Exosomes in Clinical Laboratory: From Biomarker Discovery to Diagnostic Implementation
Abstract
1. Introduction
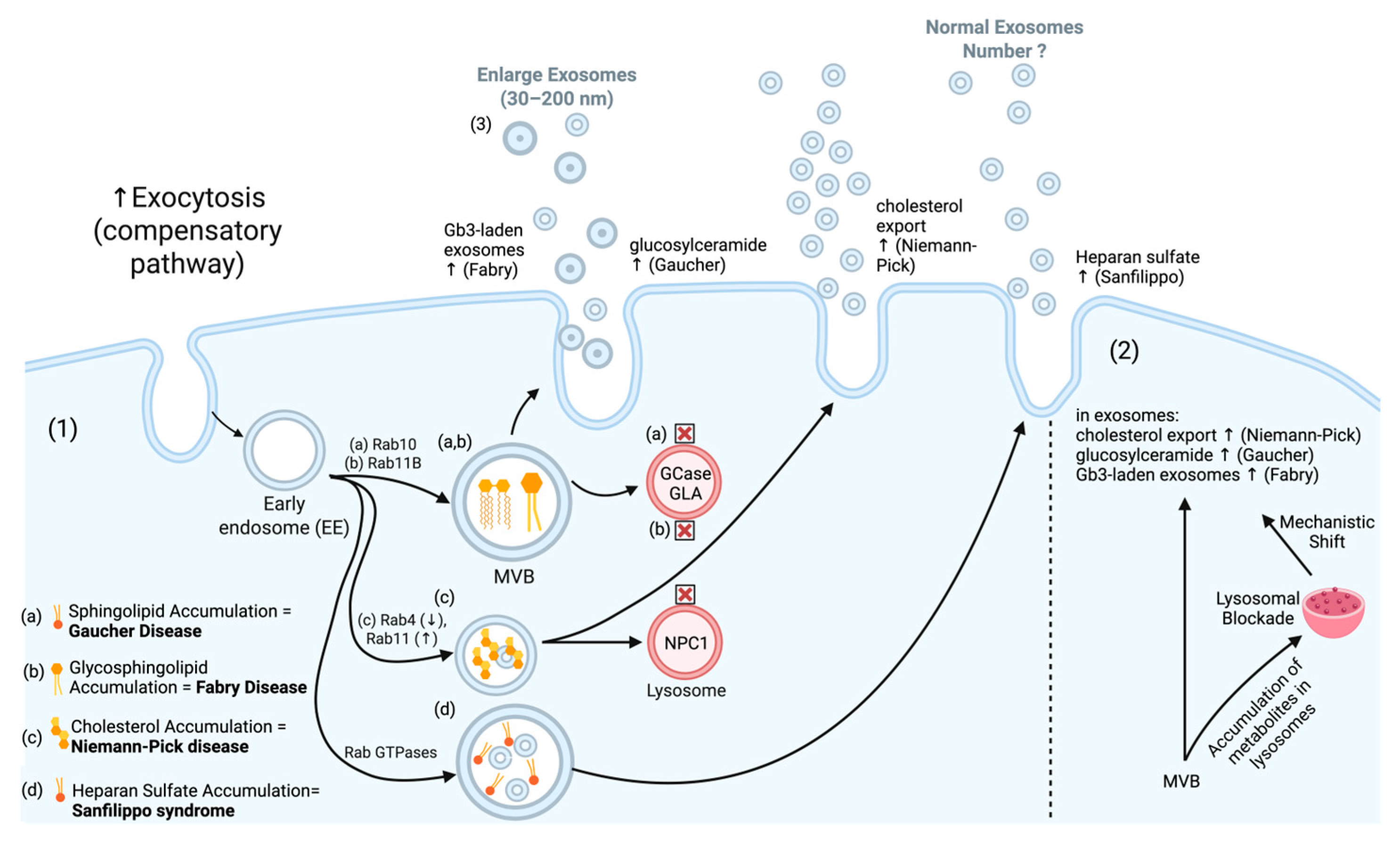
1.1. Exosome Biogenesis and Disruption in Disease
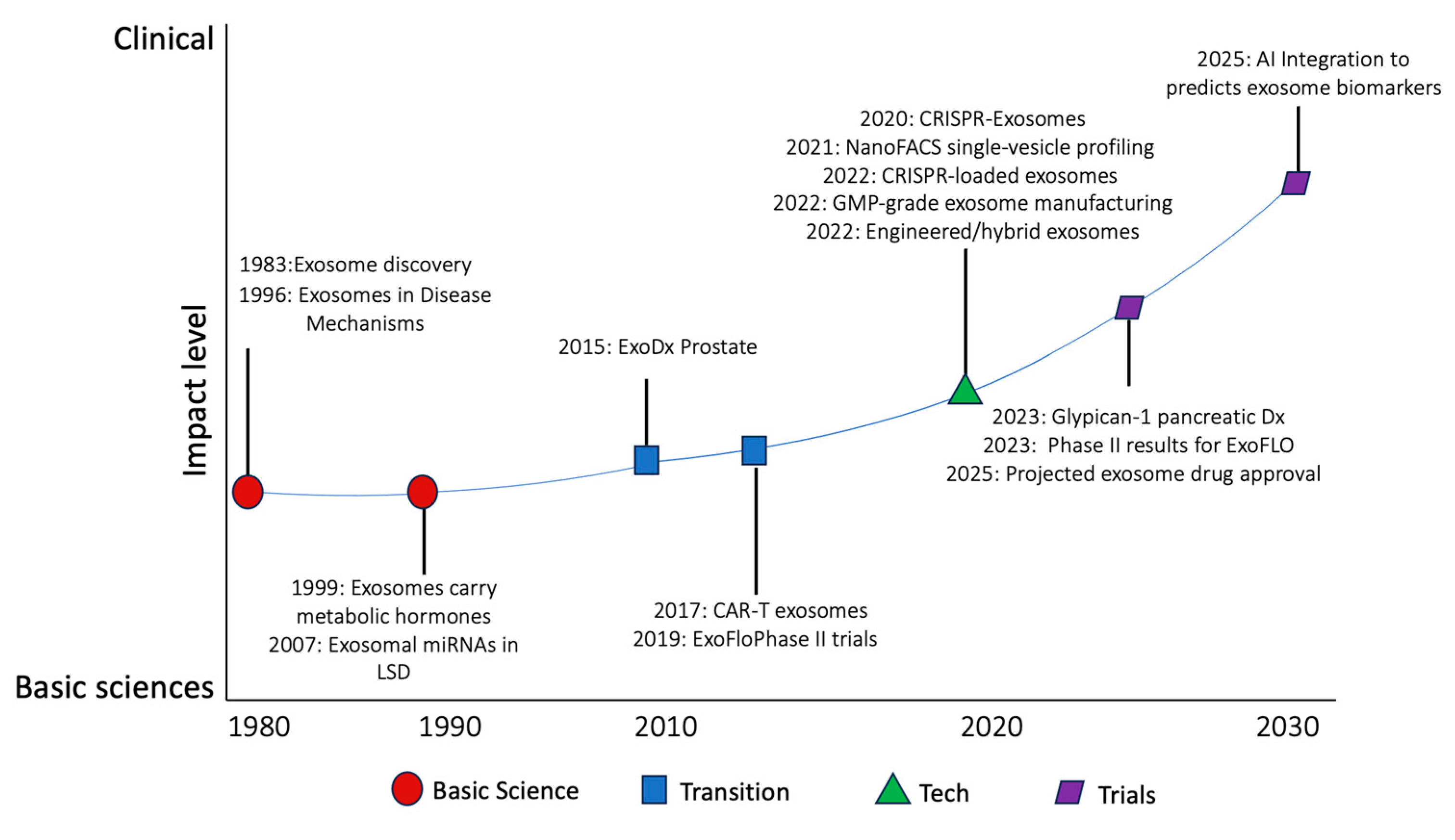
1.2. Clinical Laboratory Early-Stage Development
2. Exosome Biology in Diagnostics
2.1. Exosomal Membrane Proteins in Disease Pathogenesis
2.2. Exosomal Nucleic Acid Cargo in Disease Pathogenesis
| Functional Category | Disease-Relevant Exosomal Elements | Molecular Mechanisms | Disease Applications | Therapeutic/Diagnostic Potential |
|---|---|---|---|---|
| Regulatory RNA | miRNA [145], circRNA [146], tRFs [147], rRFs [153], snoRNA [148], Y RNA [149] | Functional Activity in Recipient Cells [145] mtDNA Released via Exosomes [154] Protected Transport [146] Targeted Delivery [147] Pathogen Transmission/Immune Evasion [151,152,155] | Cancer [145,146,147,150,156,157] Infectious Disease [151,152] Inflammatory Disease [149] Metabolic and Organ-Specific Diseases [154] | Immunomodulation [150] Therapeutic targets [157] Diagnosis of inflammatory and autoimmune diseases [149,154,158] Diagnosis of infectious diseases [151,152,155] Early detection and diagnosis [147,148,157] |
| Protein-Coding RNA | mRNAs [157] | |||
| Pathogen-Derived RNA | Viral RNA [155], Bacterial RNA [152], Parasitic RNA [152] | |||
| Genomic Material | Viral DNA [151], gDNA [150], mtDNA [154], ecDNA [159] |
2.3. Exosomal Protein Cargo in Disease Pathogenesis
2.4. Exosomal Lipids in Disease Pathogenesis
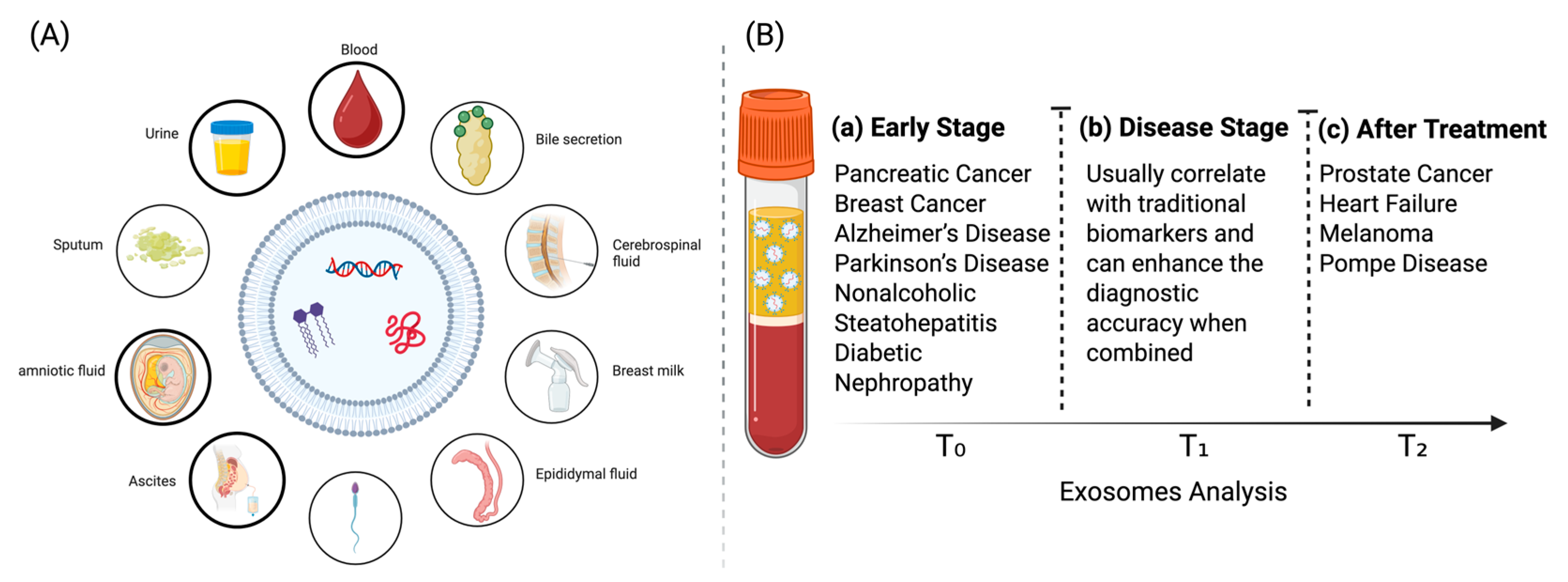
3. Clinically Exosomal Biomarkers
| Disease | Exosomal Biomarker(s) | Clinical Performance (Exosomal) | Standard Diagnostic | Advantages of Exosomal Biomarkers | Clinical Trials |
|---|---|---|---|---|---|
| Pancreatic Cancer | Glypican-1 (GPC1) [35,60] | Sensitivity: 0.88 Specificity: 0.86 AUC: 0.93 | Serum CA19-9 | Detects early-stage disease | NCT02393703, NCT06388967, NCT03791073, |
| Prostate Cancer | ExoDx Prostate IntelliScore (EPI) [65,66] | NPV: 91.3% | PSA and biopsy | Reduces biopsies by 27–30% and higher negative predictive value | NCT03235687, NCT02702856, NCT04556916 |
| Alzheimer’s Disease | Tau and toxic amyloid-β oligomers [37,203] | 96.4% correct classification | CSF biomarkers (p-Tau181, Aβ42, t-tau) | Allows detection years before clinical symptoms | NCT04388982 |
| Breast Cancer | HER2 exosomes [101,102] | AUC: 0.89 (treatment response) | Tissue biopsy and IHC | Real-time monitoring; predicts therapy efficacy | NCT01840306, NCT01344109 |
| Parkinson’s Disease | α-synuclein oligomers [214] | Sensitivity: 70.1% Specificity: 52.9% AUC: 0.654 | Dopamine transporter (DAT) scan | detects pre-symptomatic stages | NCT05320250 |
| Hepatic Disease | Exosomal miR-155 [211] | 93.6% sensitivity and 94% specificity (AUC: 0.971) | CIV, Hyp, AST | Clinical indicators of different degrees of hepatic necrosis and fibrosis | NCT06342414, NCT05871463 |
| Diabetic Nephropathy | Urinary exosomal WT-1 [207] | Predicts progression | Urinary albumin/creatinine ratio | Early prediction of renal injury | NCT06123871 |
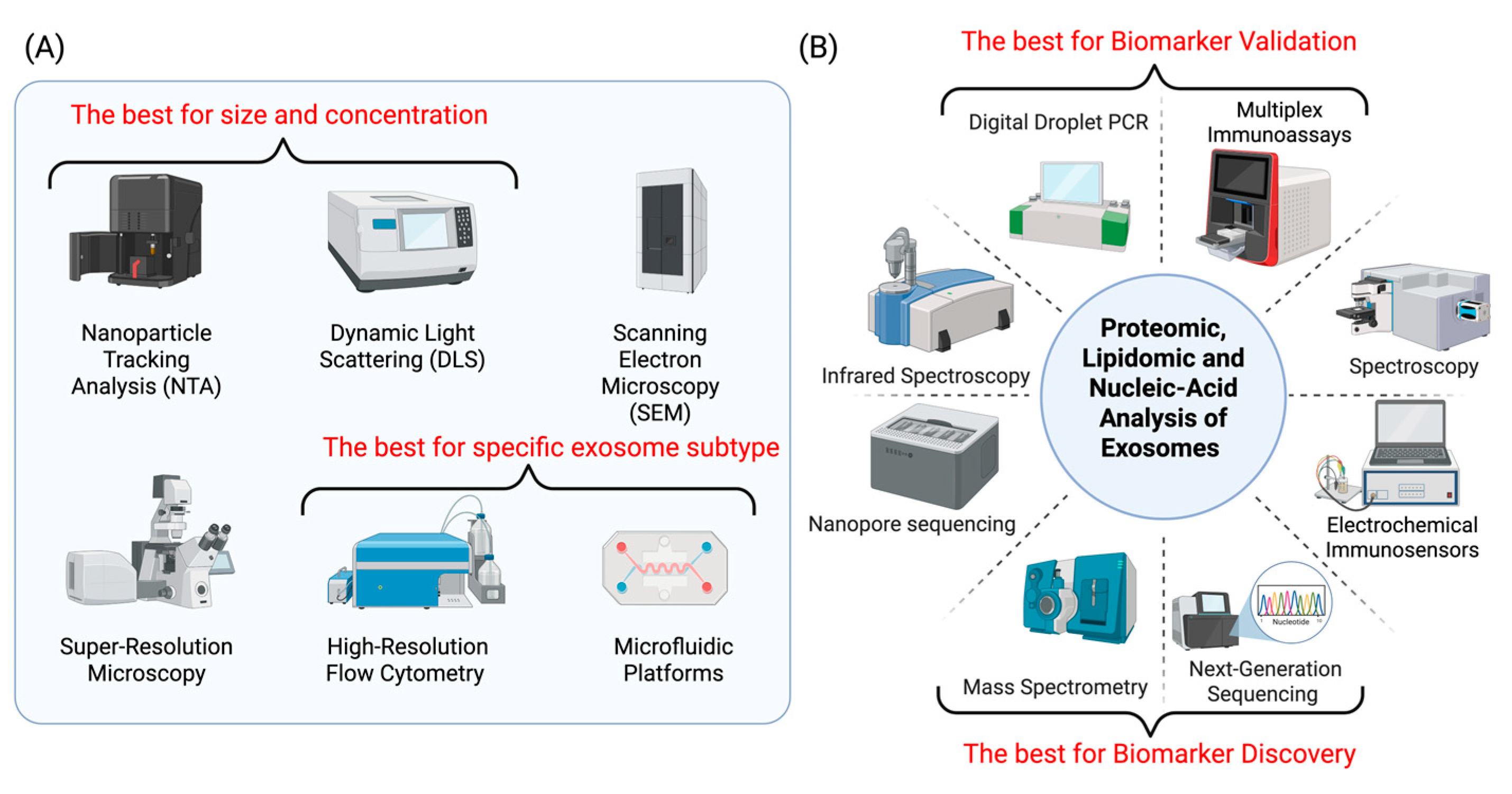
4. Translational Technologies
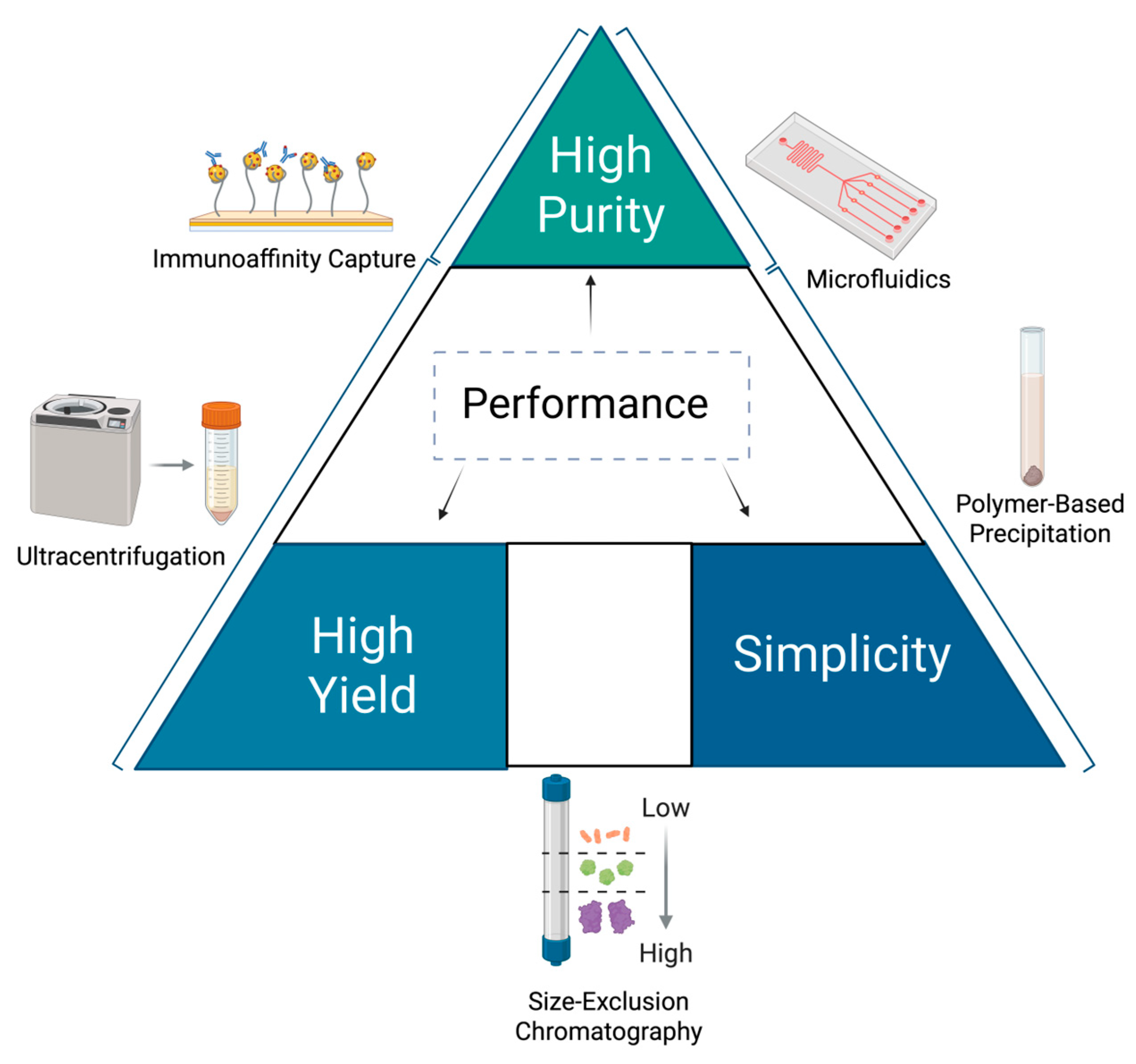
4.1. Isolation and Standardization
4.2. Analytical Platforms
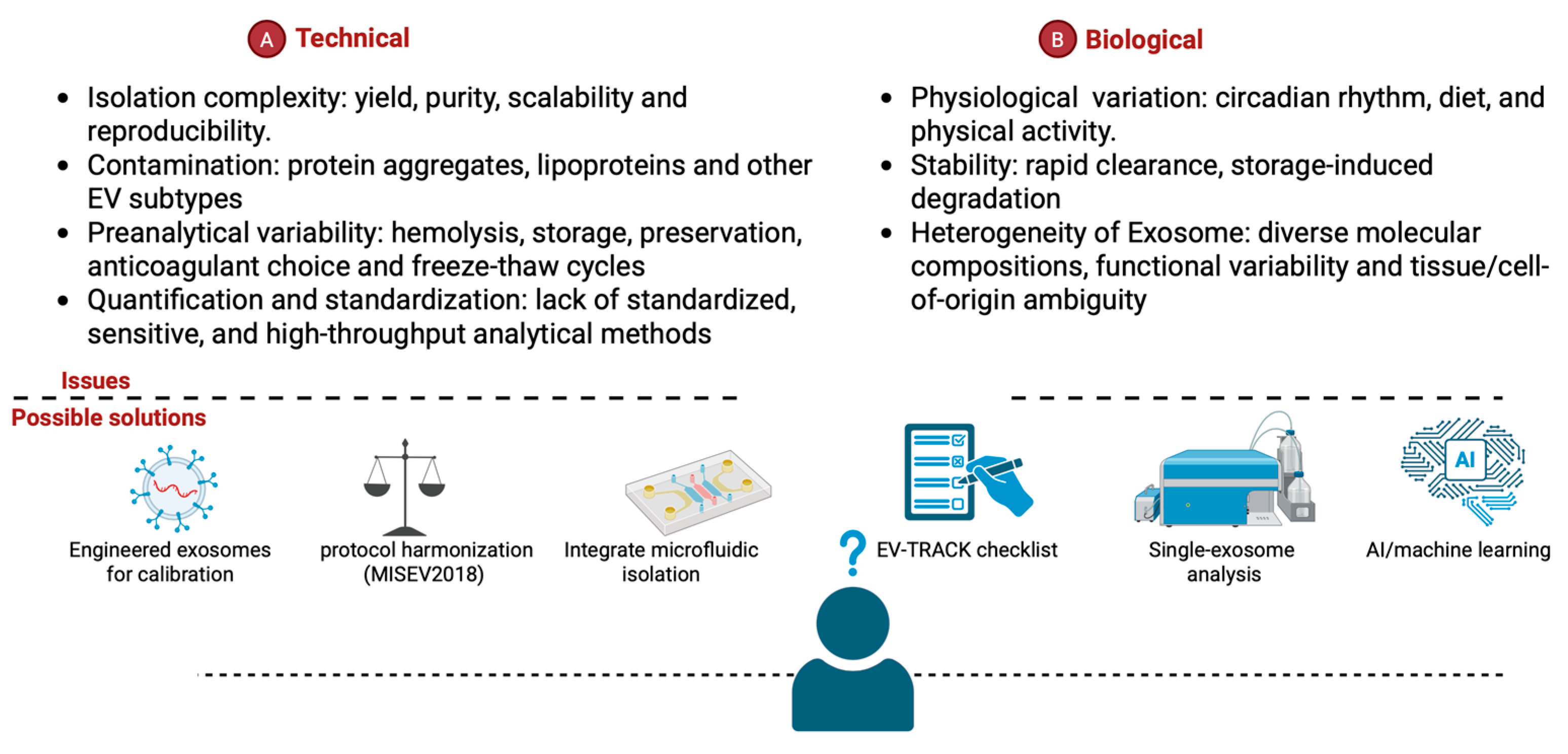
5. Technical and Implementation Challenges
5.1. Preanalytical Variables of Exosomes
5.2. Clinical Laboratory Standardization for Exosomes in Diagnostics
6. Summary and Future Directions
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of Cargo Selection in Exosome Biogenesis and Its Biomedical Applications in Cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Aaronson, S.; Behrens, U.; Orner, R.; Haines, T.H. Ultrastructure of Intracellular and Extracellular Vesicles, Membranes, and Myelin Figures Produced by Ochromonas Danica. J. Ultrastruct. Res. 1971, 35, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta (BBA) Biomembr. 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-Mediated Endocytosis of Transferrin and Recycling of the Transferrin Receptor in Rat Reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.A.; Schekman, R. Distinct Sets of SEC Genes Govern Transport Vesicle Formation and Fusion Early in the Secretory Pathway. Cell 1990, 61, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Block, M.R.; Glick, B.S.; Wilcox, C.A.; Wieland, F.T.; Rothman, J.E. Purification of an N-Ethylmaleimide-Sensitive Protein Catalyzing Vesicular Transport. Proc. Natl. Acad. Sci. USA 1988, 85, 7852–7856. [Google Scholar] [CrossRef] [PubMed]
- Brose, N.; Petrenko, A.G.; Südhof, T.C.; Jahn, R. Synaptotagmin: A Calcium Sensor on the Synaptic Vesicle Surface. Science (1979) 1992, 256, 1021–1025. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Z.; Zhang, T.; Zhang, L.; Liu, X.; Wang, M. The Role of Exosomal Molecular Cargo in Exosome Biogenesis and Disease Diagnosis. Front. Immunol. 2024, 15, 1417758. [Google Scholar] [CrossRef]
- Ofiara, L.M.; Navasakulpong, A.; Beaudoin, S.; Gonzalez, A.V. Optimizing Tissue Sampling for the Diagnosis, Subtyping, and Molecular Analysis of Lung Cancer. Front. Oncol. 2014, 4, 253. [Google Scholar] [CrossRef]
- Hirahata, T.; ul Quraish, R.; ul Quraish, A.; ul Quraish, S.; Naz, M.; Razzaq, M.A. Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer. Cancer Inform. 2022, 21. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Zhang, J.; Monga, S.P.; Dhanasekaran, R. Battle of the Biopsies: Role of Tissue and Liquid Biopsy in Hepatocellular Carcinoma. J. Hepatol. 2024, 80, 515–530. [Google Scholar] [CrossRef]
- Gilson, P.; Merlin, J.-L.; Harlé, A. Deciphering Tumour Heterogeneity: From Tissue to Liquid Biopsy. Cancers 2022, 14, 1384. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Z.; Wang, R.; Li, A.; Wang, H.; He, X.; Shen, Z. Integration of Bioinformatics Analysis and Experimental Validation Identifies Plasma Exosomal miR-103b/877-5p/29c-5p as Diagnostic Biomarkers for Early Lung Adenocarcinoma. Cancer Med. 2022, 11, 4411–4421. [Google Scholar] [CrossRef] [PubMed]
- Ashraf Malik, M.; Ishtiyaq Ali Mirza, J.; Umar, M.; Manzoor, S. CD81 + Exosomes Play a Pivotal Role in the Establishment of Hepatitis C Persistent Infection and Contribute Toward the Progression of Hepatocellular Carcinoma. Viral Immunol. 2019, 32, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood Neuron-Derived Exosomes as Biomarkers of Cognitive Impairment in HIV. Aids 2017, 31, F9–F17. [Google Scholar] [CrossRef] [PubMed]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; da Cruz e Silva, O.A.B.; Henriques, A.G. Exosome Isolation from Distinct Biofluids Using Precipitation and Column-Based Approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef]
- Yao, Y.; Jiao, D.; Li, Z.; Zhou, X.; Li, J.; Liu, Z.; Han, X. Roles of Bile-Derived Exosomes in Hepatobiliary Disease. BioMed Res. Int. 2021, 2021, 8743409. [Google Scholar] [CrossRef]
- Elkommos-Zakhary, M.; Rajesh, N.; Beljanski, V. Exosome RNA Sequencing as a Tool in the Search for Cancer Biomarkers. Noncoding RNA 2022, 8, 75. [Google Scholar] [CrossRef]
- Satyadev, N.; Rivera, M.I.; Nikolov, N.K.; Fakoya, A.O.J. Exosomes as Biomarkers and Therapy in Type 2 Diabetes Mellitus and Associated Complications. Front. Physiol. 2023, 14, 1241096. [Google Scholar] [CrossRef]
- Pérez-Macedonio, C.P.; Flores-Alfaro, E.; Alarcón-Romero, L.d.C.; Vences-Velázquez, A.; Castro-Alarcón, N.; Martínez-Martínez, E.; Ramirez, M. CD14 and CD26 from Serum Exosomes Are Associated with Type 2 Diabetes, Exosomal Cystatin C and CD14 Are Associated with Metabolic Syndrome and Atherogenic Index of Plasma. PeerJ 2022, 10, e13656. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The Lipid Composition of Extracellular Vesicles: Applications in Diagnostics and Therapeutic Delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-Free Detection and Molecular Profiling of Exosomes with a Nano-Plasmonic Sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wei, Y.; Xu, Y.; Zheng, M.; Wang, C.; Zhang, S.; Xie, X.; Ye, C.; Mi, X. Microfluidic Magnetic Detection System Combined with a DNA Framework-Mediated Immune-Sandwich Assay for Rapid and Sensitive Detection of Tumor-Derived Exosomes. Microsyst. Nanoeng. 2023, 9, 139. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Kim, J.; Chakravarty, S.; Park, S.; Gwak, H.; Kim, S.-I.; Mohammadniaei, M.; Lee, M.-H.; Hyun, K.-A.; Jung, H.-I. Detachable Microfluidic Device Implemented with Electrochemical Aptasensor (DeMEA) for Sequential Analysis of Cancerous Exosomes. Biosens. Bioelectron. 2020, 169, 112622. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal MiRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, 1804968. [Google Scholar] [CrossRef]
