Magnified Dermoscopy in Skin Cancer and Infectious Skin Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Magnified Dermoscopy in Melanocytic Lesions
3.2. Magnified Dermoscopy in Solar Lentigo and Lichen Planus-like Keratosis
3.3. Magnified Dermoscopy in Basal Cell Carcinoma
3.4. Magnified Dermoscopy in Infectious Diseases
4. Discussion
5. Current Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campos-do-Carmo, G.; Ramos-e-Silva, M. Dermoscopy: Basic Concepts. Int. J. Dermatol. 2008, 47, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.P.; Rabinovitz, H.S.; Oliviero, M.; Kopf, A.W.; Saurat, J.-H. Dermoscopy of Pigmented Skin Lesions. J. Am. Acad. Dermatol. 2005, 52, 109–121. [Google Scholar] [CrossRef]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of Melanoma and Non-Melanoma Skin Cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of Actinic Keratosis, Intraepidermal Carcinoma and Squamous Cell Carcinoma. In Actinic Keratosis; Karger Publishers: Basel, Switzerland, 2015; Volume 46, pp. 70–76. [Google Scholar]
- Errichetti, E. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy): An Up-to-Date Overview. Dermatol. Pract. Concept. 2019, 9, 169–180. [Google Scholar] [CrossRef]
- Rudnicka, L.; Olszewska, M.; Waśkiel, A.; Rakowska, A. Trichoscopy in Hair Shaft Disorders. Dermatol. Clin. 2018, 36, 421–430. [Google Scholar] [CrossRef]
- Thomas, L.; Dalle, S. Dermoscopy Provides Useful Information for the Management of Melanonychia Striata. Dermatol. Ther. 2007, 20, 3–10. [Google Scholar] [CrossRef]
- Benvenuto-Andrade, C.; Dusza, S.W.; Agero, A.L.C.; Scope, A.; Rajadhyaksha, M.; Halpern, A.C.; Marghoob, A.A. Differences Between Polarized Light Dermoscopy and Immersion Contact Dermoscopy for the Evaluation of Skin Lesions. Arch. Dermatol. 2007, 143, 329–338. [Google Scholar] [CrossRef]
- Pietkiewicz, P.; Navarrete-Dechent, C.; Togawa, Y.; Szlązak, P.; Salwowska, N.; Marghoob, A.A.; Leszczyńska-Pietkiewicz, A.; Errichetti, E. Applications of Ultraviolet and Sub-Ultraviolet Dermatoscopy in Neoplastic and Non-Neoplastic Dermatoses: A Systematic Review. Dermatol. Ther. 2024, 14, 361–390. [Google Scholar] [CrossRef]
- Puppin, D.; Salomon, D.; Saurat, J.-H. Amplified Surface Microscopy. J. Am. Acad. Dermatol. 1993, 28, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Dusi, D.; Rossi, R.; Simonacci, M.; Ferrara, G. Image Gallery: The New Age of Dermoscopy: Optical Super-High Magnification. Br. J. Dermatol. 2018, 178, e330. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Rossi, R.; Ferrara, G.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Super-high Magnification Dermoscopy Can Identify Pigmented Cells: Correlation with Reflectance Confocal Microscopy. Br. J. Dermatol. 2019, 181, e1. [Google Scholar] [CrossRef]
- Cinotti, E.; Cioppa, V.; Tognetti, L.; Perrot, J.L.; Rossi, R.; Gnone, M.; Cartocci, A.; Rubegni, P.; Cortonesi, G. Super-High Magnification Dermoscopy in 190 Clinically Atypical Pigmented Lesions. Diagnostics 2023, 13, 2238. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Campoli, M.; Liso, F.; Cicigoi, A.; Cartocci, A.; Rossi, R.; Rubegni, P.; Perrot, J.L. Super-high Magnification Dermoscopy Can Aid the Differential Diagnosis between Melanoma and Atypical Naevi. Clin. Exp. Dermatol. 2021, 46, 1216–1222. [Google Scholar] [CrossRef]
