Reduced Pineal Gland Volume in Oncology Patients: Association with Chemotherapy Duration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
- Oncology patient group: 200 participants (88 males—44% and 112 females—56%), with an average age of 56.22 ± 9.66 years.
- Control group: 200 participants (77 males—38.5% and 123 females—61.5%), with an average age of 46.51 ± 15.57 years.
2.2. Inclusion and Exclusion Criteria
2.3. Patient Data
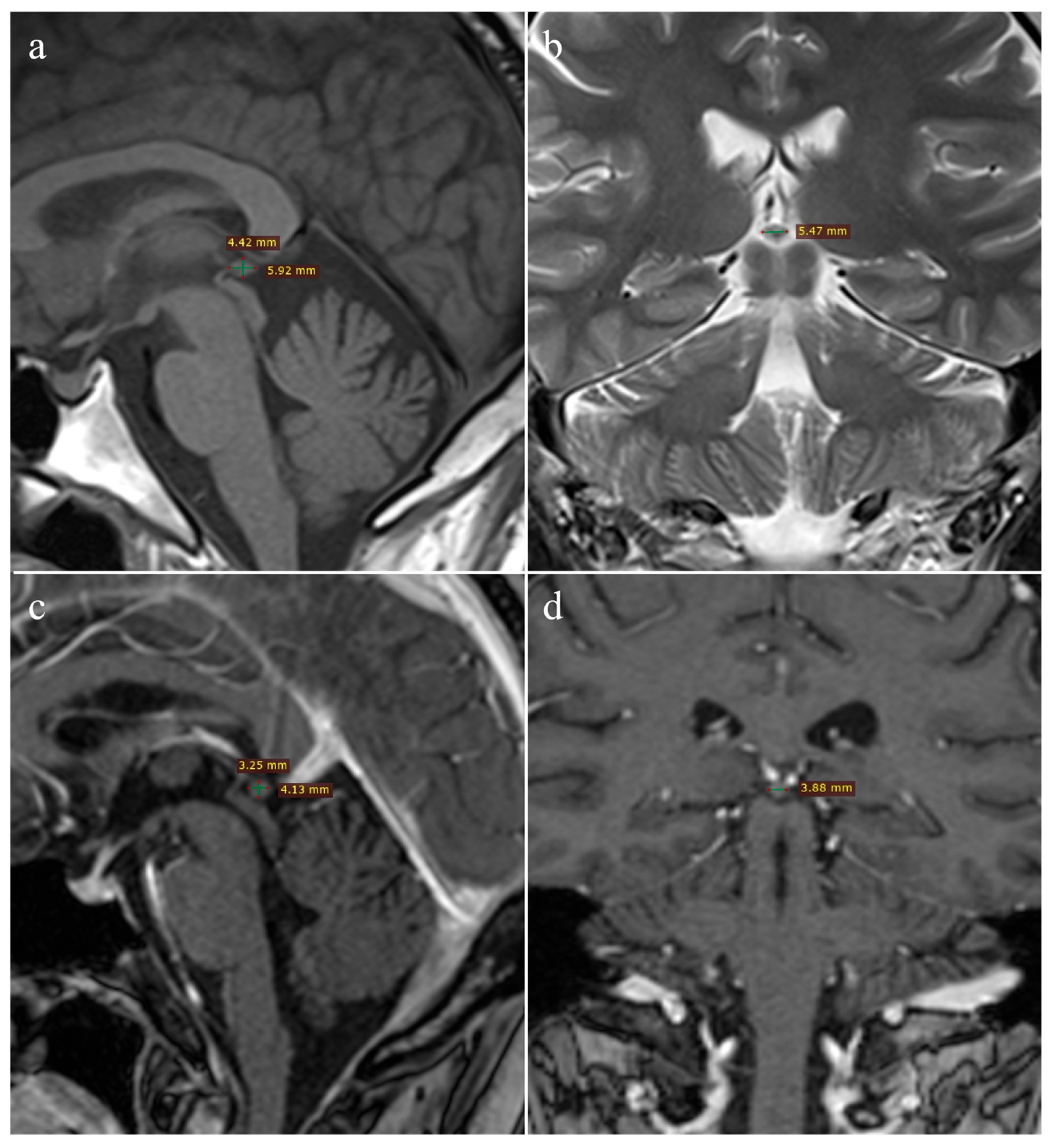
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
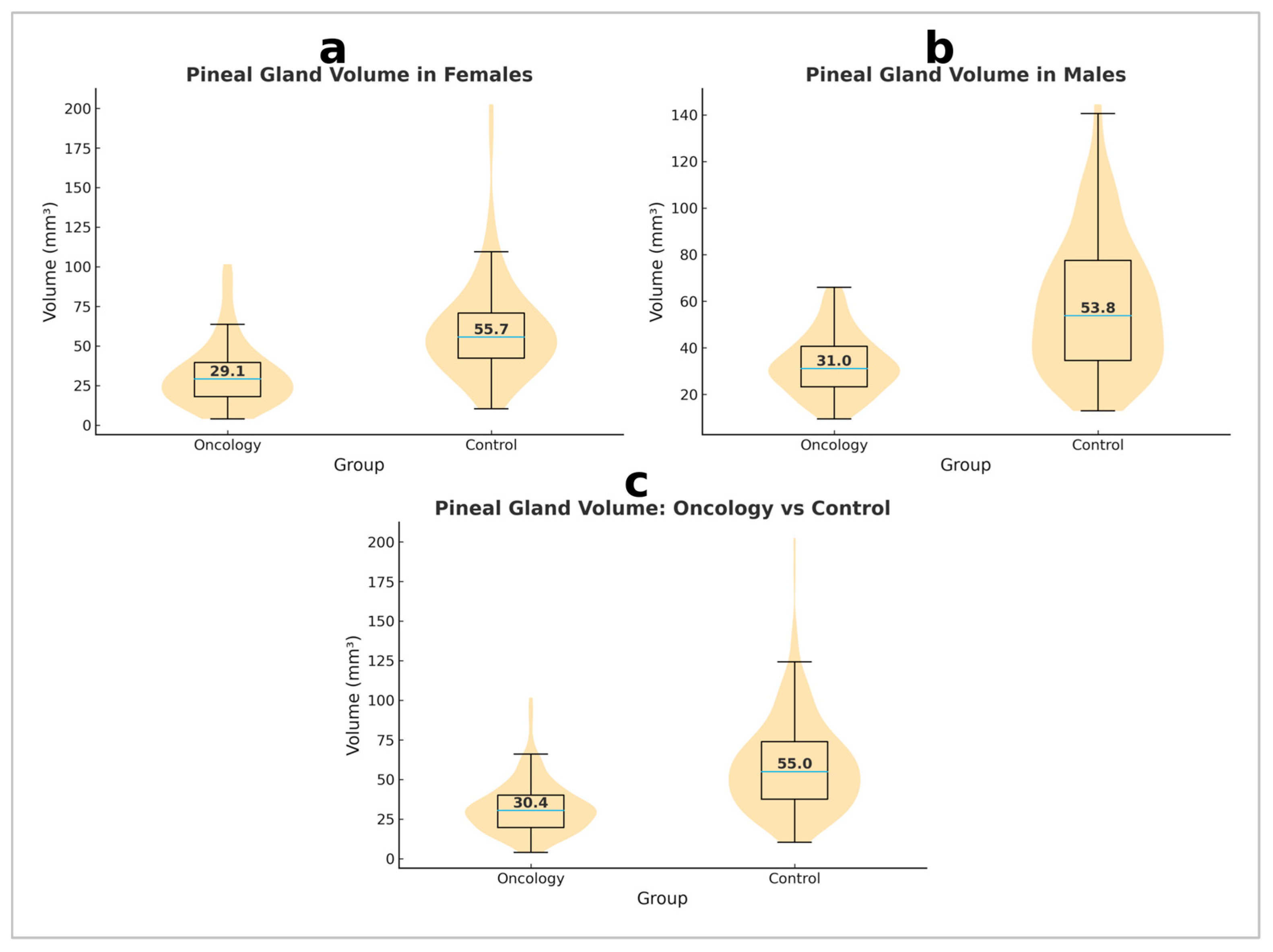
3.2. Differences in Volume Between Oncology Patients and Healthy Controls
3.3. Sex Differences in Pineal Gland Volume Within the Control Group
3.4. Sex Differences in Pineal Gland Volume Within the Oncology Group
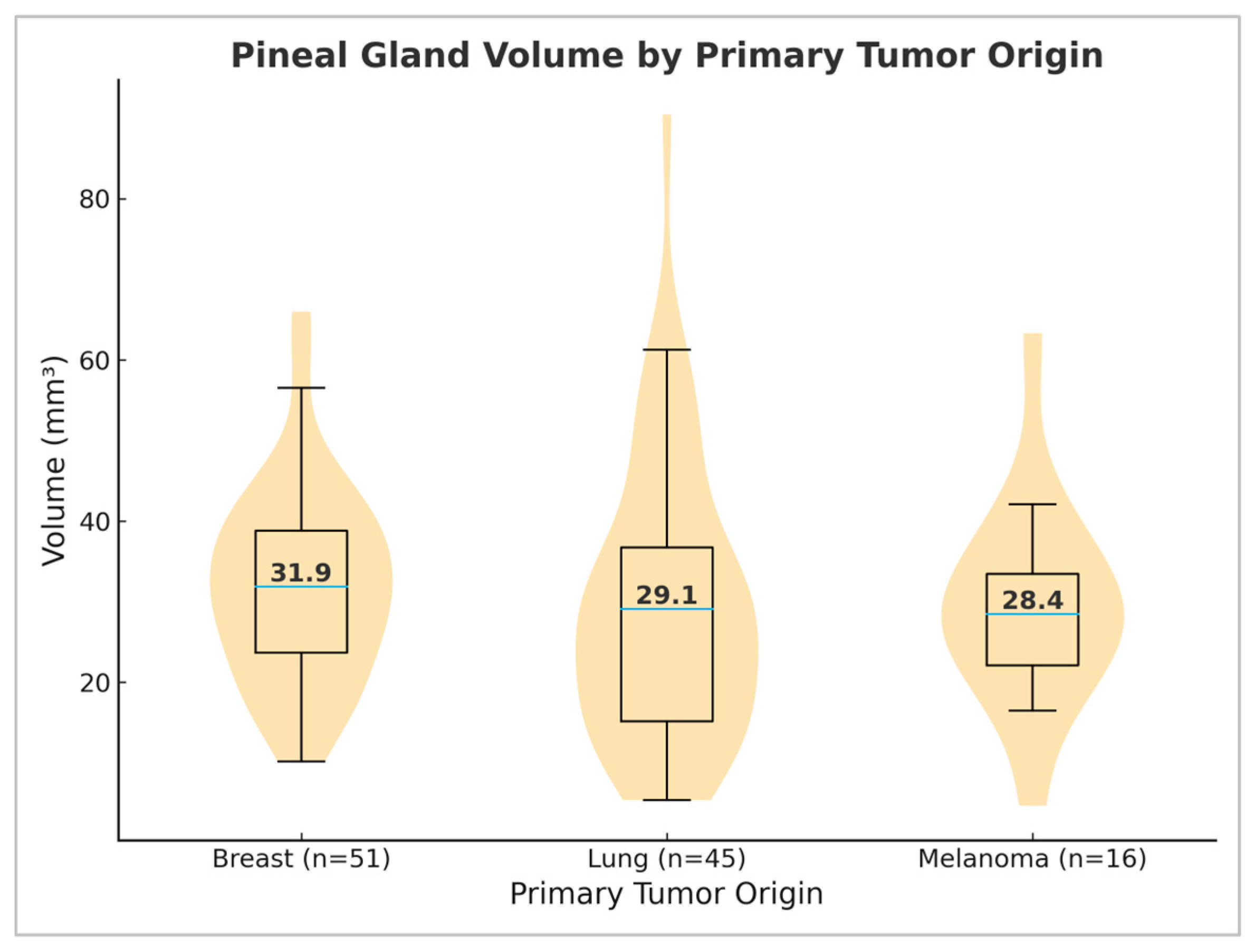
3.5. Differences in Pineal Gland Volume Depending on Primary Tumor Origin
3.6. Correlation Between Age and Pineal Gland Volume
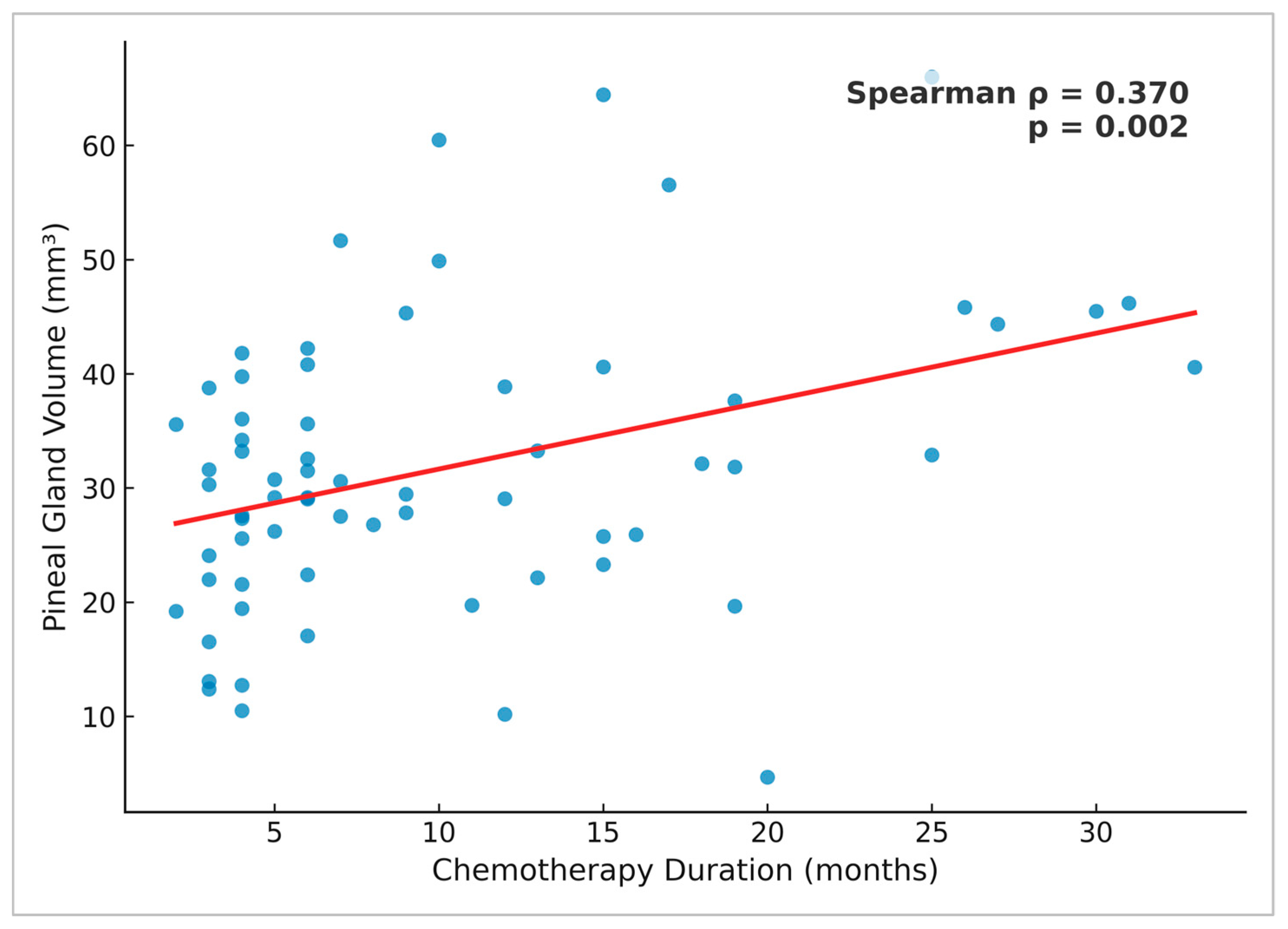
3.7. Correlation Between Chemotherapy Duration and Pineal Gland Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in cancer treatment: Current knowledge and future opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef] [PubMed]
