Clinical Spectrum, Surgical Management, and Outcomes of NR5A1-Related 46,XY Differences of Sex Development: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Steroidogenic Factor-1 (SF-1) and NR5A1 Gene
3.2. Epidemiology and Etiology
3.3. Clinical Presentation
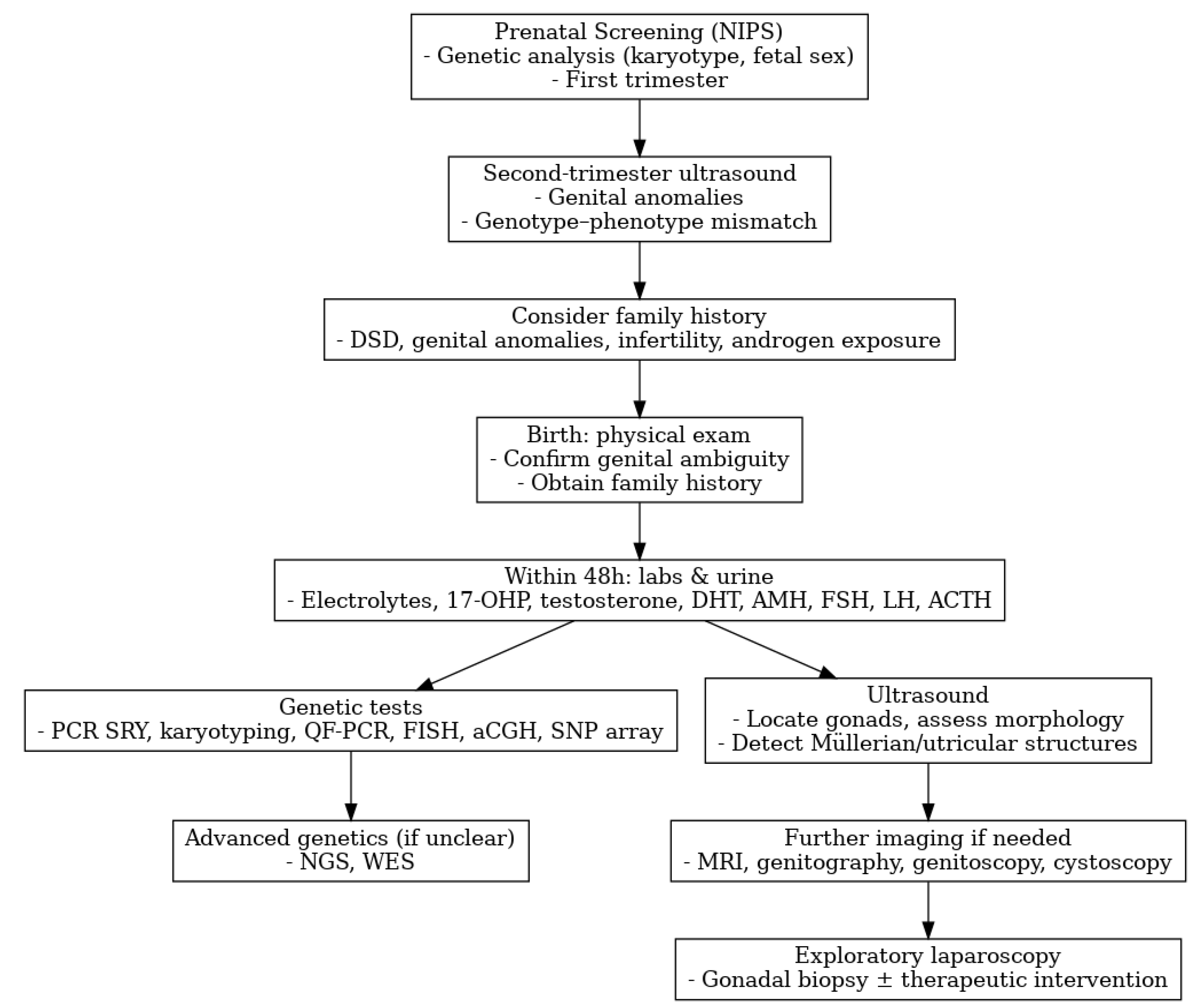
3.4. Diagnosis
3.5. Gender Assignment
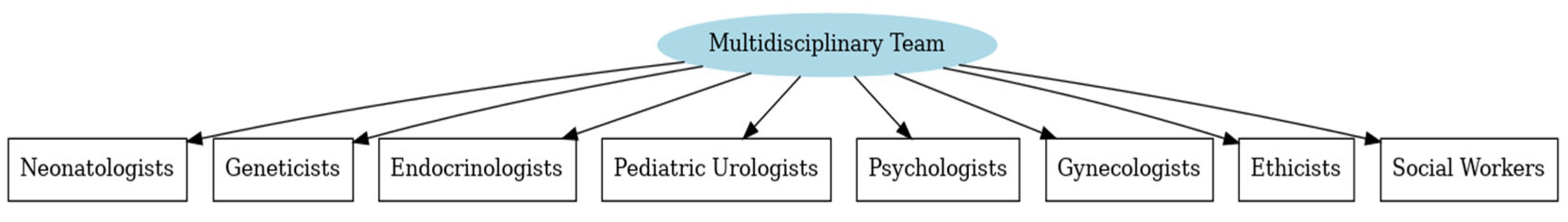
3.6. Multidisciplinary Team
3.7. Morbidity, Prognosis, and Cancer Risk
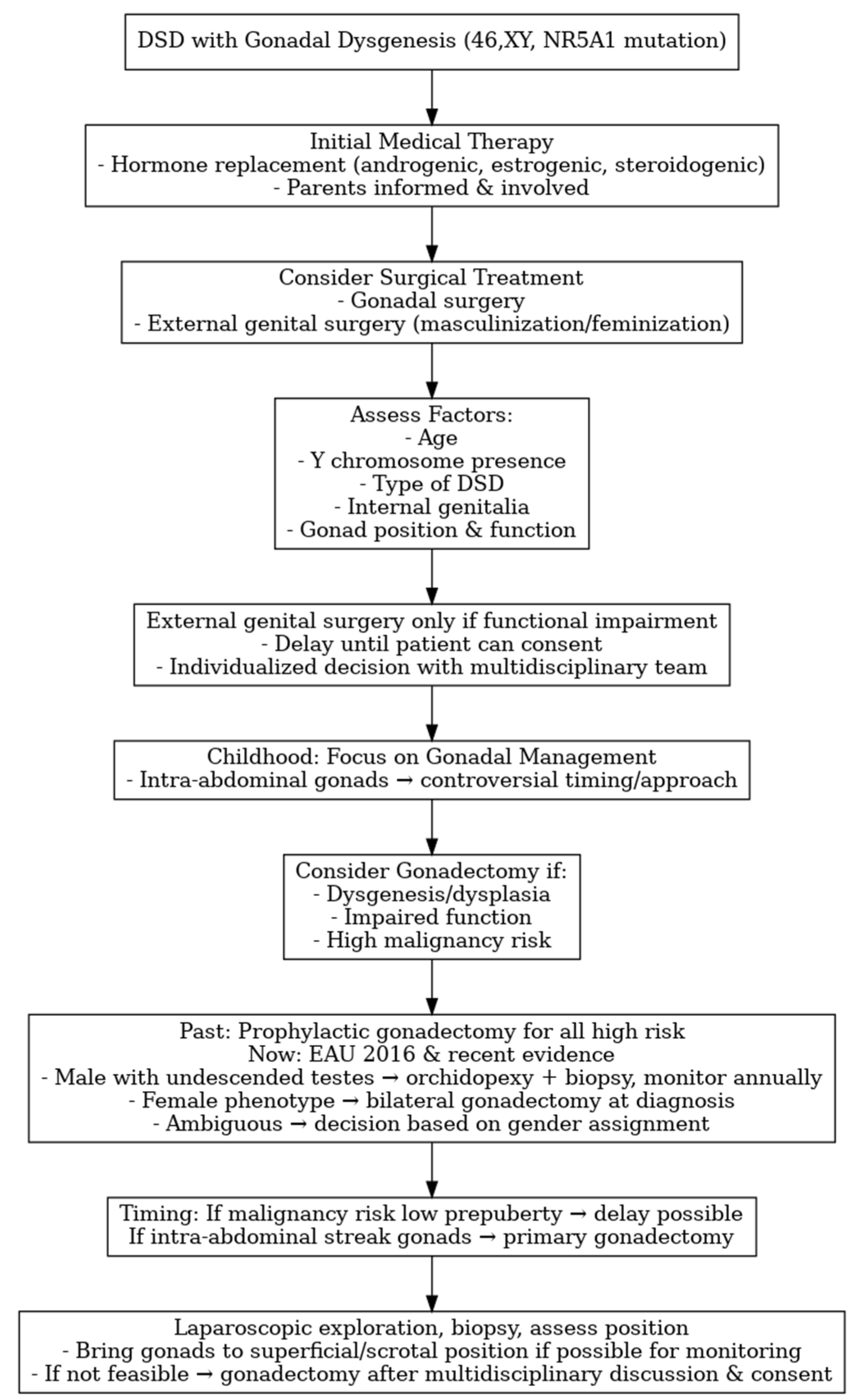
3.8. Surgical Management
3.9. Quality of Life in Children and Adolescents
3.10. Reproductive Potential in NR5A1-Related Conditions
- -
- Inheritance and recurrence risk. Most NR5A1 variants in affected families are heterozygous and behave as autosomal-dominant alleles with variable expressivity and incomplete penetrance; De Novo and (rarely) mosaic variants are also described [36]. Consequently, a heterozygous carrier has a 50% transmission risk to each child, but clinical outcomes range from severe 46,XY DSD to primary ovarian insufficiency (POI) in 46,XX or even apparently unaffected carriers, underscoring the need for individualized counseling.
