, Phentermine-topiramate, Bupropion-naltrexone, Liraglutide, Tirzepatide, etc.) | Studies conducted in vitro or on animals |
| Comparison | Placebo or other anti-obesity agents (e.g., Glucomannan, garcinia combogia, herbal teas, herbal coffees, hoodia, chitosan, chromium picolinate, conjugated linolic acid, etc.) | |
| Outcome | Gastrointestinal adverse effects (e.g., delayed gastric emptying, nausea/vomiting, constipation/diarrhea, etc.) | Outcome other than gastrointestinal adverse effects |
| Timeframe | Studies published between 2020 and 2025 | |
| Trial Type | Randomized controlled trials, non-randomized trials, cohort studies, cross-sectional studies, case–control studies, and interventional clinical trials | Systematic reviews, meta-analysis, case series, observational studies, editorials, books, and gray literature |
| Randomization | Deviation from Intended Intervention | Missing Data | Measurement of Outcome | Reporting | Overall Percentage | |
|---|---|---|---|---|---|---|
| Aronne et al., 2024 [12] |  |  |  |  |  | 100% |
| Cabrera-Rode et al., 2023 [19] |  |  |  |  |  | 90% |
| Jasterboff et al., 2023 [4] |  |  |  |  |  | 100% |
| Lundgren et al., 2021 [10] |  |  |  |  |  | 100% |
| Rodgers et al., 2021 [9] |  |  |  |  |  | 80% |
| Rubino et al., 2021 [6] |  |  |  |  |  | 100% |
| Wharton et al., 2023 [7] |  |  |  |  |  | 90% |
| Wilding et al., 2021 [13] |  |  |  |  |  | 100% |
| Zhao et al., 2024 [11] |  |  |  |  |  | 90% |
| Won et al., 2024 [15] |  |  |  |  |  | 70% |
| Key: |  | Low Risk of Bias | ||||
 | Some Concerns of Bias | |||||
| Author and Year of Publication | Type of Study | Selection (Maximum 4 Stars) | Comparability (Maximum 2 Stars) | Outcome (Maximum 3 Stars) |
|---|---|---|---|---|
| Márquez-Cruz, 2021 [3] | Cohort | **** | ** | *** |
| Rodriguez, 2024 [8] | Cohort | **** | ** | *** |
| Drug Class/Agents | Representative Studies | Summary of Findings on GI Adverse Effects | Certainty of Evidence (Narrative GRADE Assessment) | Key Limiting Factors |
|---|---|---|---|---|
| GLP-1 receptor agonists (Semaglutide, Liraglutide, Tirzepatide, Exenatide, Orforglipron) | Wilding et al. [13]; Rubino et al. [6]; Rodriguez et al. [8]; Zhao et al. [11]; Aronne et al. [12]; Lundgren et al. [10]; Rodgers et al. [9]; Wharton et al. [7]; Klausen et al. [20] | GI AEs were the most frequently reported, particularly nausea, diarrhea, vomiting, and constipation. Incidence was dose-dependent and highest during dose escalation. Most events were mild to moderate, transient, and led to discontinuation in 3–7% of participants. Gallbladder-related events (cholelithiasis, pancreatitis) occurred infrequently. | Moderate | Consistent findings across large RCTs but heterogeneity in AE definitions and absence of standardized GI severity grading; limited long-term tolerability data. |
| Sympathomimetic/Combination Therapies (Phentermine, Phentermine-Topiramate, Naltrexone-Bupropion) | Màrquez-Cruz et al. [3]; Jeon et al. [5]; Bays et al. [2] | Constipation, dry mouth, and nausea were the most common GI events. Events were generally mild, with low discontinuation rates. Some CNS-related side effects overlapped with appetite-suppressant mechanisms. | Low to Moderate | Small sample sizes; short follow up; limited GI-specific reporting; potential reporting bias in self-reported outcomes. |
| Lipase Inhibitors (Orlistat, Silibinin) | Martínez et al. [21]; Bays et al. [2] | Steatorrhea, fecal urgency, and flatulence with discharge were characteristic of orlistat use; silibinin showed fewer and milder GI events. Both agents showed predictable mechanism-related GI profiles. | Moderate | Limited sample sizes for silibinin; short-term studies; variation in AE definitions. |
| Triple-hormone receptor agonist (Retatrutide) | Jasterboff et al. [4] | GI AEs (nausea, vomiting, diarrhea) occurred in up to 82% of participants, primarily during dose escalation. Most events were transient; discontinuation due to GI effects ranged from 6–16%. | Moderate | Phase 2 data only; short follow-up; single study evidence. |
| Natural Agents/Botanicals (Lipigo, Gymnema sylvestre, Berberine) | Valero-Pérez et al. [17]; Bandala et al. [14] | Lipigo caused mild bloating in 24% of participants; Gymnema and Berberine caused mild diarrhea, nausea, or constipation, all self-limited. No severe or serious GI AEs reported. | Low | Small sample sizes; short study duration; lack of standardized AE reporting; potential publication bias. |
| Novel small-molecule agent (S-309309) | Ishibashi et al. [16] | GI symptoms reported in up to 25% of participants. Overall GI tolerability was good with no serious events. | Moderate | Early-phase data only; single small study; limited follow-up. |
| Multi-mechanism and emerging agents (Obex, natural product overviews, Vutiglabridin) | Cabrera-Rode et al. [19]; Sayed et al. [22]; Won et al. [15] | Across these heterogenous agents, GI events were generally mild and transient, including nausea, flatulence, or abdominal discomfort. Obex and Vutiglabridin demonstrated favorable tolerability profiles, while natural product reviews reported limited systemic AE data. | Low | Heterogenous interventions and study designs; small samples; lack of controlled comparison; incomplete AE reporting across studies. |
| Overall summary across all pharmacologic classes | - | Across all interventions, GI AEs were common but typically mild to moderate, transient, and related to mechanism of action. Rates of discontinuation due to GI events ranged from 3 to 16%, highest with incretin-based therapies. | Moderate | Heterogenous study designs, inconsistent adverse effect grading, and lack of gastrointestinal-specific patient reported outcomes limit comparability and confidence in pooled interpretation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takrori, E.; Peshin, S.; Singal, S. Gastrointestinal Adverse Effects of Anti-Obesity Medications in Non-Diabetic Adults: A Systematic Review. Medicina 2025, 61, 1987. https://doi.org/10.3390/medicina61111987
Takrori E, Peshin S, Singal S. Gastrointestinal Adverse Effects of Anti-Obesity Medications in Non-Diabetic Adults: A Systematic Review. Medicina. 2025; 61(11):1987. https://doi.org/10.3390/medicina61111987
Chicago/Turabian StyleTakrori, Ehab, Supriya Peshin, and Sakshi Singal. 2025. "Gastrointestinal Adverse Effects of Anti-Obesity Medications in Non-Diabetic Adults: A Systematic Review" Medicina 61, no. 11: 1987. https://doi.org/10.3390/medicina61111987
APA StyleTakrori, E., Peshin, S., & Singal, S. (2025). Gastrointestinal Adverse Effects of Anti-Obesity Medications in Non-Diabetic Adults: A Systematic Review. Medicina, 61(11), 1987. https://doi.org/10.3390/medicina61111987