- Winkler, J.K.; Kommoss, K.S.; Vollmer, A.S.; Enk, A.H.; Haenssle, H.A.; Toberer, F. Optical Super-high Magnification Dermoscopy of Benign and Malignant Melanocytic Lesions in Correlation with Histopathology. JDDG J. Dtsch. Dermatol. Ges. 2025, 23, 610–619. [Google Scholar] [CrossRef]
- Cinotti, E.; Cartocci, A.; Liso, F.G.; Cioppa, V.; Falcinelli, F.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Super-High Magnification Dermoscopy Can Help for the Diagnosis of Lentigo Maligna: A Pilot Study on 61 Cases. Dermatol. Pract. Concept. 2023, 13, e2023101. [Google Scholar] [CrossRef]
- Ravni, E.; Skowron, F.; David, C.; Cortez, C.D.; Lima, S.; Tognetti, L.; Chazelle, M.; Habougit, C.; Vercherin, P.; Perrot, M.; et al. Correlation between Super-High Magnification (400x) Dermoscopy and Reflectance Confocal Microscopy for the Diagnosis of Melanosis and Other Pigmented Genital Lesions. Eur. J. Dermatol. 2024, 34, 131–138. [Google Scholar] [CrossRef]
- Radi, G.; Rossi, R.; Diotallevi, F.; Giannoni, M.; Molinelli, E.; Paolinelli, M.; Ferrara, G.; Offidani, A. The Role of the Optical Super High Magnification Dermoscopy (O.S.H.M.D) in the Management of Melanocytic Lesions. J. Eur. Acad. Dermatol. Venereol. 2023, 37, pe122. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Szepietowski, J.C. Melanoma Cells in Optical Super-High Magnification Dermoscopy. Br. J. Dermatol. 2023, 189, e55. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Cinotti, E.; Szepietowski, J.C. Optical Super-High Magnification Dermoscopy Findings of Halo Naevus. Acta Derm. Venereol. 2023, 103, 2722. [Google Scholar] [CrossRef]
- Provvidenziale, L.; Cinotti, E.; Campoli, M.; Rubegni, P. Superhigh Magnification Dermoscopy and Management of a Pediatric Spitz Nevus Mimicking Melanoma. Ital. J. Dermatol. Venereol. 2021, 156, 111–112. [Google Scholar] [CrossRef]
- Daviti, M.; Papadimitriou, I.; Cinotti, E.; Lallas, A. Atypical Melanocytes of an Atypical Spitz Tumour Observed with Optical Super-High Magnification Dermoscopy. Br. J. Dermatol. 2024, 191, 853. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Guida, S.; Radi, G.; Rossi, R.; Lallas, A.; Cinotti, E. Correspondence of Optical Super-High Magnification Dermoscopy with Histopathology of Melanocytic Lesions. Dermatol. Pract. Concept. 2025, 15, 4817. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Ławniczak-Cielińska, D.; Cinotti, E. Optical Super-High Magnification Dermoscopy of Solar Lentigo and Lichen Planus-Like Keratosis. Dermatol. Pract. Concept. 2025, 15, 4815. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Lallas, A.; Szepietowski, J.C. Morphology of Vessels in Basal Cell Carcinoma in Optical Super-High Magnification Dermoscopy. Acta Derm. Venereol. 2023, 103, 11966. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Szepietowski, J.C. Optical Super-high Magnification Dermoscopy of Pigmented and Nonpigmented Nodular Basal Cell Carcinoma. J. Cosmet. Dermatol. 2022, 21, 6458–6460. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J.; Cinotti, E.; Lallas, A. Differentiation of Dermal Nevus and Basal Cell Carcinoma Based on Optical Super-High Magnification Dermoscopy. Dermatol. Pract. Concept. 2024, 14, e2024094. [Google Scholar] [CrossRef]