- -
- Fertility potential and options—46,XY. Spermatogenic impairment is frequent (from oligo- to azoospermia), but biological paternity is possible in selected cases, occasionally spontaneously and more often via assisted reproductive techniques (ART), including intracytoplasmic sperm injection (ICSI) with ejaculated sperm or surgically retrieved sperm (TESE/micro-TESE) when feasible. Early semen analysis and consideration of sperm banking are advisable in adolescents/young adults. If no viable sperm is available, donor sperm or adoption should be discussed. Given the 50% transmission risk and phenotype variability, couples should be offered Preimplantation Genetic Testing for Monogenic (PGT-M) or prenatal diagnosis when proceeding with biological reproduction [46].
- -
- Fertility potential and options—46,XX. Many female carriers are asymptomatic, but a relevant proportion present with diminished ovarian reserve/POI, sometimes early [46]. Time-sensitive fertility preservation (oocyte or embryo cryopreservation) should be discussed before ovarian failure, and spontaneous pregnancies have been reported in some carriers. When ovarian reserve is depleted, oocyte donation is an effective option. As above, the inheritance risk is 50% with unpredictable expressivity in offspring (risk of 46,XY DSD in sons or POI in daughters).
- -
- Shared decision-making. Current DSD consensus statements recommend that reproductive counseling accompany genetic diagnosis and long-term follow-up, integrating ART feasibility, PGT-M/prenatal testing, and psychosocial preferences within a multidisciplinary team (endocrinology, genetics, urology/gynecology, psychology) [4,5].
4. Discussion
5. Practical Guidance: When to Suspect and How to Test for NR5A1 Variants
5.1. Clinical Scenarios in Which NR5A1 Testing Should Be Considered
- -
- 46,XY newborn or infant with atypical genitalia (proximal hypospadias, micropenis, bifid scrotum, or bilateral undescended/dysgenetic testes) and normal 17-OHP levels (ruling out CAH); endocrine profile may show low/normal AMH and inhibin B with variable testosterone response to hCG.
- -
- 46,XY DSD with gonadal dysgenesis features, discordant external genitalia vs. hormonal data, or persistent Müllerian structures.
- -
- Adolescent or adult 46,XY patient with hypergonadotropic hypogonadism or primary testicular failure, particularly if childhood genital anomalies were present.
- -
- Familial clustering or sex-limited expression: 46,XY relatives with undervirilization and/or 46,XX female relatives with primary ovarian insufficiency (POI) or infertility.
- -
- 46,XX testicular or ovotesticular DSD.
- -
- Associated anomalies such as splenic malformations or, rarely, adrenal dysfunction [36].
- -
- Prenatal suspicion of ambiguous genitalia with or without family history of DSD.
5.2. Testing Strategy
- Initial assessment: Perform karyotype and baseline hormonal evaluation (LH, FSH, testosterone ± hCG test, AMH, inhibin B, 17-OHP) within a multidisciplinary DSD team.
- First-tier genetics: Order a NGS DSD multigene panel that includes NR5A1. Trio-based testing (patient and parents) is preferred to clarify inheritance.
- If panel negative or phenotype complex: Escalate to WES or Whole-Genome Sequencing (WGS) to identify additional/oligogenic variants.
- Interpretation: Apply ACMG criteria, evaluate segregation, and consider functional studies for uncertain variants. Discuss variable penetrance and the possibility of apparently unaffected carriers during counseling.
- Follow-up and management: Positive results should trigger risk-adapted gonadal management, tumor surveillance, fertility planning, and inclusion in registries such as I-DSD for structured longitudinal care.
5.3. Where Testing Should Be Performed
- -
- Genetic testing should be undertaken in accredited referral laboratories offering validated DSD multigene panels or WES.
- -
- Prefer national or regional reference centers affiliated with Endo-ERN or DSDnet networks, ensuring standardized interpretation, confirmatory testing, and integration with multidisciplinary management.
