Are HDAC and Glutamine Synthetase Expression Levels Associated with Ga68-DOTATATE PET/CT Data and Prognosis in Gastroenteropancreatic Neuroendocrine Tumours?
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Evaluation of Immunohistochemical Staining
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GEP-NETs | Gastroenteropancreatic neuroendocrine tumours |
| HDAC | Histone deacetylase enzymes |
| GS | Glutamine synthetase |
| SUVmax | Standard uptake value maksimum |
| GLS | Glutaminase |
| PET/CT | Positron emission tomography/Computed tomography |
References
- Das, S.; Dasari, A. Epidemiology, incidence, and prevalence of neuroendocrine neoplasms: Are there global differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, B.; Chen, H. Gastroenteropancreatic neuroendocrine neoplasms: Epidemiology, genetics, and treatment. Front. Endocrinol. 2024, 15, 1424839. [Google Scholar] [CrossRef]
- Perren, A.; Couvelard, A.; Scoazec, J.-Y.; Costa, F.; Borbath, I.; Fave, G.D.; Gorbounova, V.; Gross, D.; Grossman, A.; Jensen, R.T.; et al. ENETS consensus guidelines for the standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and prognostic stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; de Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Moss, S.F.; Chung, D.C.; Jensen, R.T.; Snyderwine, E. Priorities for Improving the Management of Gastroenteropancreatic Neuroendocrine Tumors. J. Natl. Cancer Inst. 2008, 100, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Grunstein, M. 25 years after the nucleosome model: Chromatin modifications. Trends Biochem. Sci. 2000, 25, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.A.; Chabner, B.A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 2009, 27, 5459–5468. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lee, D.H.; Jeon, Y.H.; Yoo, J.; Kim, S.Y.; Lee, S.W.; Cho, H.Y.; Kwon, S.H. Glutamine Synthetase as a Therapeutic Target for Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 1701. [Google Scholar] [CrossRef] [PubMed]
- Janicki, R.H.; Goldstein, L.E.O.N. Glutamine synthetase and renal ammonia metabolism. Am. J. Physiol.-Leg. Content 1969, 216, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- Jin, L.; Alesi, G.N.; Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 2016, 35, 3619–3625. [Google Scholar] [CrossRef]
- Kung, H.N.; Marks, J.R.; Chi, J.T. Glutamine synthetase is a genetic determinant of cell type–specific glutamine independence in breast epithelia. PLoS Genet. 2011, 7, e1002229. [Google Scholar] [CrossRef]
- Eom, M.; Oh, S.S.; Lkhagvadorj, S.; Han, A.; Park, K.H. HDAC1 expression in invasive ductal carcinoma of the breast and its value as a good prognostic factor. Korean J. Pathol. 2012, 46, 311. [Google Scholar] [CrossRef]
- Takahashi, Y.; Dungubat, E.; Kusano, H.; Ganbat, D.; Tomita, Y.; Odgerel, S.; Fukusato, T. Application of immunohistochemistry in the pathological diagnosis of liver tumors. Int. J. Mol. Sci. 2021, 22, 5780. [Google Scholar] [CrossRef]
- Bolzoni, M.; Chiu, M.; Accardi, F.; Vescovini, R.; Airoldi, I.; Storti, P.; Giuliani, N. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: A new attractive target. Blood 2016, 128, 667–679. [Google Scholar] [CrossRef]