- Montaruli, A.; Castelli, L.; Mulè, A.; Scurati, R.; Esposito, F.; Galasso, L.; Roveda, E. Biological rhythm and chronotype: New perspectives in health. Biomolecules 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, C.; Bartsch, H.; Blask, D.E. The Pineal Gland and Cancer: Neuroimmunoendocrine Mechanisms in Malignancy; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Hajdu, S.I.; Porro, R.S.; Lieberman, P.H.; Foote, F.W. Degeneration of the pineal gland of patients with cancer. Cancer 1972, 29, 703–710. [Google Scholar] [CrossRef]
- Lissoni, P.; Viviani, S.; Bajetta, E.; Buzzoni, R.; Barreca, A.; Mauri, R.; Resentini, M.; Morabito, F.; Esposti, D.; Esposti, G.; et al. A clinical study of the pineal gland activity in oncologic patients. Cancer 1986, 57, 837–842. [Google Scholar] [CrossRef]
- Feuer, G.M.; Kerenyi, N.A. Role of the pineal gland in the development of malignant melanoma. Neurochem. Int. 1989, 14, 265–273. [Google Scholar] [CrossRef]
- Desai, K.; Pereira, K.; Ali, H.; Thirumaran, R. Pineal gland: Sleep, malignancy, and statistics. J. Clin. Oncol. 2024, 42, 14042. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Bartsch, H.; Bartsch, C.; Mecke, D. Analysis of melatonin in patients with cancer of the reproductive system. In The Pineal Gland and Cancer; Springer: Berlin, Germany, 2001. [Google Scholar]
- Lapin, V.; Bartsch, C.; Bartsch, H.; Blask, D.E. Pineal gland and malignancy. In Neuroimmunoendocrine Mechanisms in Malignancy; Bartsch, C., Bartsch, H., Blask, D.E., Eds.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Sumida, M.; Barkovich, A.J.; Newton, T.H. Development of the pineal gland: Measurement with MR. Am. J. Neuroradiol. 1996, 17, 233–236. [Google Scholar]