- -
- Cross-border clinicians may identify suitable labs via national rare disease networks or I-DSD registry partner centers. Laboratory reports should always be discussed within the multidisciplinary team to align genotype findings with clinical and surgical strategies.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 17-OHP | 17-hydroxyprogestrone |
| aCGH | Array Comparative Genomic Hybridization |
| ACTH | Adrenocorticotropic Hormone |
| AMH | Anti-Müllerian Hormone |
| ART | Assisted Reproductive Techniques |
| CAH | Congenital Adrenal Hyperplasia |
| CAIS | Complete Androgen Insensitivity Syndrome |
| CIS | Carcinoma In Situ |
| DHT | Dihydrotestosterone |
| DSD | Differences of Sex Development |
| FISH | Fluorescence In Situ Hybridization |
| FSH | Follicle-Stimulating Hormone |
| GB | Gonadoblastoma |
| GBY | Gonadoblastoma Locus on the Y Chromosome |
| GCNIS | Germ Cell Neoplasia In Situ |
| GCT | Germ Cell Tumor |
| GD | Gonadal Dysgenesis |
| ICSI | Intracytoplasmic Sperm Injection |
| IRB | Institutional Review Board |
| LH | Luteinizing Hormone |
| MRI | Magnetic Resonance Imaging |
| NGS | Next-Generation Sequencing |
| NIPS | Non-Invasive Prenatal Screening |
| PAIS | Partial Androgen Insensitivity Syndrome |
| PCR | Polymerase Chain Reaction |
| POI | Premature Ovarian Insufficiency |
| PGT-M | Preimplantation Genetic Testing for Monogenic |
| QoL | Quality of Life |
| SF-1 | Steroidogenic Factor-1 |
| SNP | Single Nucleotide Polymorphism |
| TSPY | Testis-Specific Protein Y |
| UGT | Undifferentiated Gonadal Tissue |
| US | Ultrasound |
| WES | Whole-Exome Sequencing |
| WGS | Whole-Genome Sequencing |
References
- Hughes, I.A.; Houk, C.; Ahmed, S.F.; Lee, P.A.; LWPES Consensus Group. ESPE Consensus Group. Consensus statement on management of intersex disorders. Arch. Dis. Child. 2006, 91, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Dreger, A.D.; Chase, C.; Sousa, A.; Gruppuso, P.A.; Frader, J. Changing the nomenclature/taxonomy for intersex: A scientific and clinical rationale. J. Pediatr. Endocrinol. Metab. 2005, 18, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Houk, C.P.; Ahmed, S.F.; Hughes, I.A.; International Consensus. Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics 2006, 118, e488–e500. [Google Scholar] [CrossRef]
- Lee, P.A.; Nordenström, A.; Houk, C.P.; Ahmed, S.F.; Auchus, R.; Baratz, A.; Baratz Dalke, K.; Liao, L.M.; Lin-Su, K.; Looijenga, L.H., 3rd; et al. Global Disorders of Sex Development Update since 2006: Perceptions, Approach and Care. Horm. Res. Paediatr. 2016, 85, 158–180. [Google Scholar] [CrossRef] [PubMed]
- Radmayr, C.; Bogaert, G.; Bujons, A.; Burgu, B.; Castagnetti, M.; Silay, M.S.; O’Kelly, F.; Pakkasjärvi, N.A.; Quaedackers, J.; Rawashdeh, Y.F.H.; et al. EAU Guidelines on Paediatric Urology. In Proceedings of the EAU Annual Congress, Madrid, Spain, 21–24 March 2025; EAU Annual Congress: Madrid, Spain, 2025. ISBN 978-94-92671-29-5. [Google Scholar]
- Bertelloni, S.; Tyutyusheva, N.; Valiani, M.; D’Alberton, F.; Baldinotti, F.; Caligo, M.A.; Baroncelli, G.I.; Peroni, D.G. Disorders/Differences of Sex Development Presenting in the Newborn With 46,XY Karyotype. Front. Pediatr. 2021, 22, 62728. [Google Scholar] [CrossRef]
- Guerrero-Fernández, J.; Azcona San Julián, C.; Barreiro Conde, J.; Bermúdez de la Vega, J.A.; Carcavilla Urquí, A.; Castaño González, L.A.; Martos Tello, J.M.; Rodríguez Estévez, A.; Yeste Fernández, D.; Martínez Martínez, L.; et al. Guía de actuación en las anomalías de la diferenciación sexual (ADS) / desarrollo sexual diferente (DSD) [Management guidelines for disorders / different sex development (DSD)]. An. Pediatr. 2018, 89, 315.e1–315.e19. [Google Scholar] [CrossRef]
- Weidler, E.M.; Ochoa, B.; van Leeuwen, K. Prenatal and postnatal evaluation of differences of sex development: A user’s guide for clinicians and families. Curr. Opin. Pediatr. 2024, 36, 547–553. [Google Scholar] [CrossRef]
- Rey, R.A.; Grinspon, R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 221–238. [Google Scholar] [CrossRef]
- Luo, X.; Ikeda, Y.; Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994, 77, 481–490. [Google Scholar] [CrossRef]
- Achermann, J.C.; Ito, M.; Ito, M.; Hindmarsh, P.C.; Jameson, J.L. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 1999, 22, 125–126. [Google Scholar] [CrossRef]