- Wanek, J.; Gaisberger, M.; Beyreis, M.; Mayr, C.; Helm, K.; Primavesi, F.; Kiesslich, T. Pharmacological inhibition of class IIA HDACs by LMK-235 in pancreatic neuroendocrine tumor cells. Int. J. Mol. Sci. 2018, 19, 3128. [Google Scholar] [CrossRef]
- Sun, L.; He, Q.; Tsai, C.; Lei, J.; Chen, J.; Makcey, L.V.; Coy, D.H. HDAC inhibitors suppressed small cell lung cancer cell growth and enhanced the suppressive effects of receptor-targeting cytotoxins via upregulating somatostatin receptor II. Am. J. Transl. Res. 2018, 10, 545. [Google Scholar]
- Sun, L.; Qian, Q.; Sun, G.; Mackey, L.V.; Fuselier, J.A.; Coy, D.H.; Yu, C.Y. Valproic acid induces NET cell growth arrest and enhances tumor suppression of the receptor-targeted peptide–drug conjugate via activating somatostatin receptor type II. J. Drug. Target. 2016, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Guenter, R.; Aweda, T.; Carmona Matos, D.M.; Jang, S.; Whitt, J.; Cheng, Y.Q.; Liu, X.M.; Chen, H.; Lapi, S.E.; Jaskula-Sztul, R. Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment. Surgery 2020, 167, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Klomp, M.J.; Dalm, S.U.; van Koetsveld, P.M.; Dogan, F.; de Jong, M.; Hofland, L.J. Comparing the effect of multiple histone deacetylase inhibitors on SSTR2 expression and [111In] in-DOTATATE uptake in NET cells. Cancers 2021, 13, 4905. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.H.; Menda, Y.; Zamba, K.D.; Madsen, M.; O’Dorisio, M.S.; O’Dorisio, T.; Bushnell, D. Potential for increasing uptake of radiolabeled 68Ga-DOTATOC and 123I-MIBG in patients with midgut neuroendocrine tumors using a histone deacetylase inhibitor vorinostat. Cancer Biother. Radiopharm. 2021, 36, 632–641. [Google Scholar] [CrossRef]