- Delshad, M.; Sanaei, M.-J.; Mohammadi, M.H.; Sadeghi, A.; Bashash, D. Exosomal Biomarkers: A Comprehensive Overview of Diagnostic and Prognostic Applications in Malignant and Non-Malignant Disorders. Biomolecules 2025, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Park, D.-H.; Kang, J.-H. Exosomes as the Source of Biomarkers of Metabolic Diseases. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Weerakkody, J.S.; Schneider, R.; Miao, S.; Pitt, D. CNS Cell-Derived Exosome Signatures as Blood-Based Biomarkers of Neurodegenerative Diseases. Front. Neurosci. 2024, 18, 1426700. [Google Scholar] [CrossRef]
- Akbar, S.; Raza, A.; Mohsin, R.; Kanbour, A.; Qadri, S.; Parray, A.; Zar Gul, A.R.; Philip, A.; Vijayakumar, S.; Merhi, M.; et al. Circulating Exosomal Immuno-Oncological Checkpoints and Cytokines Are Potential Biomarkers to Monitor Tumor Response to Anti-PD-1/PD-L1 Therapy in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2023, 13, 1097117. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, M.; Nardin, C.; Chanteloup, G.; Derangere, V.; Algros, M.; Arnould, L.; Garrido, C.; Aubin, F.; Gobbo, J. Tracking the Evolution of Circulating Exosomal-PD-L1 to Monitor Melanoma Patients. J. Extracell. Vesicles 2020, 9, 1710899. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Matsubayashi, J.; Kudo, Y.; Maehara, S.; Takeuchi, S.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Nagao, T.; Ikeda, N. Serum-Derived Exosomal PD-L1 Expression to Predict Anti-PD-1 Response and in Patients with Non-Small Cell Lung Cancer. Sci. Rep. 2021, 11, 7830. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Y.; Che, X.; Zhang, M.; Li, Z.; Li, C.; Wang, S.; Wen, T.; Hou, K.; Shao, X.; et al. Anti-PD-1 Therapy Response Predicted by the Combination of Exosomal PD-L1 and CD28. Front. Oncol. 2020, 10, 760. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kapogiannis, D.; Schwartz, J.B.; Lobach, I.V.; Goetzl, L.; Abner, E.L.; Jicha, G.A.; Karydas, A.M.; Boxer, A.; Miller, B.L. Decreased Synaptic Proteins in Neuronal Exosomes of Frontotemporal Dementia and Alzheimer’s Disease. FASEB J. 2016, 30, 4141–4148. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of Preclinical Alzheimer’s Disease by a Profile of Pathogenic Proteins in Neurally Derived Blood Exosomes: A Case-control Study. Alzheimer’s Dement. 2015, 11, 600. [Google Scholar] [CrossRef]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum Neuronal Exosomes Predict and Differentiate Parkinson’s Disease from Atypical Parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating Myocardial MicroRNAs from Infarcted Hearts Are Carried in Exosomes and Mobilise Bone Marrow Progenitor Cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, Y.; Sun, L.; Zhu, W.; Lu, Y.; Zhang, J.; Mao, Y.; Chen, Q.; Zhang, F. Circulating Exosomal MiR-1-3p from Rats with Myocardial Infarction Plays a Protective Effect on Contrast-Induced Nephropathy via Targeting ATG13 and Activating the AKT Signaling Pathway. Int. J. Biol. Sci. 2021, 17, 972–985. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Dong, Z.; Su, Y.; Ji, Y.; Liao, J.; Ma, Y.; Dai, Y.; Yao, K.; Ge, J. Plasma Heat Shock Protein 70 Is Associated With the Onset of Acute Myocardial Infarction and Total Occlusion in Target Vessels. Front. Cardiovasc. Med. 2021, 8, 688702. [Google Scholar] [CrossRef]
- Paulaitis, M.; Agarwal, K.; Nana-Sinkam, P. Dynamic Scaling of Exosome Sizes. Langmuir 2018, 34, 9387–9393. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Kuo, P.-J.; Rau, C.-S.; Wu, Y.-C.; Wu, C.-J.; Lu, T.-H.; Lin, C.-W.; Tsai, C.-W.; Hsieh, C.-H. Subpopulations of Exosomes Purified via Different Exosomal Markers Carry Different MicroRNA Contents. Int. J. Med. Sci. 2021, 18, 1058–1066. [Google Scholar] [CrossRef]
- Leong, S.Y.; Ong, H.B.; Tay, H.M.; Kong, F.; Upadya, M.; Gong, L.; Dao, M.; Dalan, R.; Hou, H.W. Microfluidic Size Exclusion Chromatography (ΜSEC) for Extracellular Vesicles and Plasma Protein Separation. Small 2022, 18, 2104470. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived Exosomes from Plasma of Patients with Melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rizaldos, E.; Zhang, X.; Tadigotla, V.R.; Grimm, D.G.; Karlovich, C.; Raez, L.E.; Skog, J.K. Exosome-Based Detection of Activating and Resistance EGFR Mutations from Plasma of Non-Small Cell Lung Cancer Patients. Oncotarget 2019, 10, 2911–2920. [Google Scholar] [CrossRef]
- Guerrero-Alba, A.; Bansal, S.; Sankpal, A.N.; Mitra, G.; Rahman, M.; Ravichandran, R.; Poulson, C.; Fleming, T.P.; Smith, M.A.; Bremner, R.M.; et al. Enhanced Enrichment of Extracellular Vesicles for Laboratory and Clinical Research from Drop-Sized Blood Samples. Front. Mol. Biosci. 2024, 11, 1365783. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; You, Q.; Su, X.; Chang, Z.; Ge, M.; Mei, Q.; Yang, L.; Dong, W.; Li, L. High-Performance Detection of Exosomes Based on Synergistic Amplification of Amino-Functionalized Fe3O4 Nanoparticles and Two-Dimensional MXene Nanosheets. Sensors 2023, 23, 3508. [Google Scholar] [CrossRef] [PubMed]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The Biogenesis and Functions of Exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Babst, M.; Katzmann, D.J.; Estepa-Sabal, E.J.; Meerloo, T.; Emr, S.D. Escrt-III. Dev. Cell 2002, 3, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Dubot, P.; Astudillo, L.; Therville, N.; Sabourdy, F.; Stirnemann, J.; Levade, T.; Andrieu-Abadie, N. Are Glucosylceramide-Related Sphingolipids Involved in the Increased Risk for Cancer in Gaucher Disease Patients? Review and Hypotheses. Cancers 2020, 12, 475. [Google Scholar] [CrossRef]
- Batta, G.; Soltész, L.; Kovács, T.; Bozó, T.; Mészár, Z.; Kellermayer, M.; Szöllősi, J.; Nagy, P. Alterations in the Properties of the Cell Membrane Due to Glycosphingolipid Accumulation in a Model of Gaucher Disease. Sci. Rep. 2018, 8, 157. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kulabukhova, D.; Landa, S.; Varfolomeeva, E.; Pchelina, S.; Shtam, T. Abstract P-43: Effect of Glucocerebrosidase Dysfunction on the Pool of Plasma Exosomes of Patients with Gaucher Disease. Int. J. Biomed. 2021, 11, S31. [Google Scholar] [CrossRef]
- Tatiana, S.; Stanislav, N.; Darya, K.; Luiza, G.; Konstantin, S.; Sergey, L.; Elena, V.; Galina, S.; Nikolai, V.; Arthur, K.; et al. Altered Level of Plasma Exosomes in Patients with Gaucher Disease. Eur. J. Med. Genet. 2020, 63, 104038. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.; Goebel, C.; Runz, H.; Möbius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome Secretion Ameliorates Lysosomal Storage of Cholesterol in Niemann-Pick Type C Disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar] [CrossRef] [PubMed]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 Sorts Cholesterol into Endosomes for Storage and Distribution via Exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Guix, F.X.; Capitán, A.M.; Casadomé-Perales, Á.; Palomares-Pérez, I.; López del Castillo, I.; Miguel, V.; Goedeke, L.; Martín, M.G.; Lamas, S.; Peinado, H.; et al. Increased Exosome Secretion in Neurons Aging in Vitro by NPC1-Mediated Endosomal Cholesterol Buildup. Life Sci. Alliance 2021, 4, e202101055. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Bujanda, L. Glypican-1 in Exosomes as Biomarker for Early Detection of Pancreatic Cancer. Ann. Transl. Med. 2016, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, B.; Chen, F. Diagnostic Value of Serum Carbohydrate Antigen 19-9 in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-B. Carbohydrate Antigen 19-9 for Differential Diagnosis of Pancreatic Carcinoma and Chronic Pancreatitis. World J. Gastroenterol. 2015, 21, 4323. [Google Scholar] [CrossRef] [PubMed]
