Validation of Sarcopenic Obesity Screening Tools: A Cross-Sectional Analysis Based on ESPEN and EASO Criteria
Abstract
1. Introduction
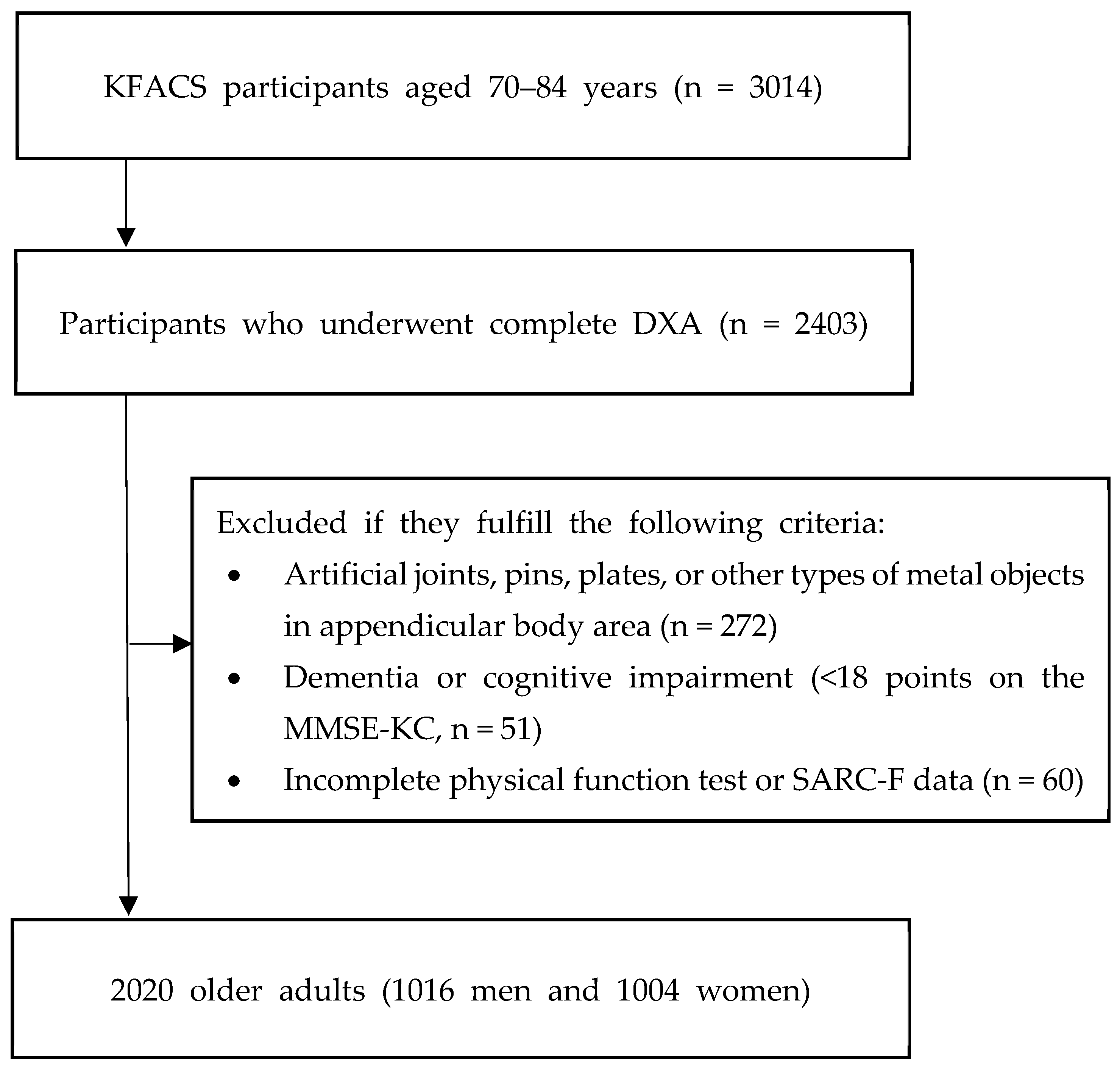
2. Materials and Methods
2.1. Study Design and Population
2.2. Sarcopenic Obesity
2.3. Screening Tools for Sarcopenic Obesity
2.3.1. Body Mass Index and Waist Circumference
2.3.2. Calf Circumference
2.3.3. SARC-F (Strength, Walking Assistance, Rise, Climb, and Falls)
2.3.4. SARC-CalF
2.4. Other Measurements
2.5. Statistical Analyses
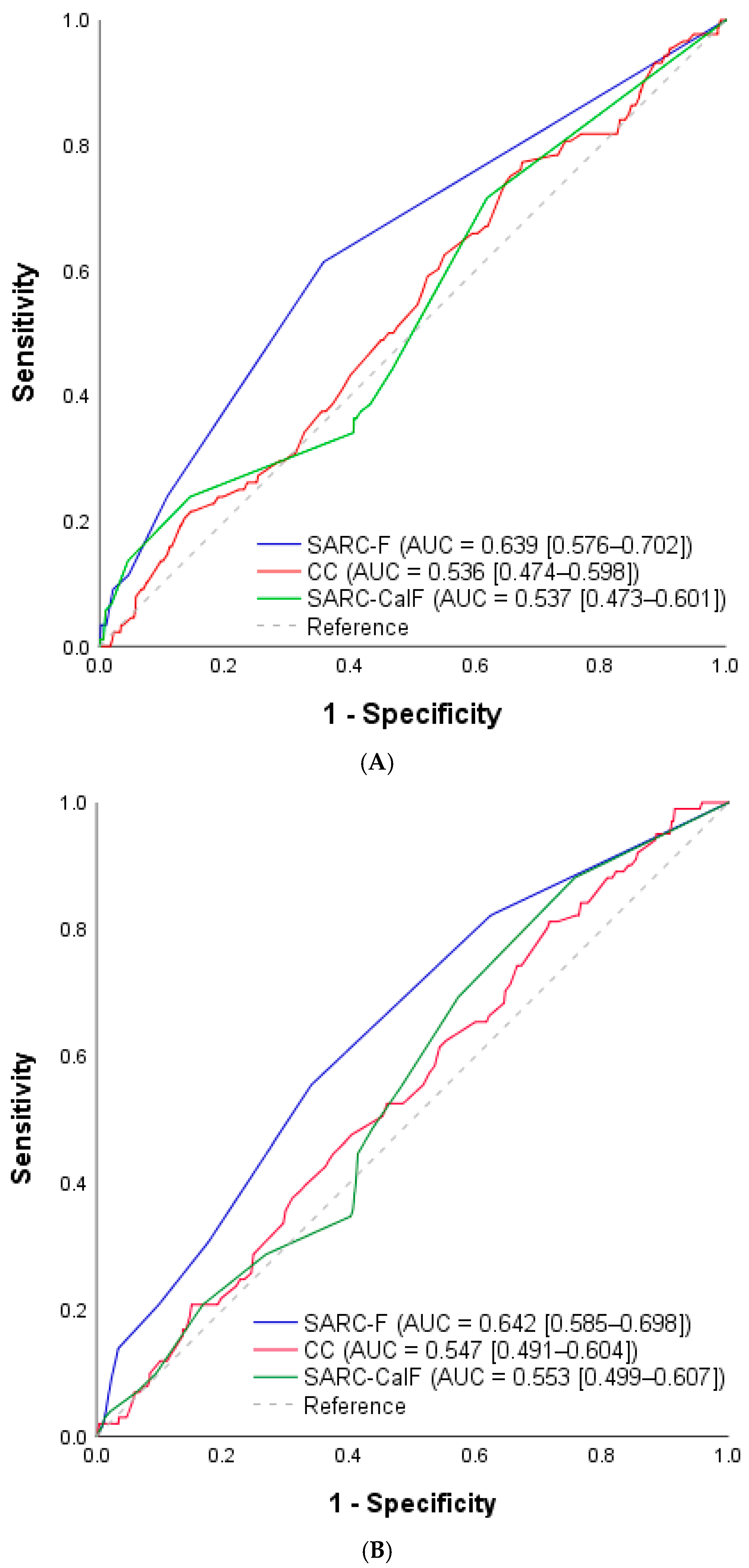
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EASO | European Association for the Study of Obesity |
| CC | Calf circumference |
| BMI | Body mass index |
| WC | Waist circumference |
| AWGS | Asian Working Group for Sarcopenia |
| KFACS | Korean Frailty and Aging Cohort Study |
| AUC | Area under the curve |
| CI | Confidence interval |
References
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic obesity: Time to meet the challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Choi, K.M. Health consequences of sarcopenic obesity: A narrative review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Cappellari, G.G.; Semolic, A.; Zanetti, M.; Vinci, P.; Ius, M.; Guarnieri, G.; Busetto, L.; Donini, L.M.; Barazzoni, R. Sarcopenic obesity in free-living older adults detected by the ESPEN-EASO consensus diagnostic algorithm: Validation in an Italian cohort and predictive value of insulin resistance and altered plasma ghrelin profile. Metabolism 2023, 145, 155595. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Erdoğan, T.; İlhan, B. SARC-F and other screening tests for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 37–42. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Głuszewska, A.; Czesak, J.; Fedyk-Łukasik, M.; Klimek, E.; Sánchez-Rodríguez, D.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. SARC-F as a case-finding tool for sarcopenia according to the EWGSOP2. National validation and comparison with other diagnostic standards. Aging Clin. Exp. Res. 2021, 33, 1821–1829. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Validating the SARC-F: A suitable community screening tool for sarcopenia? J. Am. Med. Dir. Assoc. 2014, 15, 630–634. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.; Cruz-Jentoft, A.; Arai, H.; Kritchevsky, S.; Guralnik, J.; Bauer, J.; Pahor, M.; Clark, B.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in J apanese men and women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef]