- Pogorzelska-Dyrbuś, J. “Oak-Leaf-like” Loop Vessels in Super-High Magnification Dermoscopy of Basal Cell Carcinoma. Dermatol. Pract. Concept. 2022, 12, e2022147. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Argenziano, G.; Borgia, F.; Vaccaro, F.; Vaccaro, M. Efficacy Evaluation of Scabies Treatment through Super High Magnification Dermoscopy. Ital. J. Dermatol. Venereol. 2023, 158, 161–162. [Google Scholar] [CrossRef]
- Winkler, J.K.; Toberer, F.; Enk, A.H.; Haenssle, H.A. Super-high Magnification Dermatoscopy for In-vivo Imaging of Scabies Mites. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 216–217. [Google Scholar] [CrossRef]
- Giuffrida, R.; Tognetti, L.; Guida, S.; Conforti, C.; Cinotti, E.; Zalaudek, I.; Guarneri, F. High Magnification Dermoscopy for in Vivo Identification of Larvae within Eggs in Active Scabies Infestation. Br. J. Dermatol. 2025, 193, 581. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Vaccaro, F.; Borgia, F.; Macca, L.; Portuese, S.; Vaccaro, M. Super-High Magnification Entodermoscopy: The New Era of Dermoscopy in the Field of Skin Parasitoses. Dermatol. Pract. Concept. 2024, 14, e2024021. [Google Scholar] [CrossRef]
- Cinotti, E.; Ekinde, S.; Labeille, B.; Raberin, H.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Image Gallery: Pigmented Hyphae Can Be Identified in Vivo by High and Super-high Magnification Dermoscopy. Br. J. Dermatol. 2019, 181, e4. [Google Scholar] [CrossRef]
- Cinotti, E.; Bertello, M.; Donelli, C.; Rossi, R.; Tognetti, L.; Perrot, J.L.; Rubegni, P. Super-high Magnification Dermoscopy Can Detect Demodex folliculorum. J. Eur. Acad. Dermatol. Venereol. 2023, 37, pe96. [Google Scholar] [CrossRef]
- Orsini, C.; Cortonesi, G.; Lamberti, A.; Campoli, M.; Rubegni, P.; Cinotti, E. Optical Super-High Magnification Dermoscopy: A Complementary Means in the Diagnosis of Trombiculosis. Dermatol. Pract. Concept. 2023, 13, e2023074. [Google Scholar] [CrossRef]
- Di Bartolomeo, L.; Portuese, S.; Vaccaro, F.; Borgia, F.; Vaccaro, M. Tick Bite and Super-High Magnification Dermoscopy. Dermatol. Pract. Concept. 2024, 14, e2024217. [Google Scholar] [CrossRef]
- Micantonio, T.; Neri, L.; Longo, C.; Grassi, S.; Di Stefani, A.; Antonini, A.; Coco, V.; Fargnoli, M.C.; Argenziano, G.; Peris, K. A New Dermoscopic Algorithm for the Differential Diagnosis of Facial Lentigo Maligna and Pigmented Actinic Keratosis. Eur. J. Dermatol. 2018, 28, 162–168. [Google Scholar] [CrossRef]