- Makler, A.; Asghar, W. Exosomal MiRNA Biomarker Panel for Pancreatic Ductal Adenocarcinoma Detection in Patient Plasma: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 5081. [Google Scholar] [CrossRef]
- Margolis, E.; Brown, G.; Partin, A.; Carter, B.; McKiernan, J.; Tutrone, R.; Torkler, P.; Fischer, C.; Tadigotla, V.; Noerholm, M.; et al. Predicting High-Grade Prostate Cancer at Initial Biopsy: Clinical Performance of the ExoDx (EPI) Prostate Intelliscore Test in Three Independent Prospective Studies. Prostate Cancer Prostatic Dis. 2022, 25, 296–301. [Google Scholar] [CrossRef]
- Tutrone, R.; Lowentritt, B.; Neuman, B.; Donovan, M.J.; Hallmark, E.; Cole, T.J.; Yao, Y.; Biesecker, C.; Kumar, S.; Verma, V.; et al. ExoDx Prostate Test as a Predictor of Outcomes of High-Grade Prostate Cancer—An Interim Analysis. Prostate Cancer Prostatic Dis. 2023, 26, 596–601. [Google Scholar] [CrossRef] [PubMed]
- El Fekih, R.; Franzen, K.; Hurley, J.; Haynes, B.C.; Merhej, T.; Alghamdi, A.; Hallmark, E.; Xing, S.; Kumar, S.; Choi, J.; et al. An Exosomal MRNA Urine Test for Detection and Risk Stratification of Human Kidney Transplant Rejection. Kidney Int. Rep. 2025, 10, 1131–1142. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Z.; Ma, F.; Li, T.; Liu, C.; Wang, N.; Guan, J.; He, N.; Liu, J.; Lu, S.; et al. Exosome-Based Liquid Biopsy in Early Screening and Diagnosis of Cancers. Dose Response 2025, 23. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Bai, S.; Zheng, M.; Chen, W.; Li, Y.; Yang, Y.; Zhao, Y.; Xiong, S.; Wang, R.; Cheng, B. An Exosome-Related LncRNA Signature Correlates with Prognosis, Immune Microenvironment, and Therapeutic Responses in Hepatocellular Carcinoma. Transl. Oncol. 2023, 31, 101651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, D.; Shao, Z.; Wang, X.; Xu, J.; Li, Y.; Li, K. Expression Profiles of Exosomal TRNA-Derived Fragments and Their Biological Functions in Lipomas. Front. Cell Dev. Biol. 2022, 10, 942133. [Google Scholar] [CrossRef] [PubMed]
- Rozek, W.; Kwasnik, M.; Socha, W.; Czech, B.; Rola, J. Profiling of SnoRNAs in Exosomes Secreted from Cells Infected with Influenza A Virus. Int. J. Mol. Sci. 2024, 26, 12. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Chitti, S.V.; Gummadi, S.; Kang, T.; Shahi, S.; Marzan, A.L.; Nedeva, C.; Sanwlani, R.; Bramich, K.; Stewart, S.; Petrovska, M.; et al. Vesiclepedia 2024: An Extracellular Vesicles and Extracellular Particles Repository. Nucleic Acids Res. 2024, 52, D1694–D1698. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kang, B.; Kim, O.Y.; Choi, D.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An Integrated Database of High-throughput Data for Systemic Analyses of Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chai, Z.; Pan, G.; Hao, Y.; Li, B.; Ye, T.; Li, Y.; Long, F.; Xia, L.; Liu, M. ExoBCD: A Comprehensive Database for Exosomal Biomarker Discovery in Breast Cancer. Brief. Bioinform. 2021, 22, bbaa088. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, R.; Song, C.; Hao, M.; Gao, Y.; Xin, M.; Liu, Q.; Chen, H.; Wu, X.; Sun, R.; et al. A Comprehensive Database of Exosome Molecular Biomarkers and Disease-Gene Associations. Sci. Data 2024, 11, 210. [Google Scholar] [CrossRef]
- Sharma, P.; Diergaarde, B.; Ferrone, S.; Kirkwood, J.M.; Whiteside, T.L. Melanoma Cell-Derived Exosomes in Plasma of Melanoma Patients Suppress Functions of Immune Effector Cells. Sci. Rep. 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Kuo-Elsner, W.P.; Müller, C.; Barth, K.; Peris, L.; Tenzer, S.; Möbius, W.; Werner, H.B.; Nave, K.-A.; Fröhlich, D.; et al. Oligodendrocytes Support Axonal Transport and Maintenance via Exosome Secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-Enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Berger, A.C.; Roche, P.A. T Cell-Induced Secretion of MHC Class II–Peptide Complexes on B Cell Exosomes. EMBO J. 2007, 26, 4263–4272. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Cheng, J.C.-H.; Chen, Y.-F.; Yang, J.C.-H.; Hsu, F.-M. Circulating Exosomal Integrin Β3 Is Associated with Intracranial Failure and Survival in Lung Cancer Patients Receiving Cranial Irradiation for Brain Metastases: A Prospective Observational Study. Cancers 2021, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The Role of Exosomal PD-L1 in Tumor Progression and Immunotherapy. Mol. Cancer 2019, 18, 146. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Yamamoto, M.; Yagi, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res. 2021, 19, 1583–1595. [Google Scholar] [CrossRef]
- Ben, X.-Y.; Wang, Y.-R.; Zheng, H.-H.; Li, D.-X.; Ren, R.; Ni, P.-L.; Zhang, H.-Y.; Feng, R.-J.; Li, Y.-Q.; Li, Q.-F.; et al. Construction of Exosomes That Overexpress CD47 and Evaluation of Their Immune Escape. Front. Bioeng. Biotechnol. 2022, 10, 936951. [Google Scholar] [CrossRef] [PubMed]
- Boyne, C.; Coote, A.; Synowsky, S.; Naden, A.; Shirran, S.; Powis, S.J. Characterising the HLA-I Immunopeptidome of Plasma-derived Extracellular Vesicles in Patients with Melanoma. J. Extracell. Biol. 2024, 3, e146. [Google Scholar] [CrossRef]
- Yang, C.; Ruffner, M.A.; Kim, S.; Robbins, P.D. Plasma-derived MHC Class II + Exosomes from Tumor-bearing Mice Suppress Tumor Antigen-specific Immune Responses. Eur. J. Immunol. 2012, 42, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Tey, P.Y.; Dufner, A.; Knobeloch, K.-P.; Pruneda, J.N.; Clague, M.J.; Urbé, S. Rapid Turnover of CTLA4 Is Associated with a Complex Architecture of Reversible Ubiquitylation. J. Cell Biol. 2025, 224, e202312141. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, X.; Li, X.; Fan, H.; Zhang, F.; Lv, T.; Song, Y. Expression Profiles and Clinical Value of Plasma Exosomal Tim-3 and Galectin-9 in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2018, 498, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Sanmiguel, S.; Salazar-Puerta, A.I.; Panic, A.; Dodd, D.; Francis, C.; Alzate-Correa, D.; Ortega-Pineda, L.; Lemmerman, L.; Rincon-Benavides, M.A.; Dathathreya, K.; et al. ICAM-1-Decorated Extracellular Vesicles Loaded with MiR-146a and Glut1 Drive Immunomodulation and Hinder Tumor Progression in a Murine Model of Breast Cancer. Biomater. Sci. 2023, 11, 6834–6847. [Google Scholar] [CrossRef]
- Linton, S.S.; Abraham, T.; Liao, J.; Clawson, G.A.; Butler, P.J.; Fox, T.; Kester, M.; Matters, G.L. Tumor-Promoting Effects of Pancreatic Cancer Cell Exosomes on THP-1-Derived Macrophages. PLoS ONE 2018, 13, e0206759. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; et al. ICAM-1-Mediated Adhesion Is a Prerequisite for Exosome-Induced T Cell Suppression. Dev. Cell 2022, 57, 329–343.e7. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; de Boer, M.; Blokhuis, B.; Berends, A.J.; Garssen, J.; Kraneveld, A.D.; van’t Land, B.; Willemsen, L.E.M. Epithelial-Derived Galectin-9 Containing Exosomes Contribute to the Immunomodulatory Effects Promoted by 2′-Fucosyllactose and Short-Chain Galacto- and Long-Chain Fructo-Oligosaccharides. Front. Immunol. 2022, 13, 1026031. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.E.; Lensing, S.; Chang, D.; Magpantay, L.I.; Mitsuyasu, R.; Ambinder, R.F.; Sparano, J.A.; Martínez-Maza, O.; Epeldegui, M. Plasma Extracellular Vesicles Bearing PD-L1, CD40, CD40L or TNF-RII Are Significantly Reduced after Treatment of AIDS-NHL. Sci. Rep. 2022, 12, 9185. [Google Scholar] [CrossRef] [PubMed]
- Stenqvist, A.-C.; Nagaeva, O.; Baranov, V.; Mincheva-Nilsson, L. Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J. Immunol. 2013, 191, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the Versatile Roles and Applications of EpCAM in Cancers: From Bench to Bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Rupp, A.-K.; Rupp, C.; Keller, S.; Brase, J.C.; Ehehalt, R.; Fogel, M.; Moldenhauer, G.; Marmé, F.; Sültmann, H.; Altevogt, P. Loss of EpCAM Expression in Breast Cancer Derived Serum Exosomes: Role of Proteolytic Cleavage. Gynecol. Oncol. 2011, 122, 437–446. [Google Scholar] [CrossRef]