- Kusaka, S.; Takahashi, T.; Hiyama, Y.; Kusumoto, Y.; Tsuchiya, J.; Umeda, M. Large calf circumference indicates non-sarcopenia despite body mass. J. Phys. Ther. Sci. 2017, 29, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.-C.; Liu, L.-K.; Lee, W.-J.; Peng, L.-N.; Chen, L.-K. Calf circumference as a screening instrument for appendicular muscle mass measurement. J. Am. Med. Dir. Assoc. 2018, 19, 182–184. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs. SARC-F combined with calf circumference (SARC-CalF). J. Am. Med. Dir. Assoc. 2018, 19, 277.e1–277.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Baek, K.H.; Song, K.-H.; Il Kang, M.; Park, C.Y.; Lee, W.Y.; Oh, K.W. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: The Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab. 2011, 96, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Chang, C.; Tanaka, T.; Kuroda, A.; Tsuji, T.; Akishita, M.; Iijima, K. The association between sarcopenic obesity and depressive symptoms in older Japanese adults. PLoS ONE 2016, 11, e0162898. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Balmain, Australia, 2000. [Google Scholar]
- Seo, M.H.; Kim, Y.-H.; Han, K.; Jung, J.-H.; Park, Y.-G.; Lee, S.-S.; Kwon, H.-S.; Lee, W.-Y.; Yoo, S.J. Prevalence of obesity and incidence of obesity-related comorbidities in Koreans based on National Health Insurance Service health checkup data 2006–2015. J. Obes. Metab. Syndr. 2018, 27, 46. [Google Scholar] [CrossRef]
- Barbosa-Silva, T.G.; Menezes, A.M.B.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C. Enhancing SARC-F: Improving sarcopenia screening in the clinical practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. SARC-F for sarcopenia screening in community-dwelling older adults: Are 3 items enough? Medicine 2018, 97, e11726. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Comparing mini sarcopenia risk assessment with SARC-F for screening sarcopenia in community-dwelling older adults. J. Am. Med. Dir. Assoc. 2019, 20, 53–57. [Google Scholar] [CrossRef]