- D’Onghia, M.; Falcinelli, F.; Barbarossa, L.; Pinto, A.; Cartocci, A.; Tognetti, L.; Rubegni, G.; Batsikosta, A.; Rubegni, P.; Cinotti, E. Zoom-in Dermoscopy for Facial Tumors. Diagnostics 2025, 15, 324. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Borghi, A.; Verzì, A.E.; Corazza, M.; Stinco, G.; Micali, G. Dermoscopy of Genital Diseases: A Review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2198–2207. [Google Scholar] [CrossRef]
- Guida, S.; Kaleci, S.; Rossi, R.; Radi, G.; Molinelli, E.; Pellacani, G.; Cinotti, E.; Italian Optical Super-High Magnification Dermoscopy Group. Optical Super-High Magnification Dermoscopy in the Diagnosis of Equivocal Melanocytic Lesions: Poor Agreement on Current Terminology and Future Perspectives. Dermatol. Pract. Concept. 2024, 14, e2024261. [Google Scholar] [CrossRef]
- Guida, S.; Pogorzelska-Dyrbuś, J.; Radi, G.; Giuffrida, R.; Karls, R.; Daviti, M.; Rossi, R.; Hofmann-Wellenhof, R.; Perrot, J.; Garat, H.; et al. Magnified Dermoscopy Terminology for Skin Tumours: International Dermoscopy Society Delphi Consensus. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e963–e966. [Google Scholar] [CrossRef]

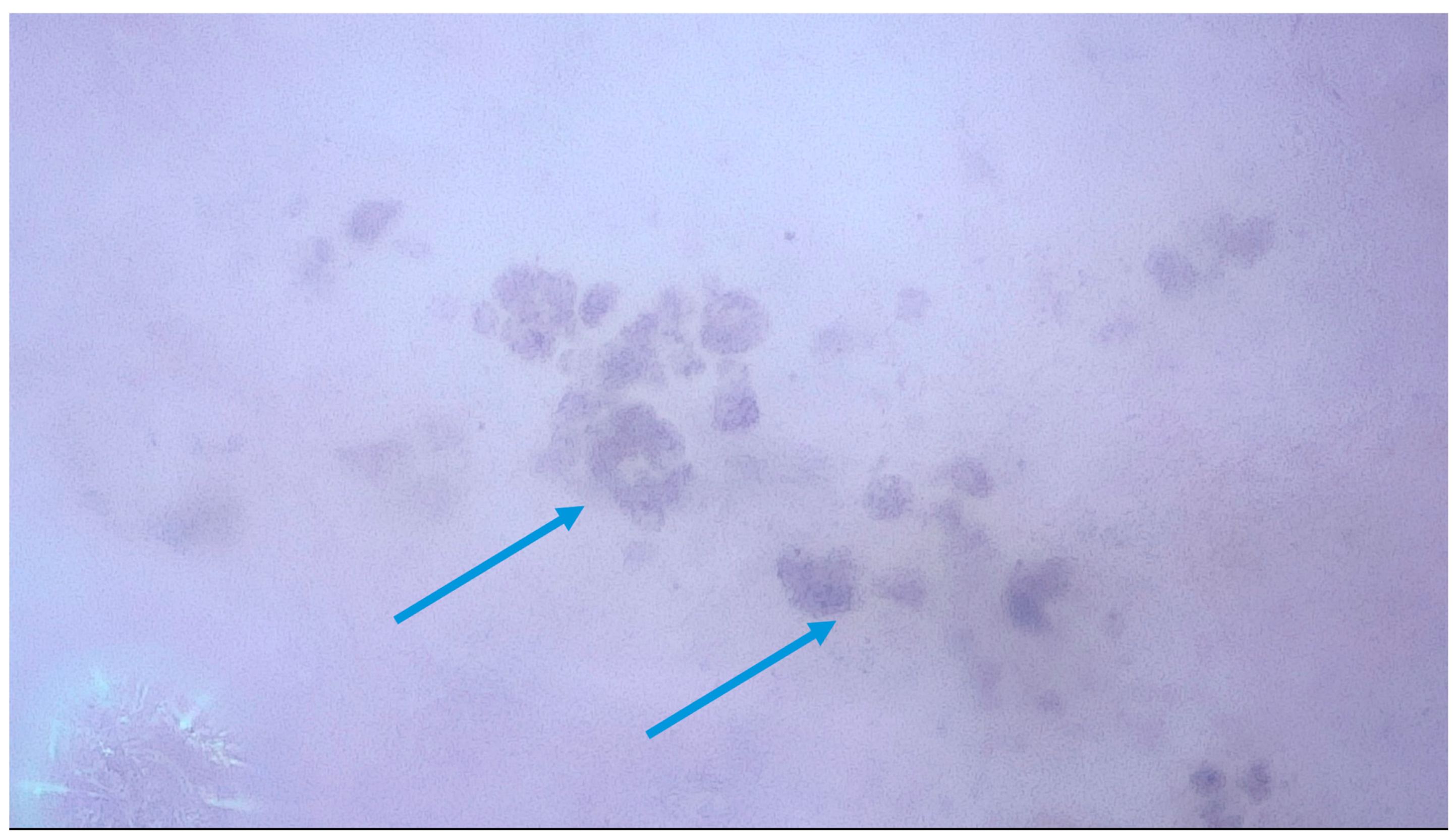

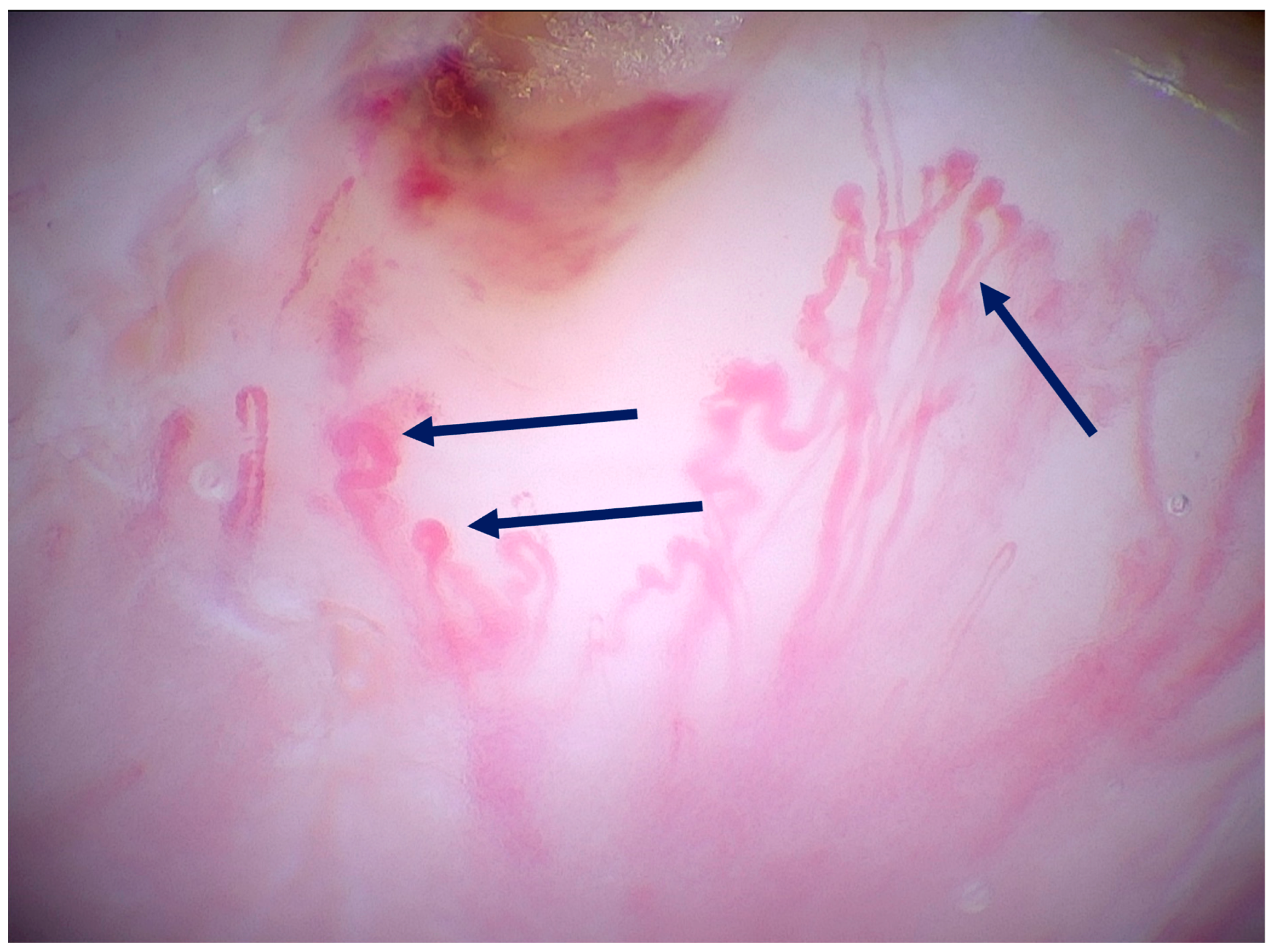
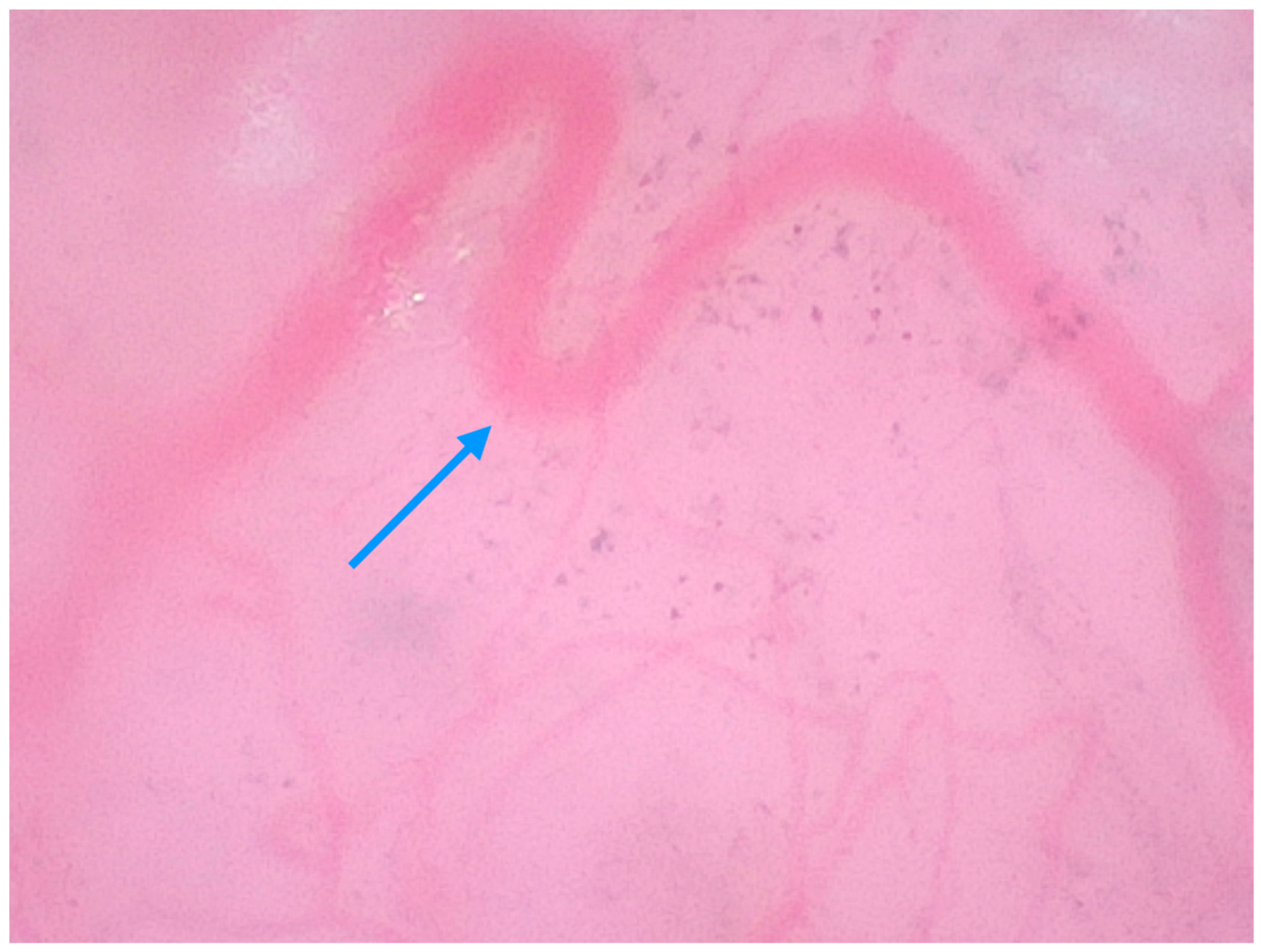
| Author and Corresponding Reference | Study Type/Case Report/Case Series | Number of Lesions and Type of Lesions | Findings in 400× Dermoscopy | Oxford Level of Evidence Ox |
|---|---|---|---|---|
| Melanocytic lesions | ||||
| Cinotti et al. (2023) [13] | Retrospective, observational multicentric study (University of Sienna and Saint-Etienne, Department of Dermatology Senigallia, Private Practice in Genoa) excluding lesions on the face. | 190 patients, 73 melanomas and 117 benign lesions (including 17 Reed/Spitz nevi)—total, 190 lesions. | Melanomas: Roundish (49.3%), dendritic (30.1%), irregularly arranged (41.1%), irregular in shape and size (52.1%) melanocytes, angled nests (22.2%). Nevi: Network with edged papillae (25.6%). Benign lesions: higher visibility of keratinocytes (93.2%), roundish nests (35.9%), lower presence of melanophages (16.2%)—no statistical relevance. Cell color (black 13 (11.1%) vs. 7 (9.6%), brown 107 (91.5%) vs. 66 (90.4%), violet/blue 45 (38.5%) vs. 29 (39.7%), out-of-focus bluish (36 (30.8%) vs. 24 (32.9%)) or grey/brown (23 (19.7) vs. 11 (15.1%)) structureless areas and vessel presence (26 (22.2%) vs. 23 (31.5%)) were not statistically significant for the differential diagnosis between melanoma and benign lesions. | III |
| Cinotti et al. (2021) [14] | Prospective, observational multicenter study (University of Siena and Saint-Etienne) | 79 patients, 51 nevi and (including 7 Reed/Spitz Nevi and 2 blue nevi) and 31 melanomas (21 invasive melanomas and 4 lentigo maligna melanomas)—total, 88 lesions. | Melanoma: Pigmented cells larger than keratinocytes (87.1%), irregular in size and shape (74.2%), out of focus (51.6%), violet/blue in color (61.3%). Presence of melanophages (48.4%), violet/blue nests (29%), out of focus blue structureless areas (61.3%). Vessels: irregular in shape (32.3%) and dilated inside dermal papillae (19.4%) Network without edge papillae (64.5%). Nevi: Cells more polygonal (91.2%), in focus (89.5%) and distributed in nests inside the network or outside dermal papillae (56.1%), network with edged papillae (38.6%). | III |