- Inubushi, S.; Kunihisa, T.; Kuniyasu, M.; Inoue, S.; Yamamoto, M.; Yamashita, Y.; Miki, M.; Mizumoto, S.; Baba, M.; Hoffman, R.M.; et al. Serum Exosomes Expressing CD9, CD63 and HER2 From Breast-Cancer Patients Decreased After Surgery of the Primary Tumor: A Potential Biomarker of Tumor Burden. Cancer Genom. Proteom. 2024, 21, 580–584. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Y.; Bai, X.; Liu, L.; Shan, Y.; Wang, F.; Yu, Z.; Zheng, C. Raman Spectroscopy and Exosome-Based Machine Learning Predicts the Efficacy of Neoadjuvant Therapy for HER2-Positive Breast Cancer. Anal. Chem. 2025, 97, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, K.; Zhang, G.; Jia, Z.; Yu, Y.; Guo, J.; Hua, Y.; Guo, F.; Li, X.; Zou, W.; et al. Enrichment of CD44 in Exosomes From Breast Cancer Cells Treated With Doxorubicin Promotes Chemoresistance. Front. Oncol. 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Shi, H.; Liu, F.; Yu, H.; Shi, H. Exosome GLUT1 Derived from Hepatocyte Identifies the Risk of Non-Alcoholic Steatohepatitis and Fibrosis. Hepatol. Int. 2023, 17, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Ching, K.L.; de Vries, M.; Gago, J.; Dancel-Manning, K.; Sall, J.; Rice, W.J.; Barnett, C.; Khodadadi-Jamayran, A.; Tsirigos, A.; Liang, F.-X.; et al. ACE2-Containing Defensosomes Serve as Decoys to Inhibit SARS-CoV-2 Infection. PLoS Biol. 2022, 20, e3001754. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Popowski, K.D.; Liu, S.; Zhu, D.; Mei, X.; Li, J.; Hu, Y.; Dinh, P.-U.C.; Wang, X.; et al. Inhalation of ACE2-Expressing Lung Exosomes Provides Prophylactic Protection against SARS-CoV-2. Nat. Commun. 2024, 15, 2236. [Google Scholar] [CrossRef]
- Simón, L.; Campos, A.; Leyton, L.; Quest, A.F.G. Caveolin-1 Function at the Plasma Membrane and in Intracellular Compartments in Cancer. Cancer Metastasis Rev. 2020, 39, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Matijašević Joković, S.; Korać, A.; Kovačević, S.; Djordjević, A.; Filipović, L.; Dobrijević, Z.; Brkušanin, M.; Savić-Pavićević, D.; Vuković, I.; Popović, M.; et al. Exosomal Prostate-Specific Membrane Antigen (PSMA) and Caveolin-1 as Potential Biomarkers of Prostate Cancer—Evidence from Serbian Population. Int. J. Mol. Sci. 2024, 25, 3533. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Gao, X.; Yuan, Y.; Zhao, J.; Zhou, S.; Wang, H.; Wang, L.; Xu, G.; Li, X.; et al. Plasma-Derived Exosomal ALIX as a Novel Biomarker for Diagnosis and Classification of Pancreatic Cancer. Front. Oncol. 2021, 11, 628346. [Google Scholar] [CrossRef] [PubMed]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Yue, S.; Mu, W.; Erb, U.; Zöller, M. The Tetraspanins CD151 and Tspan8 Are Essential Exosome Components for the Crosstalk between Cancer Initiating Cells and Their Surrounding. Oncotarget 2015, 6, 2366–2384. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, D.; Shankar, S.; Srivastava, R.K. Biomolecular Characterization of Exosomes Released from Cancer Stem Cells: Possible Implications for Biomarker and Treatment of Cancer. Oncotarget 2015, 6, 3280–3291. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, Z.; Deli, H.; Shadboorestan, A.; Sahebnasagh, R.; Motevaseli, E.; Ghahremani, M.H. Exosomes and Their Distinct Integrins Transfer the Characteristics of Oxaliplatin- and 5-FU-Resistant Behaviors in Colorectal Cancer Cells. Mol. Med. 2025, 31, 49. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma Neuronal Exosomes Serve as Biomarkers of Cognitive Impairment in HIV Infection and Alzheimer’s Disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, C.; Janzen, A.; Barber, T.R.; Seger, A.; Sommerauer, M.; Davis, J.J.; Marek, K.; Hu, M.T.; Oertel, W.H.; et al. Neuronally Derived Extracellular Vesicle α-Synuclein as a Serum Biomarker for Individuals at Risk of Developing Parkinson Disease. JAMA Neurol. 2024, 81, 59. [Google Scholar] [CrossRef]
- Tang, M.K.S.; Yue, P.Y.K.; Ip, P.P.; Huang, R.-L.; Lai, H.-C.; Cheung, A.N.Y.; Tse, K.Y.; Ngan, H.Y.S.; Wong, A.S.T. Soluble E-Cadherin Promotes Tumor Angiogenesis and Localizes to Exosome Surface. Nat. Commun. 2018, 9, 2270. [Google Scholar] [CrossRef] [PubMed]
- Worst, T.S.; von Hardenberg, J.; Gross, J.C.; Erben, P.; Schnölzer, M.; Hausser, I.; Bugert, P.; Michel, M.S.; Boutros, M. Database-Augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker. Mol. Cell. Proteom. 2017, 16, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, X.; Gu, J.; Sun, Z.; Yang, S.; Mu, Y.; Guan, M.; Chen, K.; Liu, W.; Ruan, H.; et al. CEACAM6 Serves as a Biomarker for Leptomeningeal Metastasis in Lung Adenocarcinoma. Cancer Med. 2023, 12, 4521–4529. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Liu, S.; Wang, T.; Ianni, A.; Bober, E.; Braun, T.; Xiang, R.; Yue, S. Exosomal Tetraspanins Mediate Cancer Metastasis by Altering Host Microenvironment. Oncotarget 2017, 8, 62803–62815. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from Antigen-Pulsed Dendritic Cells Induce Stronger Antigen-Specific Immune Responses than Microvesicles in Vivo. Sci. Rep. 2017, 7, 17095. [Google Scholar] [CrossRef]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune Cells-Derived Exosomes Function as a Double-Edged Sword: Role in Disease Progression and Their Therapeutic Applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Yang, J.-G.; Chen, G.-L.; Xie, Q.-H.; Fu, Q.-Y.; Xia, H.-F.; Li, Y.-C.; Huang, J.; Li, Y.; Wu, M.; et al. PD-1/CD80+ Small Extracellular Vesicles from Immunocytes Induce Cold Tumours Featured with Enhanced Adaptive Immunosuppression. Nat. Commun. 2024, 15, 3884. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as Modulators of Exosomal Alpha-Synuclein Transmission. Cell Death Dis. 2019, 10, 174. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.; Rao, A.; Braganhol, E.; Whiteside, T.L. Molecular Profiles and Immunomodulatory Activities of Glioblastoma-Derived Exosomes. Neurooncol. Adv. 2020, 2, vdaa056. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Wu, C.-C.; Wu, C.-C.; Chiang, S.-F.; Lu, Y.-T.; Yeh, C.-Y.; You, J.-F.; Chu, L.J.; Yeh, T.-S.; Yu, J.-S. Extracellular Vesicle Membrane Protein Profiling and Targeted Mass Spectrometry Unveil CD59 and Tetraspanin 9 as Novel Plasma Biomarkers for Detection of Colorectal Cancer. Cancers 2022, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Vella, L.J.; Behren, A.; Coleman, B.; Greening, D.W.; Hill, A.F.; Cebon, J. Intercellular Resistance to BRAF Inhibition Can Be Mediated by Extracellular Vesicle–Associated PDGFRβ. Neoplasia 2017, 19, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, H.; Tian, R.; Liu, H.; Wang, Z.; Wang, Z.; Tian, J.; Cui, Y.; Ren, S.; Zuo, X.; et al. Exosomal EPHA2 Derived from Highly Metastatic Breast Cancer Cells Promotes Angiogenesis by Activating the AMPK Signaling Pathway through Ephrin A1-EPHA2 Forward Signaling. Theranostics 2022, 12, 4127–4146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Li, D.; Gu, J.; Qian, D.; Qin, X.; Chen, Y. Exosome Carrying PSGR Promotes Stemness and Epithelial-Mesenchymal Transition of Low Aggressive Prostate Cancer Cells. Life Sci. 2021, 264, 118638. [Google Scholar] [CrossRef]
- Hyung, S.; Ko, J.; Heo, Y.J.; Blum, S.M.; Kim, S.T.; Park, S.H.; Park, J.O.; Kang, W.K.; Lim, H.Y.; Klempner, S.J.; et al. Patient-Derived Exosomes Facilitate Therapeutic Targeting of Oncogenic MET in Advanced Gastric Cancer. Sci. Adv. 2023, 9, eadk1098. [Google Scholar] [CrossRef]
- Pan, D.; Chen, J.; Feng, C.; Wu, W.; Wang, Y.; Tong, J.; Zhou, D. Preferential Localization of MUC1 Glycoprotein in Exosomes Secreted by Non-Small Cell Lung Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 323. [Google Scholar] [CrossRef]
- Shi, J.; Ren, Y.; Zhen, L.; Qiu, X. Exosomes from Breast Cancer Cells Stimulate Proliferation and Inhibit Apoptosis of CD133+ Cancer Cells in Vitro. Mol. Med. Rep. 2015, 11, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, H.; Heikamp, E.; Turley, H.; Dragovic, R.; Thomas, P.; Oon, C.E.; Leek, R.; Edelmann, M.; Kessler, B.; Sainson, R.C.A.; et al. New Mechanism for Notch Signaling to Endothelium at a Distance by Delta-like 4 Incorporation into Exosomes. Blood 2010, 116, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ma, J.; Zhao, H.; Wang, Q.; Guo, X.; Chen, L.; Cao, Z.; Xu, J.; Zhang, B.; Zhou, X. Serum Exosomes From Epithelial Ovarian Cancer Patients Contain LRP1, Which Promotes the Migration of Epithelial Ovarian Cancer Cell. Mol. Cell. Proteom. 2023, 22, 100520. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Long, M.; Zhou, X.; Yuan, M.; Yang, L.; Luo, W.; Cheng, Y.; Zhang, X.; Jiang, W.; et al. Single-Cell Transcriptome Sequencing-Based Analysis: Probing the Mechanisms of Glycoprotein NMB Regulation of Epithelial Cells Involved in Silicosis. Part. Fibre Toxicol. 2023, 20, 29. [Google Scholar] [CrossRef]