- Takahashi, T.; Nakamura, M.; Sasabayashi, D.; Nishikawa, Y.; Takayanagi, Y.; Nishiyama, S.; Higuchi, Y.; Furuichi, A.; Kido, M.; Noguchi, K.; et al. Reduced pineal gland volume across the stages of schizophrenia. Schizophr. Res. 2019, 206, 163–170. [Google Scholar] [CrossRef]
- Matsuoka, T.; Oya, N.; Yokota, H.; Akazawa, K.; Yamada, K.; Narumoto, J.; Alzheimer’s Disease Neuroimaging Initiative. Pineal volume reduction in patients with mild cognitive impairment who converted to Alzheimer’s disease. Psychiatry Clin. Neurosci. 2020, 74, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Barbanta, A.; Ettinger, U.; Kumari, V. Pineal abnormalities in psychosis and mood disorders: A systematic review. Brain Sci. 2023, 13, 827. [Google Scholar] [CrossRef]
- Gürbüz, A.A.; Altun, H.; Tahiroğlu, A.Y.; Mert, G.G.; Kızıldağ, B.; Arslan, S.C. Pineal gland volume in children with intellectual disability. Int. J. Dev. Neurosci. 2024, 84, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, J.W.; Suh, S.W.; Byun, S.; Han, J.H.; Bae, J.B.; Kim, J.H.; Kim, K.W. Pineal gland volume is associated with prevalent and incident isolated rapid eye movement sleep behavior disorder. Aging 2020, 12, 884–893. [Google Scholar] [CrossRef]