| Winkler et al. (2025) [15] | Retrospective, observational study (University Hospital of Heidelberg) | 99 patients, 65 nevi, 11 melanomas in situ, 22 invasive melanomas, 1 melanoma metastasis, total, 99 lesions. | Melanomas: In lesion parts (52.9%), irregular distribution of cells along pigment network (76.5%), different size of cells (79.4%), irregular vessels (32.4%), red (64.7%) and blue (55.9%) structureless areas, black-grey dots (47.1%). Nevi: Regular network (61.5%), regular distribution of cells along pigment network (60%) with the same size (72.3%) | III |
| Cinotti et al. (2023) [16] | Retrospective, observational multicentric study (University of Siena and Saint-Etienne) on facial lesions. | 61 patients, lesions on the face: 23 lentigo maligna, 3 lentigo maligna melanoma, 15 solar lentigines, 12 seborrheic keratoses, 6 lichenoid keratoses, 2 pigmented actinic keratoses—total, 61 lesions. | Lentigo maligna/lentigo maligna melanoma: Dendritic melanocytes (96.2%), roundish melanocytes (92.3%), folliculotropism of melanocytes (92.3%), melanocytes with irregular arrangement (76.9%), irregularity of melanocytes in shapes and size (50%). | III |
| Ravni et al. (2024) [17] | Cross-sectional monocentric prospective study on genital, pigmented lesions (University Hospital of Saint-Etienne) | 152 patients, 32 pathologically confirmed (1 BCC, 2 condylomas, 19 melanoses, 2 melanomas in situ, 8 nevi); no histology, only RCM and dermoscopy follow up: 164 melanoses, 11 nevi—total, 207 lesions. | Melanosis: Ring patterns and isolated round cells were observed roughly at a similar frequency between confocal microscopy and magnified dermoscopy. Dendritic cells and round cells in nests were less frequently observed with magnified dermoscopy than confocal microscopy. The concordance between magnified dermoscopy and confocal microscopy criteria for each evaluator was moderate to strong (kappa: 0.64, 0.53, 0.49) for the identification of the ring pattern, moderate to strong (kappa: 0.65, 0.75, 0.55) for the identification of the nests of round cells, strong (kappa: 0.63, 0.78, 0.76) for dendritic cells), strong (kappa: 0.73, 0.62, 0.63) for plump cellsand perfect (kappa: 1, 1, 1) for isolated round cells and spindle cells. | III |
| Cinotti et al. (2019) [12] | Image letter | Solar lentigo in magnified dermoscopy: Keratinocytes of solar lentigo appear as light brown polygons corresponding to hyper-reflective homogeneous cells in RCM. Melanoma in magnified dermoscopy: Pigmented roundish irregular structures which correlate with pagetoid spread in RCM. | V | |
| Radi et al. (2022) [18] | Case report | 20-year-old patient with genital lesion–dermal nevus. | Dermal nevus findings: Circular melanocytes, regular and uniform in size, some more pigmented than others, which appear as small circles, non-pigmented center surrounded by a peripheral pigmented ring, while others present full, solid small circles with a uniformly pigmented center and periphery. | V |