- Fabbri-Scallet, H.; de Sousa, L.M.; Maciel-Guerra, A.T.; Guerra-Júnior, G.; de Mello, M.P. Mutation update for the NR5A1 gene involved in DSD and infertility. Hum. Mutat. 2020, 41, 58–68. [Google Scholar] [CrossRef]
- Wei, X.; Li, S.; He, Y. NR5A1-related 46,XY partial gonadal dysgenesis: A case report and literature review. Medicine 2023, 102, e36725. [Google Scholar] [CrossRef]
- Faienza, M.F.; Chiarito, M.; Baldinotti, F.; Canale, D.; Savino, C.; Paradies, G.; Corica, D.; Romeo, C.; Tyutyusheva, N.; Caligo, M.A.; et al. NR5A1 Gene Variants: Variable Phenotypes, New Variants, Different Outcomes. Sex. Dev. 2019, 13, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L. A novel heterozygous SF1/NR5A1 gene variant causes 46,XY DSD-gonadal dysgenesis with hypergonadotropic hypogonadism without adrenal insufficiency. Genes. Dis. 2023, 11, 101160. [Google Scholar] [CrossRef]
- Luppino, G.; Wasniewska, M.; Coco, R.; Pepe, G.; Morabito, L.A.; Li Pomi, A.; Corica, D.; Aversa, T. Role of NR5A1 Gene Mutations in Disorders of Sex Development: Molecular and Clinical Features. Curr. Issues. Mol. Biol. 2024, 46, 4519–4532. [Google Scholar] [CrossRef] [PubMed]
- Camats, N.; Flück, C.E.; Audí, L. Oligogenic Origin of Differences of Sex Development in Humans. Int. J. Mol. Sci. 2020, 21, 1809. [Google Scholar] [CrossRef]
- Morohashi, K.I.; Inoue, M.; Baba, T. Coordination of Multiple Cellular Processes by NR5A1/Nr5a1. Endocrinol. Metab. 2020, 35, 756–764. [Google Scholar]
- Hattori, A.; Fukami, M. Nuclear Receptor Gene Variants Underlying Disorders/Differences of Sex Development through Abnormal Testicular Development. Biomolecules 2023, 13, 691. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008, 453, 930–934. [Google Scholar] [CrossRef]
- Josso, N.; Picard, J.Y. Genetics of anti-Müllerian hormone and its signaling pathway. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101634. [Google Scholar] [CrossRef]
- Josso, N.; Rey, R.A. What Does AMH Tell Us in Pediatric Disorders of Sex Development? Front. Endocrinol. 2020, 11, 619. [Google Scholar] [CrossRef]
- Tremblay, J.J.; Robert, N.M. Role of nuclear receptors in INSL3 gene transcription in Leydig cells. Ann. N. Y. Acad. Sci. 2005, 1061, 183–189. [Google Scholar] [CrossRef]
- Takayama, K.; Sasano, H.; Fukaya, T.; Morohashi, K.; Suzuki, T.; Tamura, M.; Costa, M.J.; Yajima, A. Immunohistochemical localization of Ad4-binding protein with correlation to steroidogenic enzyme expression in cycling human ovaries and sex cord stromal tumors. J. Clin. Endocrinol. Metab. 1995, 80, 2815–2821. [Google Scholar]
- Suntharalingham, J.P.; Buonocore, F.; Duncan, A.J.; Achermann, J.C. DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 607–619. [Google Scholar] [CrossRef]
- Lin, L.; Philibert, P.; Ferraz-de-Souza, B.; Kelberman, D.; Homfray, T.; Albanese, A.; Molini, V.; Sebire, N.J.; Einaudi, S.; Conway, G.S.; et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J. Clin. Endocrinol. Metab. 2007, 92, 991–999. [Google Scholar] [CrossRef]
- Tuhan, H.; Anik, A.; Catli, G.; Onay, H.; Aykut, A.; Abaci, A.; Bober, E. A novel mutation in steroidogenic factor (SF1/NR5A1) gene in a patient with 46 XY DSD without adrenal insufficiency. Andrologia 2017, 49, e12589. [Google Scholar] [CrossRef] [PubMed]
- Mönig, I.; Schneidewind, J.; Johannsen, T.H.; Juul, A.; Werner, R.; Lünstedt, R.; Birnbaum, W.; Marshall, L.; Wünsch, L.; Hiort, O. Pubertal development in 46,XY patients with NR5A1 mutations. Endocrine 2022, 75, 601–613. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Eliades, A.; Papatheodoropoulou, A.; Sertedaki, A.; Sinopidis, X.; Tzelepi, V.; Jang, S.; Seo, G.H.; Chrysis, D. 46,ΧΥ DSD in an adolescent with a novel de novo variant of the NR5A1 gene-case report and literature review. Hormones 2025, 24, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cools, M.; Hoebeke, P.; Wolffenbuttel, K.P.; Stoop, H.; Hersmus, R.; Barbaro, M.; Wedell, A.; Brüggenwirth, H.; Looijenga, L.H.; Drop, S.L. Pubertal androgenization and gonadal histology in two 46,XY adolescents with NR5A1 mutations and predominantly female phenotype at birth. Eur. J. Endocrinol. 2012, 166, 341–349. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J.; Baker, P.J.; Johnston, H. The foetal Leydig cell-- differentiation, function and regulation. Int. J. Androl. 2006, 29, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Domenice, S.; Batista, R.L.; Arnhold, I.J.P.; Sircili, M.H.; Costa, E.M.F.; Mendonca, B.B. 46,XY Differences of Sexual Development. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar] [PubMed]