- Batın, S.; Ekinci, Y.; Gürbüz, K.; Payas, A.; Kurtoğlu, E.; Uçar, İ.; Seber, T.; Arık, M.; Yılmaz, H.; Unur, E. The role of pineal gland volume in the development of scoliosis. Eur. Spine J. 2023, 32, 181–189. [Google Scholar] [CrossRef]
- Scharenberg, K.; Liss, L. The histologic structure of the human pineal body. Prog. Brain Res. 1965, 10, 193–217. [Google Scholar]
- Vuković, M.; Nosek, I.; Boban, J.; Kozić, D. Pineal gland volume loss in females with multiple sclerosis. Front. Neuroanat. 2024, 18, 1386295. [Google Scholar] [CrossRef]
- Acer, N.; Ilıca, A.T.; Turgut, A.T.; Özçelik, Ö.; Yıldırım, B.; Turgut, M. Comparison of three methods for the estimation of pineal gland volume using magnetic resonance imaging. Sci. World J. 2012, 2012, 123412. [Google Scholar] [CrossRef]
- Schmitz, S.A.; Platzek, I.; Kunz, D.; Mahlberg, R.; Wolf, K.J.; Heidenreich, J.O. Computed tomography of the human pineal gland for study of the sleep–wake rhythm: Reproducibility of a semi-quantitative approach. Acta Radiol. 2006, 47, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, D.; Tang, Y.; Fan, L.; Lin, X.; Yu, T.; Qi, H.; Li, Z.; Liu, S. The pineal volume: A three-dimensional volumetric study in healthy young adults using 3.0 T MR data. Int. J. Dev. Neurosci. 2009, 27, 655–660. [Google Scholar] [CrossRef]