| Pogorzelska-Dyrbuś et al. (2023) [19] | Case report | 38-year-old patient with a melanoma. | Melanoma findings: The presence of scattered irregular round pigmented cells of different sizes and a nest of pigmented cells which correspond to pagetoid spread on pathology. | V |
| Pogorzelska-Dyrbuś et. al. (2023) [20] | Case report | 6-year-old patient with a halo nevus. | Halo nevus findings: Remnants of nests of melanocytes with a dilated vessel, the structure of a hair with completely depigmented cortex and white medulla at the periphery of the lesion along the normally pigmented hair. | V |
| Provvidenziale et al. (2020) [21] | Case report | 11-year-old boy with a Spitz nevus on the right forearm. | Spitz nevus findings: Irregular in shape and size, dark brown and black pigmented cells, regular light brown pigmented cells. In pathology, they might correspond to melanocyte proliferation and pigmented keratinocytes. | V |
| Daviti et al. (2024) [22] | Case report | 64-year-old patient with an atypical Spitz tumor. | Atypical Spitz tumor findings: Scattered, irregular, pigmented roundish structures and roundish structures with projections that are heterogenous in size. | V |
| Pogorzelska-Dyrbuś et al. (2025) [23] | Case series | Comparison between magnified dermoscopy features in dermal nevus, junctional nevus, compound nevus, atypical nevus and melanoma. | Dermal nevus: Numerous brown roundish structures with a darker rim and an internal lighter part, in darker types, purple multi-shaped larger pigmented structures are observed, histopathologically corresponding to melanophages. Junctional nevus: Polygonal and roundish structures histopathologically corresponding to either keratinocytes or melanocytes, which are difficult to distinguish with magnified dermoscopy. Compound nevus: Numerous pigmented cells are visible, the majority most probably corresponding to keratinocytes. Atypical nevus: Melanocytes are larger, either round or spindle-shaped. Melanoma: Melanocytes in melanoma are larger and heterogeneous in shape and size, unevenly scattered. | IV |
| Solar lentigo and lichen planus-like keratosis | ||||
| Pogorzelska-Dyrbuś et al. (2025) [24] | Case series | A 73-year-old female patient with a solar lentigo on her left cheek, and a 55-year-old female patient with lplk on her right arm. | Solar lentigo: Brown-reddish uniform polygonal structures corresponding to keratinocytes that contoured follicular openings, areas of dense arrangement of brown polygonal structures with well-defined borders. Lichen planus-like keratosis: Numerous blue-purple large structures corresponding to melanophages with straight linear vessels. | V |
| Basal Cell Carcinoma (BCC) | ||||