- Adachi, M.; Hasegawa, T.; Tanaka, Y.; Asakura, Y.; Hanakawa, J.; Muroya, K. Spontaneous virilization around puberty in NR5A1-related 46,XY sex reversal: Additional case and a literature review. Endocr. J. 2018, 65, 1187–1192. [Google Scholar] [CrossRef]
- Ferraz-de-Souza, B.; Lin, L.; Achermann, J.C. Steroidogenic factor-1 (SF-1, NR5A1) and human disease. Mol. Cell. Endocrinol. 2011, 336, 198–205. [Google Scholar] [CrossRef]
- Schimmer, B.P.; White, P.C. Minireview: Steroidogenic factor 1: Its roles in differentiation, development, and disease. Mol. Endocrinol. 2010, 24, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Kouri, C.; Sommer, G.; Martinez de Lapiscina, I.; Elzenaty, R.N.; Tack, L.J.W.; Cools, M.; Ahmed, S.F.; Flück, C.E.; SF1next study group. Clinical and genetic characteristics of a large international cohort of individuals with rare NR5A1/SF-1 variants of sex development. EBioMedicine 2024, 99, 104941. [Google Scholar] [CrossRef] [PubMed]
- Atta, I.; Ibrahim, M.; Parkash, A.; Lone, S.W.; Khan, Y.N.; Raza, J. Etiological diagnosis of undervirilized male/XY disorder of sex development. J. Coll. Physicians Surg. Pak. 2014, 24, 714–718. [Google Scholar] [PubMed]
- Wisniewski, A.B.; Batista, R.L.; Costa, E.M.F.; Finlayson, C.; Sircili, M.H.P.; Dénes, F.T.; Domenice, S.; Mendonca, B.B. Management of 46,XY Differences/Disorders of Sex Development (DSD) Throughout Life. Endocr. Rev. 2019, 40, 1547–1572. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Zalel, Y.; Smith, E.; Mazkereth, R.; Aviram, A.; Lipitz, S.; Achiron, R. Prenatal diagnosis of sex differentiation disorders: The role of fetal ultrasound. J. Clin. Endocrinol. Metab. 2002, 87, 4547–4553. [Google Scholar] [CrossRef]
- Pedace, L.; Laino, L.; Preziosi, N.; Valentini, M.S.; Scommegna, S.; Rapone, A.M.; Guarino, N.; Boscherini, B.; De Bernardo, C.; Marrocco, G.; et al. Longitudinal hormonal evaluation in a patient with disorder of sexual development, 46,XY karyotype and one NR5A1 mutation. Am. J. Med. Genet. A 2014, 164, 2938–2946. [Google Scholar] [CrossRef]
- Kulle, A.; Krone, N.; Holterhus, P.M.; Schuler, G.; Greaves, R.F.; Juul, A.; de Rijke, Y.B.; Hartmann, M.F.; Saba, A.; Hiort, O.; et al. Steroid hormone analysis in diagnosis and treatment of DSD: Position paper of EU COST Action BM 1303 ‘DSDnet’. Eur. J. Endocrinol. 2017, 176, P1–P9. [Google Scholar] [CrossRef]
- Bertelloni, S.; Dati, E.; Baldinotti, F.; Toschi, B.; Marrocco, G.; Sessa, M.R.; Michelucci, A.; Simi, P.; Baroncelli, G.I. NR5A1 gene mutations: Clinical, endocrine and genetic features in two girls with 46,XY disorder of sex development. Horm. Res. Paediatr. 2014, 81, 104–108. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Tong, Y.; Li, M.; Meng, L.; Song, Q.; Xin, Y. A novel c.64G > T (p.G22C) NR5A1 variant in a Chinese adolescent with 46,XY disorders of sex development: A case report. BMC Pediatr. 2023, 23, 182. [Google Scholar] [CrossRef]
- Hughes, L.A.; McKay-Bounford, K.; Webb, E.A.; Dasani, P.; Clokie, S.; Chandran, H.; McCarthy, L.; Mohamed, Z.; Kirk, J.M.W.; Krone, N.P.; et al. Next generation sequencing (NGS) to improve the diagnosis and management of patients with disorders of sex development (DSD). Endocr. Connect. 2019, 8, 100–110. [Google Scholar] [CrossRef]
- Guerra-Junior, G.; Andrade, K.C.; Barcelos, I.H.K.; Maciel-Guerra, A.T. Imaging Techniques in the Diagnostic Journey of Disorders of Sex Development. Sex. Dev. 2018, 12, 95–99. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Achermann, J.C.; Arlt, W.; Balen, A.; Conway, G.; Edwards, Z.; Elford, S.; Hughes, I.A.; Izatt, L.; Krone, N.; et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin. Endocrinol. 2016, 84, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Tafazzoli, K.; Wünsch, L.; Bouteleux, M.; Lindert, J.; Schulz, T.; Birnbaum, W.; Marshall, L.; Hiort, O.; Tüshaus, L. Endoscopy and Laparoscopy in Disorders of Sex Development. Sex. Dev. 2018, 12, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A. A perspective on the approach to the intersex child born with genital ambiguity. J. Pediatr. Endocrinol. Metab. 2004, 17, 133–140. [Google Scholar] [CrossRef]