- van Niel, G.; Bergam, P.; Di Cicco, A.; Hurbain, I.; Lo Cicero, A.; Dingli, F.; Palmulli, R.; Fort, C.; Potier, M.C.; Schurgers, L.J.; et al. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015, 13, 43–51. [Google Scholar] [CrossRef] [PubMed]
- He, J.-G.; Wu, X.-X.; Li, S.; Yan, D.; Xiao, G.-P.; Mao, F.-G. Exosomes Derived from MicroRNA-540-3p Overexpressing Mesenchymal Stem Cells Promote Immune Tolerance via the CD74/Nuclear Factor-KappaB Pathway in Cardiac Allograft. World J. Stem Cells 2024, 16, 1022–1046. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wang, Y.; Zhao, D.; Sun, C.; Zhou, S.; Xu, D.; Zhao, J. Exosomes Derived from CXCR4-Overexpressing BMSC Promoted Activation of Microvascular Endothelial Cells in Cerebral Ischemia/Reperfusion Injury. Neural Plast. 2020, 2020, 8814239. [Google Scholar] [CrossRef]
- Srinivasan, S.; Su, M.; Ravishankar, S.; Moore, J.; Head, P.; Dixon, J.B.; Vannberg, F. TLR-Exosomes Exhibit Distinct Kinetics and Effector Function. Sci. Rep. 2017, 7, 41623. [Google Scholar] [CrossRef]
- Li, T.; Tao, Z.; Zhu, Y.; Liu, X.; Wang, L.; Du, Y.; Cao, J.; Wang, B.; Zhang, J.; Hu, X. Exosomal Annexin A6 Induces Gemcitabine Resistance by Inhibiting Ubiquitination and Degradation of EGFR in Triple-Negative Breast Cancer. Cell Death Dis. 2021, 12, 684. [Google Scholar] [CrossRef]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Wu, B.; Li, T.; Beer, L.A.; Sharma, G.; Li, M.; Lee, C.N.; Liu, S.; Yang, C.; Huang, L.; et al. HRS Phosphorylation Drives Immunosuppressive Exosome Secretion and Restricts CD8+ T-Cell Infiltration into Tumors. Nat. Commun. 2022, 13, 4078. [Google Scholar] [CrossRef]
- Edgar, J.R.; Manna, P.T.; Nishimura, S.; Banting, G.; Robinson, M.S. Tetherin Is an Exosomal Tether. Elife 2016, 5, e17180. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The Role of Exosomal MiRNAs in Cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Han, Z.; Chen, H.; Guo, Z.; Shen, J.; Luo, W.; Xie, F.; Wan, Y.; Wang, S.; Li, J.; He, J. Circular RNAs and Their Role in Exosomes. Front. Oncol. 2022, 12, 848341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Song, X.; Wang, L.; Zhang, Y.; Tang, Y.; Wang, S.; Li, L.; Wu, Y.; Song, X.; Xie, L. Plasma Exosomal TRNA-Derived Fragments as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 1037523. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; You, Q.; Zhu, F.; Zhang, Y. Serum Exosomal Small Nucleolar RNA (SnoRNA) Signatures as a Predictive Biomarker for Benign and Malignant Pulmonary Nodules. Cancer Cell Int. 2024, 24, 341. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, T.A.P.; Mol, S.; de Bruin, S.; Peters, A.; Zhang, X.; Lindenbergh, M.F.S.; Beuger, B.M.; van Stalborch, A.D.; Spaan, T.; de Jong, E.C.; et al. Y-RNA Subtype Ratios in Plasma Extracellular Vesicles Are Cell Type- Specific and Are Candidate Biomarkers for Inflammatory Diseases. J. Extracell. Vesicles 2020, 9, 1764213. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of Double-Stranded Genomic DNA Spanning All Chromosomes with Mutated KRAS and P53 DNA in the Serum Exosomes of Patients with Pancreatic Cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Liu, J.; Kong, X.; Yin, Y.; Jia, Z.; Zhang, X.; Peng, B.; Ji, M.; Pan, W. Exosomal HBV-DNA for Diagnosis and Treatment Monitoring of Chronic Hepatitis B. Open Life Sci. 2023, 18, 20220585. [Google Scholar] [CrossRef]
- Singh, P.P.; Li, L.; Schorey, J.S. Exosomal RNA from Mycobacterium Tuberculosis -Infected Cells Is Functional in Recipient Macrophages. Traffic 2015, 16, 555–571. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, Y.; Xie, Y.; Yu, X.; Zhang, S.; Ge, J.; Li, Z.; Ye, G.; Guo, J. Extracellular Vesicles-Associated TRNA-Derived Fragments (TRFs): Biogenesis, Biological Functions, and Their Role as Potential Biomarkers in Human Diseases. J. Mol. Med. 2022, 100, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Konaka, H.; Kato, Y.; Hirano, T.; Tsujimoto, K.; Park, J.; Koba, T.; Aoki, W.; Matsuzaki, Y.; Taki, M.; Koyama, S.; et al. Secretion of Mitochondrial DNA via Exosomes Promotes Inflammation in Behçet’s Syndrome. EMBO J. 2023, 42, e112573. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.A.; Sampey, G.C.; Lepene, B.; Akpamagbo, Y.; Barclay, R.A.; Iordanskiy, S.; Hakami, R.M.; Kashanchi, F. Presence of Viral RNA and Proteins in Exosomes from Cellular Clones Resistant to Rift Valley Fever Virus Infection. Front. Microbiol. 2016, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Peng, W.; Wang, R.; Bai, S.; Cao, M.; Xiong, S.; Li, Y.; Yang, Y.; Liang, J.; Liu, L.; et al. Exosome-Derived TRNA Fragments TRF-GluCTC-0005 Promotes Pancreatic Cancer Liver Metastasis by Activating Hepatic Stellate Cells. Cell Death Dis. 2024, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Pang, H.; Shi, X.; Li, J.; Wang, Y.; Luo, S.; Lin, J.; Yu, H.; Xiao, Y.; Li, X.; et al. Plasma-Derived Exosomal MRNA Profiles Associated with Type 1 Diabetes Mellitus. Front. Immunol. 2022, 13, 995610. [Google Scholar] [CrossRef]
- Hou, C.-C.; Bao, H.-F.; Shen, Y.; Ye, J.-F.; Ma, H.-F.; Guan, J.-L. Expression of MiRNAs Derived from Plasma Exosomes in Patients with Intestinal Behçet’s Syndrome. Clin. Exp. Rheumatol. 2021, 40, 1480–1490. [Google Scholar] [CrossRef]
- Hong, J.; Zheng, S.; Jiang, D. The Contributions of Extrachromosomal DNA Elements in Neoplasm Progression. Am. J. Cancer Res. 2021, 11, 2417–2429. [Google Scholar]
- Yuyama, K.; Igarashi, Y. Exosomes as Carriers of Alzheimer’s Amyloid-ß. Front. Neurosci. 2017, 11, 229. [Google Scholar] [CrossRef]
- Iguchi, Y.; Eid, L.; Parent, M.; Soucy, G.; Bareil, C.; Riku, Y.; Kawai, K.; Takagi, S.; Yoshida, M.; Katsuno, M.; et al. Exosome Secretion Is a Key Pathway for Clearance of Pathological TDP-43. Brain 2016, 139, 3187–3201. [Google Scholar] [CrossRef]
- Wang, S.; Kelly, K.; Brotchie, J.M.; Koprich, J.B.; West, A.B. Exosome Markers of LRRK2 Kinase Inhibition. npj Park. Dis. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome Release of β-Catenin: A Novel Mechanism That Antagonizes Wnt Signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Sturm, R.; Henrich, D.; Lupu, L.; Rottluff, K.; Marzi, I.; Leppik, L. Diagnostic and Prognostic Potential of Exosomal Cytokines IL-6 and IL-10 in Polytrauma Patients. Int. J. Mol. Sci. 2023, 24, 11830. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-mediated Effects and Applications in Inflammatory Bowel Disease. Biol. Rev. 2020, 95, 1287–1307. [Google Scholar] [CrossRef]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Greco, M.F.; Rizzuto, A.S.; Zarà, M.; Cafora, M.; Favero, C.; Solazzo, G.; Giusti, I.; Adorni, M.P.; Zimetti, F.; Dolo, V.; et al. PCSK9 Confers Inflammatory Properties to Extracellular Vesicles Released by Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2022, 23, 13065. [Google Scholar] [CrossRef] [PubMed]
- Blandin, A.; Amosse, J.; Froger, J.; Hilairet, G.; Durcin, M.; Fizanne, L.; Ghesquière, V.; Prieur, X.; Chaigneau, J.; Vergori, L.; et al. Extracellular Vesicles Are Carriers of Adiponectin with Insulin-Sensitizing and Anti-Inflammatory Properties. Cell Rep. 2023, 42, 112866. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Puntigam, L.; Hofmann, L.; Jeske, S.S.; Beccard, I.J.; Doescher, J.; Laban, S.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.-N.; et al. Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers 2020, 12, 2110. [Google Scholar] [CrossRef]
- Han, Y.; Bai, X.; Wang, X. Exosomal Myeloperoxidase as a Biomarker of Deep Venous Thrombosis. Ann. Transl. Med. 2022, 10, 9. [Google Scholar] [CrossRef]