- Dogliotti, L.; Berruti, A.; Buniva, T.; Torta, M.; Bottini, A.; Tampellini, M.; Terzolo, M.; Faggiuolo, R.; Angeli, A. Melatonin and human cancer. J. Steroid Biochem. Mol. Biol. 1990, 37, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, L.; Cohen, M.; Roselle, D.; Reichert, C.; Lippman, M.; Chabner, B. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 1982, 216, 1003–1005. [Google Scholar] [CrossRef]
- Ziegler, I.; Maier, K.; Fink, M. Pteridine-binding I-acid glycoprotein from blood of patients with neoplastic diseases. Cancer Res. 1982, 42, 1567–1572. [Google Scholar]
- Pourhanifeh, M.H.; Mahdavinia, M.; Reiter, R.J.; Asemi, Z. Potential use of melatonin in skin cancer treatment: A review of current biological evidence. J. Cell. Physiol. 2019, 234, 12142–12148. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Z.; Sun, H.; Song, J.; Chen, X.; Huang, J.; Lin, X.; Zhou, R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020, 242, 117191. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Friedrich, M.; Schulte, T.; Petrowski, K.; Tibubos, A.N.; Hartung, T.J. The Pittsburgh Sleep Quality Index (PSQI) applied to cancer patients: Psychometric properties and factors affecting sleep quality. Cancer Investig. 2025, 43, 103–113. [Google Scholar] [CrossRef] [PubMed]
| Group | n (%) | Age ( ± SD) |
|---|---|---|
| Oncology patients | 200 (100%) | 56.22 ± 9.66 |
| Males | 88 (44%) | 57.22 ± 9.45 |
| Females | 112 (56%) | 55.45 ± 9.79 |
| Healthy controls | 200 (100%) | 46.51 ± 15.57 |
| Males | 77 (38.5%) | 48.51 ± 16.21 |
| Females | 123 (61.5%) | 45.27 ± 15.09 |
| Sex | Oncology Patients | Healthy Controls | p-Value |