- Cashman, S.; Reidy, P.; Cody, K.; Lemay, C. Developing and measuring progress toward collaborative, integrated, interdisciplinary health care teams. J. Interprof. Care 2004, 18, 183–196. [Google Scholar] [CrossRef]
- The Whoqol Group. The World Health Organization Quality of Life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- Wunsch, G.; Gourbin, C. Mortality, morbidity and health in developed societies: A review of data sources. Genus 2018, 74, 2. [Google Scholar] [CrossRef] [PubMed]
- Das, D.V.; Jabbar, P.K.; Gomez, R.; Nambisan, B.; Bhuvitha, M.S.; Nair, A.; Jayakumari, C. Prevalence, distribution, and risk markers for the development of gonadal germ cell tumors in patients with certain types of disorders of sexual differentiation with Y chromosome-A retrospective study. Indian J. Cancer 2023, 60, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Cools, M.; Wolffenbuttel, K.P.; Drop, S.L.; Oosterhuis, J.W.; Looijenga, L.H. Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex. Dev. 2011, 5, 167–180. [Google Scholar] [CrossRef]
- Cools, M.; Drop, S.L.; Wolffenbuttel, K.P.; Oosterhuis, J.W.; Looijenga, L.H. Germ cell tumors in the intersex gonad: Old paths, new directions, moving frontiers. Endocr. Rev. 2006, 27, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Wolffenbuttel, K.P.; Hersmus, R.; Stoop, H.; Biermann, K.; Hoebeke, P.; Cools, M.; Looijenga, L.H. Gonadal dysgenesis in disorders of sex development: Diagnosis and surgical management. J. Pediatr. Urol. 2016, 12, 411–416. [Google Scholar] [CrossRef]
- Hewitt, J.; Zacharin, M. Hormone replacement in disorders of sex development: Current thinking. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Barthold, J.S. Disorders of sex differentiation: A pediatric urologist’s perspective of new terminology and recommendations. J. Urol. 2011, 185, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Vidal, I.; Gorduza, D.B.; Haraux, E.; Gay, C.L.; Chatelain, P.; Nicolino, M.; Mure, P.Y.; Mouriquand, P. Surgical options in DSD with ambigous genitalia. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 311–324. [Google Scholar] [CrossRef]
- van der Zwan, Y.G.; Biermann, K.; Wolffenbuttel, K.P.; Cools, M.; Looijenga, L.H. Gonadal maldevelopment as risk factor for germ cell cancer: Towards a clinical decision model. Eur. Urol. 2015, 67, 692–701. [Google Scholar] [CrossRef]
- Cools, M.; Looijenga, L.H.; Wolffenbuttel, K.P.; T’Sjoen, G. Managing the risk of germ cell tumourigenesis in disorders of sex development patients. Endocr. Dev. 2014, 27, 185–196. [Google Scholar]
- Mouriquand, P.D.; Gorduza, D.B.; Gay, C.L.; Meyer-Bahlburg, H.F.; Baker, L.; Baskin, L.S.; Bouvattier, C.; Braga, L.H.; Caldamone, A.C.; Duranteau, L.; et al. Surgery in disorders of sex development (DSD) with a gender issue: If (why), when, and how? J. Pediatr. Urol. 2016, 12, 139–149. [Google Scholar] [CrossRef]
- Selveindran, N.M.; Syed Zakaria, S.Z.; Jalaludin, M.Y.; Rasat, R. Quality of life in children with disorders of sex development. Horm. Res. Paediatr. 2017, 88, 324–330. [Google Scholar] [CrossRef]
- Pilan, B.Ş.; Özbaran, B.; Çelik, D.; Özcan, T.; Özen, S.; Gökşen, D.; Ulman, İ.; Avanoğlu, A.; Tiryaki, S.; Onay, H.; et al. Quality of life and psychological well-being in children and adolescents with disorders of sex development. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 23–33. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, L.; Wan, X.; Li, H.; You, Q.; Gao, L.; Feng, J. Quality of life evaluation in juveniles with disorders of sexual development. Pediatr. Surg. Int. 2012, 28, 1119–1123. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Rode, C.A. The PedsQL: Measurement model for the pediatric quality of life inventory. Med. Care. 1999, 37, 126–139. [Google Scholar] [CrossRef]
- Waehre, A.; Heggeli, C.; Hald, K.; Myhre, A.G.; Diseth, T. A 15-20-year follow-up of mental health, psychosocial functioning and quality of life in a single center sample of individuals with differences in sex development. Health Psychol. Behav. Med. 2022, 10, 837–854. [Google Scholar] [CrossRef]