- McNamara, R.P.; Costantini, L.M.; Myers, T.A.; Schouest, B.; Maness, N.J.; Griffith, J.D.; Damania, B.A.; MacLean, A.G.; Dittmer, D.P. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. mBio 2018, 9. [Google Scholar] [CrossRef]
- El Safadi, D.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef]
- Desai, P.P.; Narra, K.; James, J.D.; Jones, H.P.; Tripathi, A.K.; Vishwanatha, J.K. Combination of Small Extracellular Vesicle-Derived Annexin A2 Protein and MRNA as a Potential Predictive Biomarker for Chemotherapy Responsiveness in Aggressive Triple-Negative Breast Cancer. Cancers 2022, 15, 212. [Google Scholar] [CrossRef]
- Cheng, W.; Liao, T.; Lin, C.; Yuan, L.E.; Lan, H.; Lin, H.; Teng, H.; Chang, H.; Lin, C.; Yang, C.; et al. RAB27B-activated Secretion of Stem-like Tumor Exosomes Delivers the Biomarker MicroRNA-146a-5p, Which Promotes Tumorigenesis and Associates with an Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Int. J. Cancer 2019, 145, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral Trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 14787. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cerrotti, G.; Sagini, K.; Delo, F.; Buratta, S.; Pellegrino, R.M.; Alabed, H.B.R.; Fratini, F.; Emiliani, C.; Urbanelli, L. Evidence of Lysosomal β-Hexosaminidase Enzymatic Activity Associated with Extracellular Vesicles: Potential Applications for the Correction of Sandhoff Disease. J. Funct. Biomater. 2024, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal Tau with Seeding Activity Is Released from Alzheimer’s Disease Synapses, and Seeding Potential Is Associated with Amyloid Beta. Lab. Investig. 2021, 101, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-G.; Liu, C.; Su, K.; Yu, S.; Zhang, L.; Zhang, S.; Wang, J.; Cao, X.; Grizzle, W.; Kimberly, R.P. A Membrane Form of TNF-α Presented by Exosomes Delays T Cell Activation-Induced Cell Death. J. Immunol. 2006, 176, 7385–7393. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.-P.; Xu, C.; Guo, A. TGF-Β1-Containing Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Proliferation, Migration and Fibrotic Activity in Rotator Cuff Tenocytes. Regen. Ther. 2020, 15, 70–76. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; del Rosario, I.; Paul, K.C.; Wong, D.Y.; Duarte Folle, A.; Markovic, D.; Palma, J.-A.; et al. α-Synuclein in Blood Exosomes Immunoprecipitated Using Neuronal and Oligodendroglial Markers Distinguishes Parkinson’s Disease from Multiple System Atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; West, A.B. Ser(P)-1292 LRRK2 in Urinary Exosomes Is Elevated in Idiopathic Parkinson’s Disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.; Xiao, Y.; Su, G.; Chen, Y.; Cui, Z. Cancer-Testis Antigen LDH-C4 in Tissue, Serum, and Serum-Derived Exosomes Serves as a Promising Biomarker in Lung Adenocarcinoma. Front. Oncol. 2022, 12, 912624. [Google Scholar] [CrossRef]
- Yuan, M.; Zheng, X.; Zheng, S.; Li, H.; Zhang, X.; Chen, Y.; Zhang, X.; Han, B.; Wei, W.; Wu, J.; et al. Exosomal PKM2: A Noninvasive Diagnostic Marker Linking Macrophage Metabolic Reprogramming to Gastric Cancer Pathogenesis. Cancer Sci. 2025, 116, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, Q.; Zeng, H.; Zhao, Q.; Guo, Z.; Zhong, R.; Xie, M.; Cai, X.; Su, J.; He, Z.; et al. Exosomal CA125 as A Promising Biomarker for Ovarian Cancer Diagnosis. J. Cancer 2020, 11, 6445–6453. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.R.; Rebiai, R.; Kwak, A.; Marshall, J.; Wozniak, D.; Scesa, G.; Nguyen, D.; Rue, E.; Pathmasiri, K.C.; Pijewski, R.; et al. The Pathogenic Sphingolipid Psychosine Is Secreted in Extracellular Vesicles in the Brain of a Mouse Model of Krabbe Disease. ASN Neuro. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Ohmi, Y.; Yesmin, F.; Kambe, M.; Kawamoto, Y.; Bhuiyan, R.H.; Mizutani, M.; Hashimoto, N.; Tsuchida, A.; Ohkawa, Y.; et al. Crucial Roles of Exosomes Secreted from Ganglioside GD3/GD2-Positive Glioma Cells in Enhancement of the Malignant Phenotypes and Signals of GD3/GD2-Negative Glioma Cells. Nagoya J. Med. Sci. 2024, 86, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cortez, L.; Kamali-Jamil, R.; Sim, V.; Wille, H.; Kar, S. Implications of Exosomes Derived from Cholesterol-Accumulated Astrocytes in Alzheimer’s Disease Pathology. Dis. Model Mech. 2021, 14, dmm048929. [Google Scholar] [CrossRef]
- Shen, M.; Di, K.; He, H.; Xia, Y.; Xie, H.; Huang, R.; Liu, C.; Yang, M.; Zheng, S.; He, N.; et al. Progress in Exosome Associated Tumor Markers and Their Detection Methods. Mol. Biomed. 2020, 1, 3. [Google Scholar] [CrossRef]
- Wang, L.; Lin, G.; Zuo, Z.; Li, Y.; Byeon, S.K.; Pandey, A.; Bellen, H.J. Neuronal Activity Induces Glucosylceramide That Is Secreted via Exosomes for Lysosomal Degradation in Glia. Sci. Adv. 2022, 8, eabn3326. [Google Scholar] [CrossRef] [PubMed]
- Melero-Fernandez de Mera, R.M.; Villaseñor, A.; Rojo, D.; Carrión-Navarro, J.; Gradillas, A.; Ayuso-Sacido, A.; Barbas, C. Ceramide Composition in Exosomes for Characterization of Glioblastoma Stem-Like Cell Phenotypes. Front. Oncol. 2022, 11, 788100. [Google Scholar] [CrossRef]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced Release of Acid Sphingomyelinase-Enriched Exosomes Generates a Lipidomics Signature in CSF of Multiple Sclerosis Patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef]
- Mosaad, E.; Peiris, H.N.; Holland, O.; Morean Garcia, I.; Mitchell, M.D. The Role(s) of Eicosanoids and Exosomes in Human Parturition. Front. Physiol. 2020, 11, 594313. [Google Scholar] [CrossRef] [PubMed]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen Enrichment in Exosomes Secreted by a Nematode Parasite versus Those Derived from Its Mouse Host: Implications for Exosome Stability and Biology. J. Extracell. Vesicles 2016, 5, 30741. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Bian, Y.; Song, Y.; Zhang, Q.; Wan, X. Exosome-Contained APOH Associated With Antiphospholipid Syndrome. Front. Immunol. 2021, 12, 604222. [Google Scholar] [CrossRef]
- Scavo, M.P.; Negro, R.; Arrè, V.; Depalo, N.; Carrieri, L.; Rizzi, F.; Mastrogiacomo, R.; Serino, G.; Notarnicola, M.; De Nunzio, V.; et al. The Oleic/Palmitic Acid Imbalance in Exosomes Isolated from NAFLD Patients Induces Necroptosis of Liver Cells via the Elongase-6/RIP-1 Pathway. Cell Death Dis. 2023, 14, 635. [Google Scholar] [CrossRef]
- Sharma, R.; Huang, X.; Brekken, R.A.; Schroit, A.J. Detection of Phosphatidylserine-Positive Exosomes for the Diagnosis of Early-Stage Malignancies. Br. J. Cancer 2017, 117, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Lobasso, S.; Tanzarella, P.; Mannavola, F.; Tucci, M.; Silvestris, F.; Felici, C.; Ingrosso, C.; Corcelli, A.; Lopalco, P. A Lipidomic Approach to Identify Potential Biomarkers in Exosomes From Melanoma Cells With Different Metastatic Potential. Front. Physiol. 2021, 12, 748895. [Google Scholar] [CrossRef] [PubMed]
- van de Vlekkert, D.; Demmers, J.; Nguyen, X.-X.; Campos, Y.; Machado, E.; Annunziata, I.; Hu, H.; Gomero, E.; Qiu, X.; Bongiovanni, A.; et al. Excessive Exosome Release Is the Pathogenic Pathway Linking a Lysosomal Deficiency to Generalized Fibrosis. Sci. Adv. 2019, 5, eaav3270. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, D.H.; Kim, S.A. Exosome Secretion and Cellular Signaling Change in a Fabry Disease Cell Model Induced by Gene-Silencing. Vivo 2024, 38, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Ribeiro, B.; Silva, H.G.; Sampaio-Marques, B.; Fraga, A.G.; Azevedo, O.; Pedrosa, J.; Ludovico, P. Inflammation and Exosomes in Fabry Disease Pathogenesis. Cells 2024, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Rozas, A.; Fernández-Simón, E.; Lleixà, M.C.; Belmonte, I.; Pedrosa-Hernandez, I.; Montiel-Morillo, E.; Nuñez-Peralta, C.; Llauger Rossello, J.; Segovia, S.; De Luna, N.; et al. Identification of Serum MicroRNAs as Potential Biomarkers in Pompe Disease. Ann. Clin. Transl. Neurol. 2019, 6, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial Exosomes Facilitate α-Synuclein Transmission in Parkinson’s Disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s Disease Pathology Propagation by Exosomes Containing Toxic Amyloid-Beta Oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Manda, S.V.; Kataria, Y.; Tatireddy, B.R.; Ramakrishnan, B.; Ratnam, B.G.; Lath, R.; Ranjan, A.; Ray, A. Exosomes as a Biomarker Platform for Detecting Epidermal Growth Factor Receptor–Positive High-Grade Gliomas. J. Neurosurg. 2018, 128, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes Derived from Alcohol-Treated Hepatocytes Horizontally Transfer Liver Specific MiRNA-122 and Sensitize Monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Han, J.-A.; Kang, S.M.; Jeong, S.W.; Ryu, T.; Park, H.S.; Yoo, J.-J.; Lee, S.H.; Kim, S.G.; Kim, Y.S.; et al. Clinical Impact of Serum Exosomal MicroRNA in Liver Fibrosis. PLoS ONE 2021, 16, e0255672. [Google Scholar] [CrossRef]