| Pogorzelska-Dyrbuś et al. (2023) [25] | Prospective, observational study in a private practice clinic (Tychy). | 41 patients, 41 BCCs: nodular (61%), superficial (26.8%), multifocal (9.8%), infiltrative (2.4%). | BCC findings: The percentage of looped vessels was significantly higher in magnified dermoscopy than in standard dermoscopy (63.4% vs. 29.2%). Arborizing vessels were seen at the same frequency in both magnifications (53.7%). Pigmented structures were more common in magnified dermoscopy than in standard dermoscopy (56.1% vs. 34.1%), with the identification of individual cells, including melanophages. | III |
| Pogorzelska-Dyrbuś et al. (2022) [26] | Case series | 2 cases of pigmented BCC (skin of abdomen in a 68-year-old male and left cheek in a 57-year-old female), 2 cases of nonpigmented BCCs (42-year-old female with a lesion on the back and 68-year-old male with a lesion on the forearm). | Pigmented BCCs: Tree-like vessels and linear vessels, arranged around blue-grey globules formed by nests of tumor cells in both cases. Non-pigmented BCC: Shiny white-red structureless area with looped vessels, with extremely branched loop seen in both cases. | V |
| Pogorzelska Dyrbuś et al. (2025) [27] | Case series | 2 cases of dermal nevi (abdomen of a 36-year-old male, face 45-year-old female) and 2 cases of nodular BCCs (55-year-old and 45-year-old females). | Dermal nevi: Prominent stem and looped vessels, but also multiple small pale brown circular structures of the same size, possibly corresponding to melanocytes in the upper part of dermal nests. BCCs: Branched vessels and brown globules located focally at the periphery, fine pigmented structures and looped vessels. | |
| Pogorzelska-Dyrbuś et al. (2022) [28] | Case series | 2 cases of BCC: nodular (back of an 81-year-old male patient) and superficial (abdomen of a 49-year-old male patient). | Nodular BCC: Looped vessels with extremely branched loops resembling oak leaves, which were distributed throughout the entire lesion. Superficial BCC: Numerous loop vessels forming the so-called “oak leaves”, distributed across the entire lesion. | V |
| Infectoscopy | ||||
| Scabies | ||||
| Di Bartolomeo et al. (2023) [29] | Prospective observational study (University of Messina, Italy) | 22 patients were observed to evaluate treatment efficacy of benzyl benzoate 25% cream after failed 5% permetrine treatment. | Magnified dermoscopy findings: Intestinal peristalsis or movement of surface structures were suggested as signs of mite vitality, while translucent appearance of “delta wing” (head and legs) or visualization of the epimeres of anterior legs at higher magnification were associated with degradation of the mite. | IV |
| Winkler et al. (2022) [30] | Case report | 75-year-old patient with treatment resistance lesions on upper trunk and axillary folds. | Magnified dermoscopy findings: Head, extremities and intestine of the mite more visible in classic dermoscopy. | V |
| Giuffrida et al. (2025) [31] | Image letter | Observation of scabies mites in magnified dermoscopy. | Magnified dermoscopy findings: Revealed details of head, extremities and intestine of the mite and larvae within eggs as sign of active infestation, invisible at lower magnifications, substantially obviating the need for mineral oil preparation. | V |