- Jürgensen, M.; Lux, A.; Wien, S.B.; Kleinemeier, E.; Hiort, O.; Thyen, U. Health-related quality of life in children with disorders of sex development (DSD). Eur. J. Pediatr. 2014, 173, 893–903. [Google Scholar] [CrossRef]
- Ediati, A.; Verrips, G.H.W.; Juniarto, A.Z.; Faradz, S.M.H.; Drop, S.L.S.; Dessens, A.B. Quality of Life in Late-Treated Patients With Disorders of Sex Development: Insights for Patient-Centered Care. Front. Pediatr. 2019, 6, 434. [Google Scholar] [CrossRef]
- Ediati, A.; Juniarto, A.Z.; Birnie, E.; Okkerse, J.; Wisniewski, A.; Drop, S.; Faradz, S.M.H.; Dessens, A. Social stigmatisation in late identified patients with disorders of sex development in Indonesia. BMJ. Paediatr. Open. 2017, 1, e000130. [Google Scholar] [CrossRef] [PubMed]
- Cools, M.; Nordenström, A.; Robeva, R.; Hall, J.; Westerveld, P.; Flück, C.; Köhler, B.; Berra, M.; Springer, A.; Schweizer, K.; et al. Caring for individuals with a difference of sex development (DSD): A Consensus Statement. Nat. Rev. Endocrinol. 2018, 14, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Fraccascia, B.; Sodero, G.; Pane, L.C.; Malavolta, E.; Gola, C.; Pane, L.; Paradiso, V.F.; Nanni, L.; Rigante, D.; Cipolla, C. Complete Androgen Insensitivity Syndrome in a Young Girl with Primary Amenorrhea and Suspected Delayed Puberty: A Case-Based Review of Clinical Management, Surgical Follow-Up, and Oncological Risk. Diseases 2024, 12, 235. [Google Scholar] [CrossRef] [PubMed]
| DSD Category | Definition/Key Features | Main Examples |
|---|---|---|
| 46,XX DSD | Conditions with a 46,XX karyotype and virilized or ambiguous external genitalia, usually due to androgen excess during fetal life. | Congenital adrenal hyperplasia (21-hydroxylase deficiency), maternal androgen exposure, aromatase deficiency |
| 46,XY DSD | Conditions with a 46,XY karyotype and undervirilized or ambiguous genitalia due to defects in testicular development, androgen synthesis, or androgen action. | Partial/complete gonadal dysgenesis, 5a-reductase type 2 deficiency, 17B-HSD deficiency, partial/complete androgen insensitivity syndrome |
| Sex chromosome DSD | Mosaic or structural abnormalities of sex chromosomes resulting in gonadal dysgenesis or mixed gonadal development. Highly variable phenotype from male to ambiguous; asymmetrical gonads; one gonad may be testicular, the other a streak gonad. | 45,X/46,XY mixed gonadal dysgenesis, 45,X/46,XY Turner variants, 47,XXY (Klinefelter syndrome) |
| Ovotesticular DSD | Presence of both ovarian and testicular tissue, either separate or combined as an ovotestis in the same individual, previously referred to as “true hermaphroditism” | 46,XX ovotesticular DSD, 46,XY ovotesticular DSD, chromosomal mosaicism (46,XX/46,XY) |
| Non-hormonal/Non-chromosomal DSD | Anatomical or structural abnormalities of sex development unrelated to chromosomal or hormonal dysfunction. | Cloacal exstrophy, bladder exstrophy-epispadias complex, aphallia, severe micropenis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicario, S.; Escolino, M.; Esposito, G.; Porcaro, M.; Di Mase, R.; Azizoglu, M.; Esposito, C. Clinical Spectrum, Surgical Management, and Outcomes of NR5A1-Related 46,XY Differences of Sex Development: A Narrative Review. Medicina 2025, 61, 1965. https://doi.org/10.3390/medicina61111965
Vicario S, Escolino M, Esposito G, Porcaro M, Di Mase R, Azizoglu M, Esposito C. Clinical Spectrum, Surgical Management, and Outcomes of NR5A1-Related 46,XY Differences of Sex Development: A Narrative Review. Medicina. 2025; 61(11):1965. https://doi.org/10.3390/medicina61111965
Chicago/Turabian StyleVicario, Stefania, Maria Escolino, Giorgia Esposito, Mauro Porcaro, Raffaella Di Mase, Mustafa Azizoglu, and Ciro Esposito. 2025. "Clinical Spectrum, Surgical Management, and Outcomes of NR5A1-Related 46,XY Differences of Sex Development: A Narrative Review" Medicina 61, no. 11: 1965. https://doi.org/10.3390/medicina61111965
APA StyleVicario, S., Escolino, M., Esposito, G., Porcaro, M., Di Mase, R., Azizoglu, M., & Esposito, C. (2025). Clinical Spectrum, Surgical Management, and Outcomes of NR5A1-Related 46,XY Differences of Sex Development: A Narrative Review. Medicina, 61(11), 1965. https://doi.org/10.3390/medicina61111965