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary Exosomal Wilms’ Tumor-1 as a Potential Biomarker for Podocyte Injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F553–F559. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Cortés-Hernández, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. MiR-29c in Urinary Exosomes as Predictor of Early Renal Fibrosis in Lupus Nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Rostami Hir, S.; Alizadeh, Z.; Mazinani, M.; Mahlooji Rad, M.; Fazlollahi, M.R.; Kazemnejad, A.; Zavaran Hosseini, A.; Moin, M. Exosomal MicroRNAs as Biomarkers in Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20. [Google Scholar] [CrossRef]
- Zou, J.; Peng, H.; Liu, Y. The Roles of Exosomes in Immunoregulation and Autoimmune Thyroid Diseases. Front. Immunol. 2021, 12, 757674. [Google Scholar] [CrossRef]
- Niu, L.-J.; Zhang, Y.-M.; Huang, T.; Sun, X.-F.; Luo, S.-X. Exosomal MicroRNA-155 as a Biomarker for Hepatic Fibrosis Diagnosis and Progression. Ann. Transl. Med. 2021, 9, 137. [Google Scholar] [CrossRef]
- Zhu, X.G.; Zhang, T.N.; Wen, R.; Liu, C.F. Overexpression of MiR-150-5p Alleviates Apoptosis in Sepsis-Induced Myocardial Depression. Biomed. Res. Int. 2020, 2020, 3023186. [Google Scholar] [CrossRef]
- Ye, R.; Lin, Q.; Xiao, W.; Mao, L.; Zhang, P.; Zhou, L.; Wu, X.; Jiang, N.; Zhang, X.; Zhang, Y.; et al. MiR-150-5p in Neutrophil-Derived Extracellular Vesicles Associated with Sepsis-Induced Cardiomyopathy in Septic Patients. Cell Death Discov. 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma Exosomal α-Synuclein Is Likely CNS-Derived and Increased in Parkinson’s Disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef] [PubMed]
- McFaul, M.; Ventura, C.; Evans, S.; Dundar, H.; Rumpler, M.J.; McCloskey, C.; Lowe, D.; Vlassov, A. V Urine Exosome MRNA-Based Test for Monitoring Kidney Allograft Rejection: Effects of Sample Transportation and Storage, and Interference Substances. World J. Methodol. 2023, 13, 492–501. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Li, B.; Yang, S.; Li, W.; Zhao, S.; Ning, J.; Zhou, X.; Cheng, F. Exosomes on the Development and Progression of Renal Fibrosis. Cell Prolif. 2024, 57, e13677. [Google Scholar] [CrossRef]
- Zarà, M.; Amadio, P.; Campodonico, J.; Sandrini, L.; Barbieri, S.S. Exosomes in Cardiovascular Diseases. Diagnostics 2020, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Makler, A.; Asghar, W. Exosomal Biomarkers for Cancer Diagnosis and Patient Monitoring. Expert Rev. Mol. Diagn. 2020, 20, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Arora, S.; Singh, A.K.; Kumar, A. Extracellular Vesicle-Associated MiRNAs in Cornea Health and Disease: Diagnostic Potential and Therapeutic Implications. Targets 2025, 3, 32. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, Opportunity, and Perspective on Exosome Isolation—Efforts for Efficient Exosome-Based Theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Lu, Y.; Luo, X.; Huang, Y.; Xie, T.; Pilarsky, C.; Dang, Y.; Zhang, J. Microfluidic Technology for the Isolation and Analysis of Exosomes. Micromachines 2022, 13, 1571. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Van Deun, J.; Hendrix, A. Is Your Article EV-TRACKed? J. Extracell. Vesicles 2017, 6, 1379835. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke II, D.J. Exosomes: Improved Methods to Characterize Their Morphology, RNA Content, and Surface Protein Biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef]
- Gómez-Serrano, M.; Preußer, C.; Stelter, K.; Pogge von Strandmann, E. The More the Better—Determining the Optimal Range When Performing Single-Vesicle Phenotyping. Trillium Extracell. Vesicles 2021, 1, 26–33. [Google Scholar] [CrossRef]
- Kashkanova, A.D.; Blessing, M.; Gemeinhardt, A.; Soulat, D.; Sandoghdar, V. Precision Size and Refractive Index Analysis of Weakly Scattering Nanoparticles in Polydispersions. Nat. Methods 2022, 19, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.S.; Ahn, Y.; Im, Y.-J.; Kim, S.-S.; Lee, K.-H.; Kim, J.; Choi, Y.; Lee, D.; Kang, E.; Jin, G.; et al. The Characterization of Exosomes from Fibrosarcoma Cell and the Useful Usage of Dynamic Light Scattering (DLS) for Their Evaluation. PLoS ONE 2021, 16, e0231994. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Garcia-Rico, E.; O’Loghlen, A.; Giannini, V.; Alvarez-Puebla, R.A. Surface-Enhanced Raman Scattering (SERS) Spectroscopy for Sensing and Characterization of Exosomes in Cancer Diagnosis. Cancers 2021, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Gajda-Walczak, A.; Ruzycka-Ayoush, M.; Targonska, A.; Mosieniak, G.; Glogowski, M.; Szumera-Cieckiewicz, A.; Prochorec-Sobieszek, M.; Bamburowicz-Klimkowska, M.; Nowicka, A.M.; et al. Parallel SPR and QCM-D Quantitative Analysis of CD9, CD63, and CD81 Tetraspanins: A Simple and Sensitive Way to Determine the Concentration of Extracellular Vesicles Isolated from Human Lung Cancer Cells. Anal. Chem. 2023, 95, 9520–9530. [Google Scholar] [CrossRef]
- Zini, J.; Saari, H.; Ciana, P.; Viitala, T.; Lõhmus, A.; Saarinen, J.; Yliperttula, M. Infrared and Raman Spectroscopy for Purity Assessment of Extracellular Vesicles. Eur. J. Pharm. Sci. 2022, 172, 106135. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Qiu, X.; Mei, Q.; Luo, Y.; Fu, W. Rapid and Sensitive Exosome Detection with CRISPR/Cas12a. Anal. Bioanal. Chem. 2020, 412, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Pan, H.; Yang, M.; Li, B.; Zhu, R.; Xiao, Y.; Sun, W.; Guo, Z.; Liu, S.; Ge, S.; et al. A Universal and Programmable Platform for Fluorescent Detection and Profiling of Exosomes Based on Bispecific Aptamer-Programmed DNAzyme-Switched CRISPR/Cas12a. New J. Chem. 2025, 49, 8666–8674. [Google Scholar] [CrossRef]
- Youssef, E.; Palmer, D.; Fletcher, B.; Vaughn, R. Exosomes in Precision Oncology and Beyond: From Bench to Bedside in Diagnostics and Therapeutics. Cancers 2025, 17, 940. [Google Scholar] [CrossRef]
- Kretschmer, A.; Kajau, H.; Margolis, E.; Tutrone, R.; Grimm, T.; Trottmann, M.; Stief, C.; Stoll, G.; Fischer, C.A.; Flinspach, C.; et al. Validation of a CE-IVD, Urine Exosomal RNA Expression Assay for Risk Assessment of Prostate Cancer Prior to Biopsy. Sci. Rep. 2022, 12, 4777. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Arora, S. Navigating the Global Regulatory Landscape for Exosome-Based Therapeutics: Challenges, Strategies, and Future Directions. Pharmaceutics 2025, 17, 990. [Google Scholar] [CrossRef] [PubMed]
- Stridfeldt, F.; Cavallaro, S.; Hååg, P.; Lewensohn, R.; Linnros, J.; Viktorsson, K.; Dev, A. Analyses of Single Extracellular Vesicles from Non-Small Lung Cancer Cells to Reveal Effects by Epidermal Growth Factor Inhibitor Treatments. Talanta 2022, 259, 124553. [Google Scholar] [CrossRef] [PubMed]
- Palmulli, R.; van Niel, G. To Be or Not to Be… Secreted as Exosomes, a Balance Finely Tuned by the Mechanisms of Biogenesis. Essays Biochem. 2018, 62, 177–191. [Google Scholar] [CrossRef] [PubMed]
- López-Guerrero, J.A.; Valés-Gómez, M.; Borrás, F.E.; Falcón-Pérez, J.M.; Vicent, M.J.; Yáñez-Mó, M. Standardising the Preanalytical Reporting of Biospecimens to Improve Reproducibility in Extracellular Vesicle Research—A GEIVEX Study. J. Extracell. Biol. 2023, 2, e76. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, E.; Park, J.; Choi, S.; Lee, M.-S.; Park, J. Pre-Analytical Handling Conditions and Protein Marker Recovery from Urine Extracellular Vesicles for Bladder Cancer Diagnosis. PLoS ONE 2023, 18, e0291198. [Google Scholar] [CrossRef]