- Massironi, S.; Gallo, C.; Coltro, L.; Dell’Anna, G.; Preatoni, P.; Danese, S. Clinical and biological heterogeneity of Grade 2 digestive neuroendocrine neoplasms: Prognostic significance of the 10% Ki-67 index cutoff and implications for treatment strategies. A longitudinal study. J. Endocrinol. Investig. 2025, 48, 1483–1493. [Google Scholar] [CrossRef]
- Refardt, J.; Klomp, M.J.; van Koetsveld, P.M.; Dogan, F.; Konijnenberg, M.; Brabander, T.; Hofland, J. Effect of epigenetic treatment on SST2 expression in neuroendocrine tumour patients. Clin. Transl. Med. 2022, 12, e957. [Google Scholar] [CrossRef]
- Hsu, L.; Tappel, A.L. The intracellular distribution of glutamine synthetase in rat liver and the effect of metals on its activity. J. Cell Comp. Physiol. 1964, 64, 265–270. [Google Scholar] [CrossRef]
- Meister, A.; Griffith, O.W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: Possible chemotherapeutic implications. Cancer Treat. Rep. 1979, 63, 1115–1121. [Google Scholar] [PubMed]
- Gibbs, C.S.; Campbell, K.E.; Wilson, R.H. Sequence of a human glutamine synthetase cDNA. Nucleic Acids Res. 1987, 15, 6293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Salloum, R.M.; Austgen, T.R.; Bland, J.B.; Bland, K.I.; Copeland, E.M., III; Souba, W.W. Tumor regulation of hepatic glutamine metabolism. J. Parenter. Enter. Nutr. 1991, 15, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Espat, N.J.; Bland, K.I.; Copeland, E.M.; Souba, W.W. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann. Surg. 1993, 217, 655–667. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Yi, C.; Liu, Y.; He, Q. [13N] Ammonia positron emission tomographic/computed tomographic imaging targeting glutamine synthetase expression in prostate cancer. Mol. Imaging 2015, 14, 7290-2014. [Google Scholar] [CrossRef]
- Ippolito, J.E.; Piwnica-Worms, D. A fluorescence-coupled assay for gamma aminobutyric acid (GABA) reveals metabolic stress-induced modulation of GABA content in neuroendocrine cancer. PLoS ONE 2014, 9, e88667. [Google Scholar] [CrossRef]
- Castegna, A.; Menga, A. Glutamine synthetase: Localization dictates outcome. Genes 2018, 9, 108. [Google Scholar] [CrossRef]
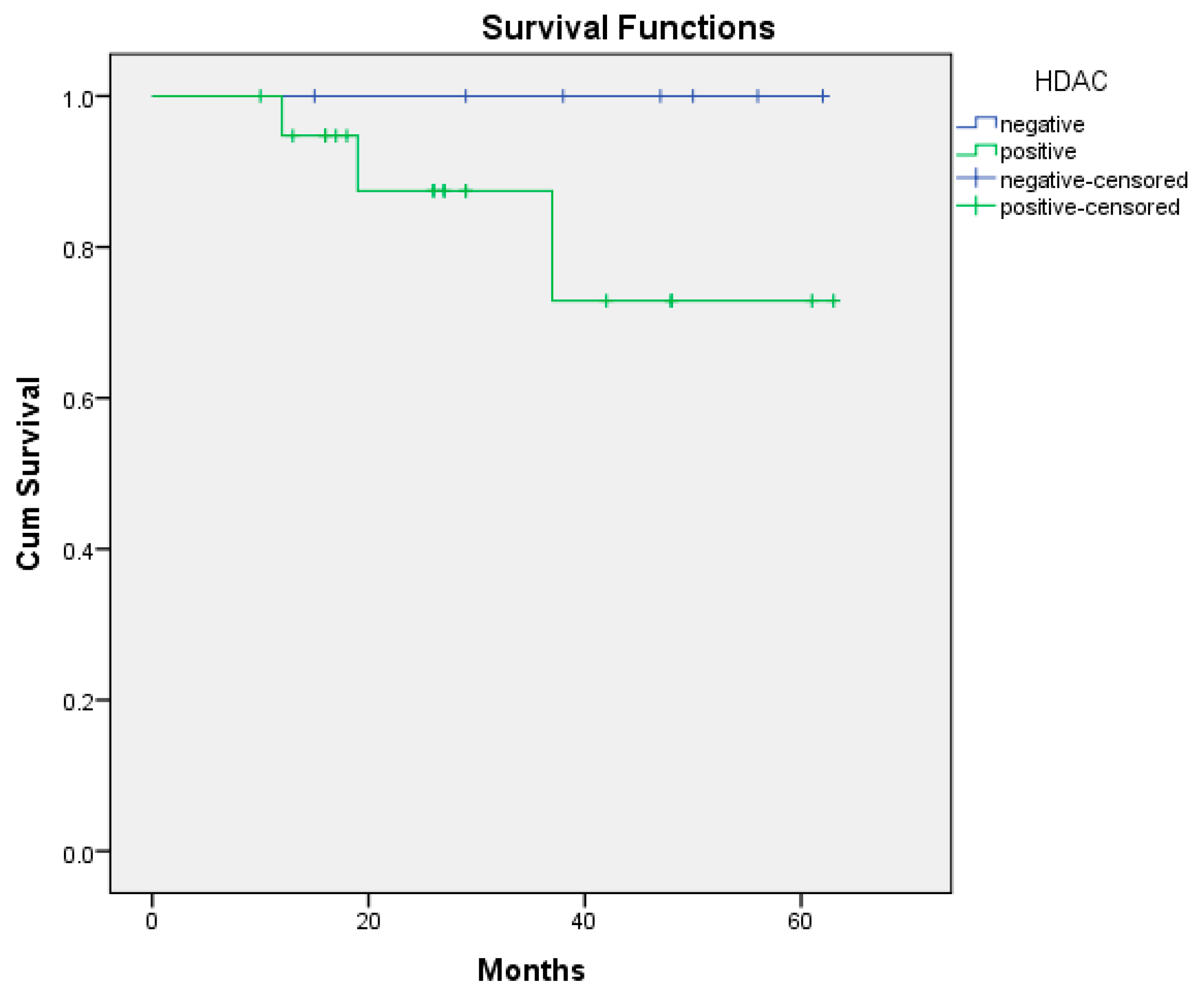
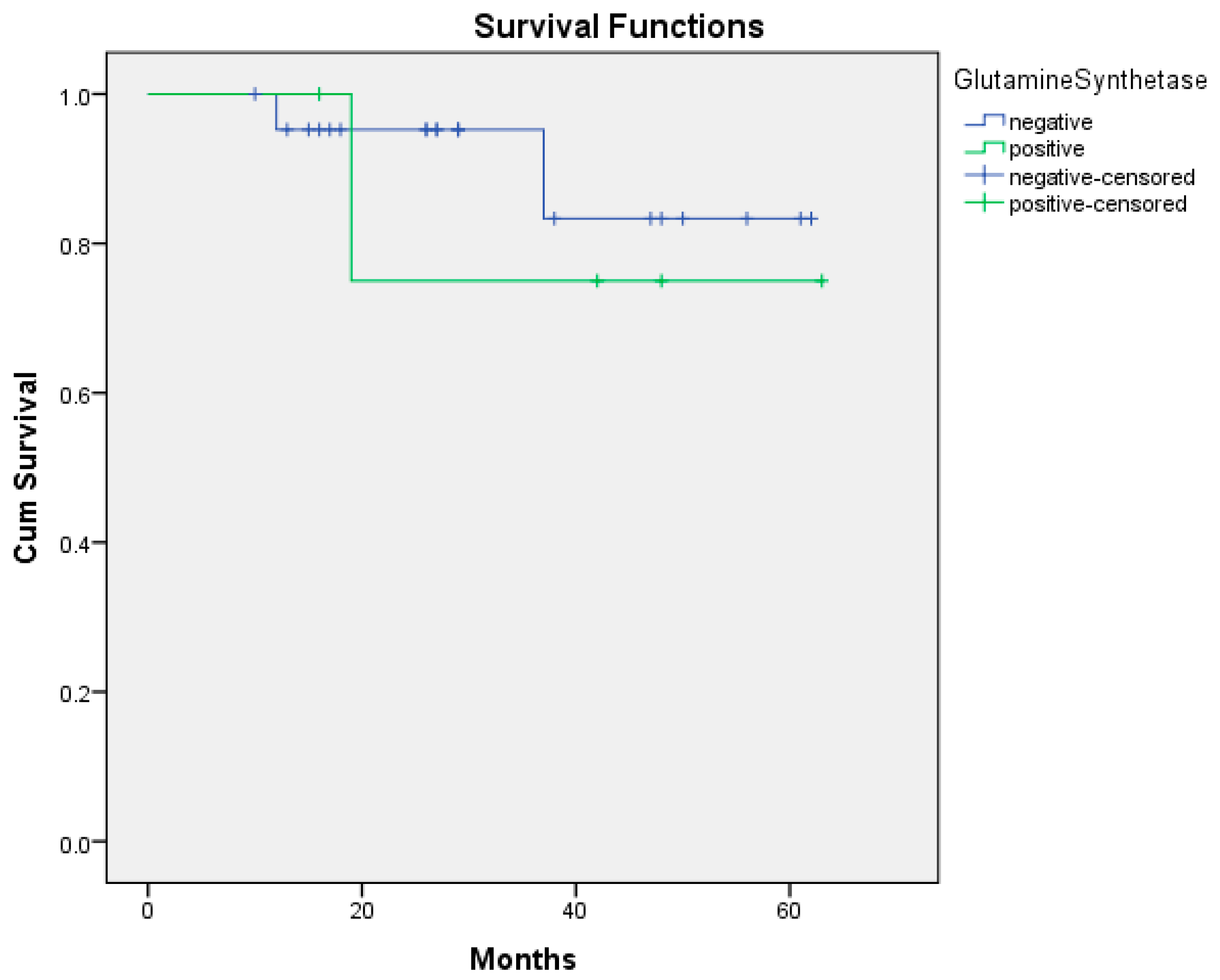
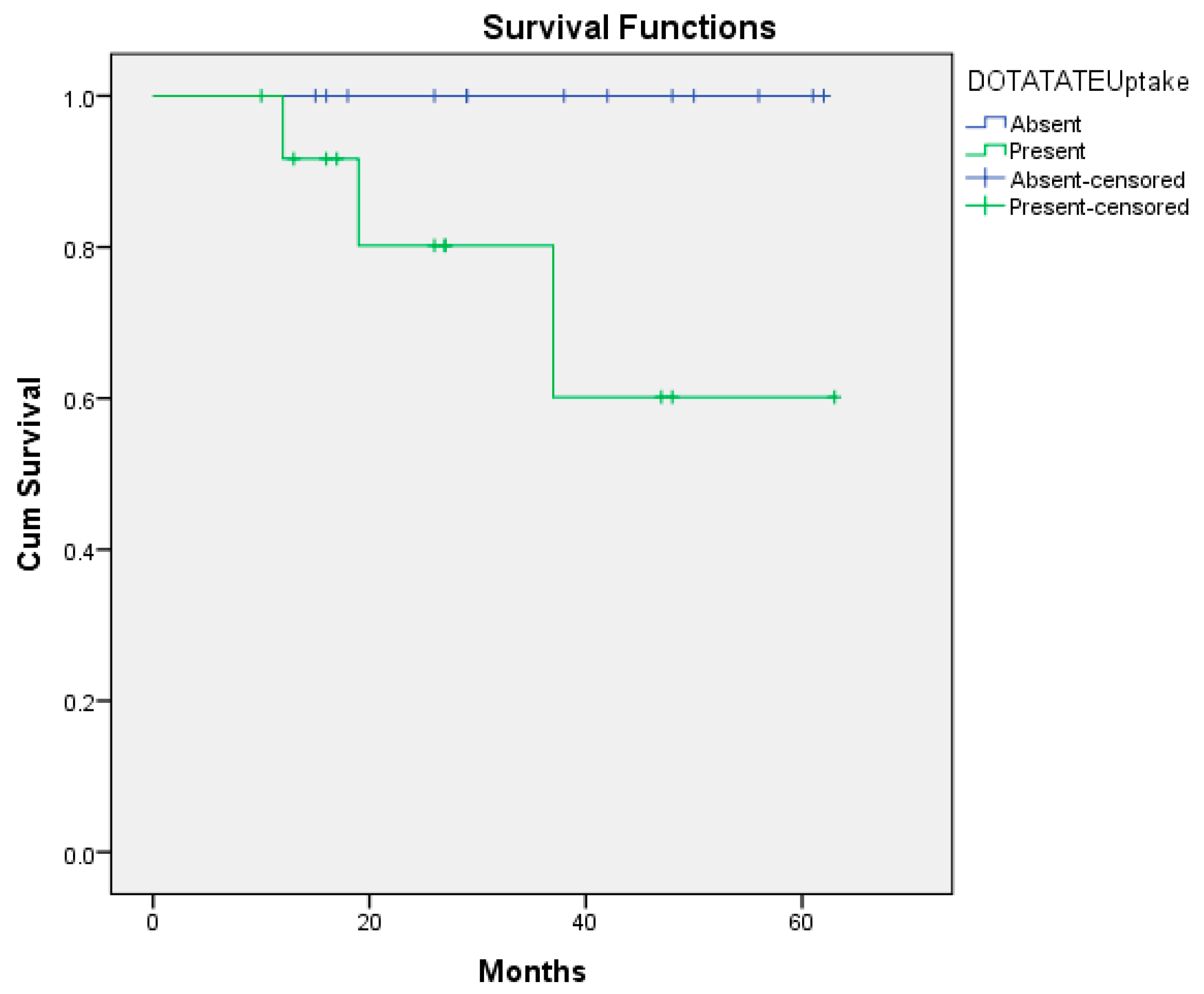
| HDAC-Positive | HDAC-Negative | GS-Positive | GS-Negative | |
|---|---|---|---|---|
| Positive DOTATATE Uptake | 12 (% 80) | 3 (% 20) | 3 (% 20) | 12 (% 80) |
| Negative DOTATATE Uptake | 8 (% 66.7) | 4 (% 33.3) | 2 (% 16.7) | 10 (% 83.3) |
| Sex | ||||
| Female | 12 (% 80) | 3 (% 20) | 3 (% 20) | 12 (% 80) |
| Male | 8 (% 66.7) | 4 (% 33.3) | 2 (% 16.7) | 10 (% 83.3) |
| Age Median (Min–Max) | 57.7 (26–94) | 52 (45–75) | 55 (41–94) | 57 (26–76) |
| Presence of metastasis | ||||
| Yes | 7 (% 87.5) | 1 (% 12.5) | 2 (% 25) | 6 (% 75) |
| No | 13 (% 68.4) | 6 (% 31.6) | 3 (% 15.8) | 16 (% 84.2) |
| Which organ | ||||
| Duodenum | 2 (% 100) | 0 (% 0) | 0 (% 0) | 2 (% 100) |