| Other infectious diseases | ||||
| Di Bartolomeo et al. (2024) [32] | Case series | Three patients with history of itch (50-year-old male, 2-year-old child, 40-year-old male) who were diagnosed with Pthirus pubis, Pediculus capitis, scabies and demodex spp. | Magnified dermoscopy findings: mouthparts with antennae, the digestive system, spiracles, terminal claws, hair on the dorsal surface, air tubes seen in Pthirus pubis specimen. In Pediculus capitis: antennae; mouthparts; eyes; digestive tract, legs were seen along vital nit, presenting the dome-shaped operculum. In third patient, scabiei mite with mouthparts and anterior legs, spines on abdomen and posterior legs visible through transparent body were detected, along with Demodex folliculorum tails. | V |
| Cinotti et al. (2019) [33] | Case Report | 45-year-old male patient with a lesion on the palm, diagnosed as tinea egra caused by Hortaea werneckii. | Magnified dermoscopy findings: brown elongated hyphae and two-celled spindle-shaped blastoconidia. | V |
| Cinotti et al. (2022) [34] | Case report | 55-year-old male patient with papules on the face diagnosed as demodicosis. | Magnified dermoscopy findings: elongated and round white structures inside hair follicles which correspond to the image of Demodex folliculorum. | V |
| Orsini et al. (2023) [35] | Case report | 27-year-old male patient with diffuse erythematous nodules, diagnosed as trombiculosis. | Magnified dermoscopy findings: six-legged golden colored parasites, strongly attached to the skin, which allowed us to diagnose an infestation of the larval stage of Neotrombicula autumnalis. | V |
| Di Bartolomeo et al. (2025) [36] | Case report | 50-year-old female with a new lesion on her breast. After examination, a diagnosis of a tick bite was set. | Magnified dermoscopy findings: dorsal partial scutum of the tick, demonstrating that it was a female belonging to the Ixodidae family, and the intact rostrum, thus proving the correct removal of tick. | V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korecka, K.; Pogorzelska-Dyrbuś, J.; Polańska, A.; Dańczak-Pazdrowska, A.; Lallas, A. Magnified Dermoscopy in Skin Cancer and Infectious Skin Diseases. Medicina 2025, 61, 1970. https://doi.org/10.3390/medicina61111970
Korecka K, Pogorzelska-Dyrbuś J, Polańska A, Dańczak-Pazdrowska A, Lallas A. Magnified Dermoscopy in Skin Cancer and Infectious Skin Diseases. Medicina. 2025; 61(11):1970. https://doi.org/10.3390/medicina61111970
Chicago/Turabian StyleKorecka, Katarzyna, Joanna Pogorzelska-Dyrbuś, Adriana Polańska, Aleksandra Dańczak-Pazdrowska, and Aimilios Lallas. 2025. "Magnified Dermoscopy in Skin Cancer and Infectious Skin Diseases" Medicina 61, no. 11: 1970. https://doi.org/10.3390/medicina61111970
APA StyleKorecka, K., Pogorzelska-Dyrbuś, J., Polańska, A., Dańczak-Pazdrowska, A., & Lallas, A. (2025). Magnified Dermoscopy in Skin Cancer and Infectious Skin Diseases. Medicina, 61(11), 1970. https://doi.org/10.3390/medicina61111970