- Danielson, K.M.; Estanislau, J.; Tigges, J.; Toxavidis, V.; Camacho, V.; Felton, E.J.; Khoory, J.; Kreimer, S.; Ivanov, A.R.; Mantel, P.-Y.; et al. Diurnal Variations of Circulating Extracellular Vesicles Measured by Nano Flow Cytometry. PLoS ONE 2016, 11, e0144678. [Google Scholar] [CrossRef]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Jafari, N.; Tamadon, A.; Ghaffarzadeh, A.; Rahbarghazi, R.; Mahdipour, M. Different Storage and Freezing Protocols for Extracellular Vesicles: A Systematic Review. Stem Cell Res. Ther. 2024, 15, 453. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Effect of Exosome Isolation Methods on Physicochemical Properties of Exosomes and Clearance of Exosomes from the Blood Circulation. Eur. J. Pharm. Biopharm. 2016, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Juthani, N.; Doyle, P.S. Quantitative and Multiplex Detection of Extracellular Vesicle-Derived MicroRNA via Rolling Circle Amplification within Encoded Hydrogel Microparticles. Adv. Healthc. Mater. 2022, 11, 2102332. [Google Scholar] [CrossRef]
- Ram Kumar, R.M. Exosome- Machine Learning Integration in Biomedicine: Advancing Diagnosis and Biomarker Discovery. Curr. Med. Chem. 2024, 31, 5760–5771. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, T.; Kong, J.; Chen, H. ChatExosome: An Artificial Intelligence (AI) Agent Based on Deep Learning of Exosomes Spectroscopy for Hepatocellular Carcinoma (HCC) Diagnosis. Anal. Chem. 2025, 97, 4643–4652. [Google Scholar] [CrossRef]
- Verma, N.; Arora, S.; Singh, A.K.; Ahmed, J. Unlocking the Potential of Exosomes ‘Extracellular Vesicles’: Drug Delivery Advancements and Therapeutics in Ocular Diseases. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Ubanako, P.; Mirza, S.; Ruff, P.; Penny, C. Exosome-Mediated Delivery of SiRNA Molecules in Cancer Therapy: Triumphs and Challenges. Front. Mol. Biosci. 2024, 11, 1447953. [Google Scholar] [CrossRef]
- Do, M.A.; Levy, D.; Brown, A.; Marriott, G.; Lu, B. Targeted Delivery of Lysosomal Enzymes to the Endocytic Compartment in Human Cells Using Engineered Extracellular Vesicles. Sci. Rep. 2019, 9, 17274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Q.; Tan, S.; Cai, J.; Ye, X.; Su, G.; Yang, P. Effects of Plasma-Derived Exosomal MiRNA-19b-3p on Treg/T Helper 17 Cell Imbalance in Behçet’s Uveitis. Investig. Opthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Parsaei, A.; Moradi, S.; Masoumi, M.; Davatchi, F.; Najafi, A.; Kooshki, A.M.; Hajighadery, A.; Akhlaghi, M.; Faezi, T.; Kavosi, H. Predictive Value of Erythrocyte Sedimentation Rate and C-Reactive Protein in Behcet’s Disease Activity and Manifestations: A Cross-Sectional Study. BMC Rheumatol. 2022, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- MENTESOGLU, D.; ATAKAN, N. The Association between Behçet Disease Activity and Elevated Systemic Immune–Inflammation Index: A Retrospective Observational Study in a Tertiary Care Hospital. Natl. Med. J. India 2024, 37, 74. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Seo, D.; Choi, Y. Extracellular Vesicle Identification Using Label-Free Surface-Enhanced Raman Spectroscopy: Detection and Signal Analysis Strategies. Molecules 2020, 25, 5209. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; López-Cobo, S.; Reyburn, H.T.; Costa-García, A.; López-Martín, S.; Yáñez-Mó, M.; Cernuda-Morollón, E.; Paschen, A.; Valés-Gómez, M.; Blanco-López, M.C. Development of a Rapid Lateral Flow Immunoassay Test for Detection of Exosomes Previously Enriched from Cell Culture Medium and Body Fluids. J. Extracell. Vesicles 2016, 5, 31803. [Google Scholar] [CrossRef]
| Functional Category | Disease-Relevant Exosomal Elements | Molecular Mechanisms | Disease Applications | Therapeutic/Diagnostic Potential |
|---|---|---|---|---|
| Cell Adhesion and Migration | Tetraspanins [110,111,112], Integrins [84,113,114], ICAM-1 [93,94,95], EpCAM [99,100], L1CAM [115,116], Cadherin [117], CD44 [103], CLDN4 [118], Glypican-1 [35,60], CEACAM5/6 [119] | Metastasis by mediating uptake [114,120] Systemic immunosuppression [86] Immune evasion [87] Induces regulatory cytokines [96] Inhibiting viral entry [105] | Organotropism in many cancers [114] Tetraspanin and metastasis [120] Distinguish benign from cancerous [60] Immunosuppressive correlation [86] Resistance to therapies [105] Response to drug assessment [86] | Exosome inhibitors may inhibit metastasis [87,105,114] Engineered ACE2-exosomes for COVID-19 [106] |
| Immune Modulation and Antigen Presentation | MHC-I/II [89,90], CD86 [121,122], TIM-3 [92], CD80 [123], PD-L1 [85,86], CD47 [87,88], Galectin-9 [96], CD40/CD40L [97], FASL and TRAIL [98], Alpha-Synuclein [124], CTLA-4 [91,125], CD59 [126] | |||
| Signaling Receptors and Disease-Associated Proteins | HER2 [101,102], PDGFRβ [127], EPHA2 [128], PSGR [129], MET [130], MUC1 [131], Prominin-1 [132], Notch receptors [133], LRP1 [134], GPNMB [135], ACE2 [105,106], GLUT-1 [104], ApoE [136], CD74 [137], CXCR [138], TLR [139] | |||
| Vesicle Biogenesis, Structure, and Trafficking | Flotillin-1/2 [57], Annexins [140,141], Caveolin-1 [107,108], ALIX [109], HRS [142], Tetherin [143], LAMP1 [144] |
| Functional Category | Disease-Relevant Exosomal Elements | Molecular Mechanisms | Disease Applications | Therapeutic/Diagnostic Potential |
|---|---|---|---|---|
| Pathological Protein Propagation | Tau [177], Amyloid-β [160] TDP-43, [161], LRRK2 [162] | Spread of Pathogenic Proteins [160,177] Delivery of Signaling and enzymes [164,168,176,178] Direct Ligand–Receptor Signaling [167,178] Antagonism of Signaling Molecules [163] Pathological Protein Clearance [161] | Neurodegenerative Diseases [161,162,177] Cancer [161,179,180] Inflammatory and Autoimmune Diseases [165,181] Infectious Diseases [172,175] Metabolic Diseases [166,168] Cardiovascular Diseases [167,170] Immune Regulation [161,169] | Targeting Pathogenic Exosomes [177] Engineering Exosomes as Therapeutics [176] Cancer Early Detection [35] Cancer Chemotherapy Response [173] |
| Oncogenic Signaling and Metabolic Regulation | β-catenin [163], TGF-β [179], LDH-C4 [182], PKM2 [183], EGFR Hexosaminidase A [176], FABP4 [166], PCSK9 [167], Adiponectin [168] | |||
| Immune System Modulation | IL [164], TNF [178], Calprotectin [165], Nef [171], NS1 [172], MPO [170], | |||
| Cellular Structure and Transport | Annexin [173], Rab [174], Fibronectin [175], EpCAM [100], Glypican-1 [35], CA-125 [184] |
| Functional Category | Disease-Relevant Exosomal Elements | Molecular Mechanisms | Disease Applications | Therapeutic/Diagnostic Potential |
|---|---|---|---|---|
| Membrane Structure and Dynamics | Ceramide [190], Sphingomyelin [191], Cholesterol [187], Glucosylceramide [189], Lactosylceramide [188], Gangliosides [186] | Eicosanoid Delivery Plasmalogen [192] Enrichment [193] Ceramide-Mediated Biogenesis/Signaling [191] Glucosylceramide Shuttling [189] Cholesterol Delivery [187] Malignancy Transfer [186] | Cancer [188,190,194,195] Multiple Sclerosis [191] Alzheimer’s Disease [187] | Inhibiting Exosome Biogenesis [190,191] Engineering Exosomes [189,193] Lipidomics-Based Liquid Biopsies [188,195] |
| Mitochondrial Function and Energy Metabolism | Cardiolipin [194], Fatty Acids [195] | |||
| Cellular Signaling and Inflammation | Phosphatidylserine [196], Phosphatidic Acid [195,197], Eicosanoids [192] | |||
| Metabolic Regulation | Plasmalogens [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljohani, M.A. Exosomes in Clinical Laboratory: From Biomarker Discovery to Diagnostic Implementation. Medicina 2025, 61, 1930. https://doi.org/10.3390/medicina61111930
Aljohani MA. Exosomes in Clinical Laboratory: From Biomarker Discovery to Diagnostic Implementation. Medicina. 2025; 61(11):1930. https://doi.org/10.3390/medicina61111930
Chicago/Turabian StyleAljohani, Majdi A. 2025. "Exosomes in Clinical Laboratory: From Biomarker Discovery to Diagnostic Implementation" Medicina 61, no. 11: 1930. https://doi.org/10.3390/medicina61111930
APA StyleAljohani, M. A. (2025). Exosomes in Clinical Laboratory: From Biomarker Discovery to Diagnostic Implementation. Medicina, 61(11), 1930. https://doi.org/10.3390/medicina61111930