|---|---|---|---|
| Males | 31.96 ± 20.71 | 60.72 ± 32.09 | <0.001 |
| Females | 32.77 ± 13.00 | 58.35 ± 28.70 | <0.001 |
| Total | 32.41 ± 16.79 | 59.26 ± 29.99 | <0.001 |
| Group | Correlation Coefficient (ρ/r *) | p-Value |
|---|---|---|
| Oncology patients | −0.087 | 0.220 |
| Males | −0.044 | 0.683 |
| Females | −0.163 | 0.086 |
| Healthy controls | 0.080 | 0.259 |
| Males | 0.039 | 0.734 |
| Females | 0.152 * | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarošković, M.; Vuković, M.; Vasić, J.; Nosek, I.; Kozić, D. Reduced Pineal Gland Volume in Oncology Patients: Association with Chemotherapy Duration. Medicina 2025, 61, 1923. https://doi.org/10.3390/medicina61111923
Šarošković M, Vuković M, Vasić J, Nosek I, Kozić D. Reduced Pineal Gland Volume in Oncology Patients: Association with Chemotherapy Duration. Medicina. 2025; 61(11):1923. https://doi.org/10.3390/medicina61111923
Chicago/Turabian StyleŠarošković, Milica, Miloš Vuković, Jelena Vasić, Igor Nosek, and Duško Kozić. 2025. "Reduced Pineal Gland Volume in Oncology Patients: Association with Chemotherapy Duration" Medicina 61, no. 11: 1923. https://doi.org/10.3390/medicina61111923
APA StyleŠarošković, M., Vuković, M., Vasić, J., Nosek, I., & Kozić, D. (2025). Reduced Pineal Gland Volume in Oncology Patients: Association with Chemotherapy Duration. Medicina, 61(11), 1923. https://doi.org/10.3390/medicina61111923