| Small intestine | 0 (% 0) | 1 (% 100) | 0 (% 0) | 1 (% 100) |
| Jejenum | 1 (% 100) | 0 (% 0) | 0 (% 0) | 1 (% 100) |
| Colon | 1 (% 100) | 0 (% 0) | 0 (% 0) | 1 (% 100) |
| Gastric | 9 (% 75) | 3 (% 25) | 3 (% 25) | 9 (% 75) |
| Pancreas | 4 (% 80) | 1 (% 20) | 2 (% 40) | 3 (% 60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, O.; Yuceer, R.O.; Hasbek, Z.; Ozer, H.; Seker, K.; Yılmaz, M.; Uçar, M. Are HDAC and Glutamine Synthetase Expression Levels Associated with Ga68-DOTATATE PET/CT Data and Prognosis in Gastroenteropancreatic Neuroendocrine Tumours? Medicina 2025, 61, 1952. https://doi.org/10.3390/medicina61111952
Ulas O, Yuceer RO, Hasbek Z, Ozer H, Seker K, Yılmaz M, Uçar M. Are HDAC and Glutamine Synthetase Expression Levels Associated with Ga68-DOTATATE PET/CT Data and Prognosis in Gastroenteropancreatic Neuroendocrine Tumours? Medicina. 2025; 61(11):1952. https://doi.org/10.3390/medicina61111952
Chicago/Turabian StyleUlas, Ozge, Ramazan Oguz Yuceer, Zekiye Hasbek, Hatice Ozer, Kerim Seker, Mukaddes Yılmaz, and Mahmut Uçar. 2025. "Are HDAC and Glutamine Synthetase Expression Levels Associated with Ga68-DOTATATE PET/CT Data and Prognosis in Gastroenteropancreatic Neuroendocrine Tumours?" Medicina 61, no. 11: 1952. https://doi.org/10.3390/medicina61111952
APA StyleUlas, O., Yuceer, R. O., Hasbek, Z., Ozer, H., Seker, K., Yılmaz, M., & Uçar, M. (2025). Are HDAC and Glutamine Synthetase Expression Levels Associated with Ga68-DOTATATE PET/CT Data and Prognosis in Gastroenteropancreatic Neuroendocrine Tumours? Medicina, 61(11), 1952. https://doi.org/10.3390/medicina61111952