- Dodds, R.; Murray, J.; Robinson, S.; Sayer, A. The identification of probable sarcopenia in early old age based on the SARC-F tool and clinical suspicion: Findings from the 1946 British birth cohort. Eur. Geriatr. Med. 2020, 11, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sacar, D.E.; Kilic, C.; Karan, M.; Bahat, G. Ability of SARC-F to find probable sarcopenia cases in older adults. J. Nutr. Health Aging 2021, 25, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Drey, M.; Ferrari, U.; Schraml, M.; Kemmler, W.; Schoene, D.; Franke, A.; Freiberger, E.; Kob, R.; Sieber, C. German version of SARC-F: Translation, adaption, and validation. J. Am. Med. Dir. Assoc. 2020, 21, 747–751.e1. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Lee, Y.; Kim, B.; Yoon, T.Y.; Won, C.W. Calf circumference as a simple screening marker for diagnosing sarcopenia in older Korean adults: The Korean Frailty and Aging Cohort Study (KFACS). J. Korean Med. Sci. 2018, 33, e151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Valencak, T.G.; Shan, T. Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. iScience 2024, 27, 109221. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Lim, J.P.; Chew, J.; Tan, A.W.K. Calf circumference as a case-finding tool for sarcopenia: Influence of obesity on diagnostic performance. J. Am. Med. Dir. Assoc. 2020, 21, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Mazocco, L.; Chagas, P.; Barbosa-Silva, T.G.; Gonzalez, M.C.; Schwanke, C.H.A. Accuracy of SARC-F and SARC-CalF for sarcopenia screening in older women from southern Brazil. Nutrition 2020, 79, 110955. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Low, N.A.; Merchant, R.A. Prevalence of sarcopenia in pre-frail community dwelling older adult and utility of SARC-F, SARC-CalF and calf circumference in case finding. J. Frailty Sarcopenia Falls 2020, 5, 53. [Google Scholar] [CrossRef]
| Men (n = 1016) | Women (n = 1004) | Total (n = 2020) | p Value | |
|---|---|---|---|---|
| Age (years) | 76.4 ± 3.9 | 75.4 ± 3.9 | 75.9 ± 3.9 | <0.01 * |
| Height (cm) | 165.0 ± 5.6 | 152.0 ± 5.2 | 158.5 ± 8.4 | <0.01 * |
| Weight (kg) | 65.3 ± 8.9 | 56.7 ± 7.6 | 61.09± 9.4 | <0.01 * |
| Hypertension | 540 (53.1) | 582 (58.0) | 1122 (55.5) | 0.29 |
| Dyslipidemia | 248 (24.4) | 418 (41.6) | 666 (33.0) | <0.01 * |
| Diabetes mellitus | 251 (24.7) | 193 (19.2) | 444 (22.0) | <0.01 * |
| Knee osteoarthritis | 109 (10.7) | 291 (29.0) | 400 (19.8) | <0.01 * |
| Depression | 19 (1.9) | 32 (3.2) | 51 (2.5) | 0.06 |
| Cardiovascular diseases | 58 (5.7) | 31 (3.1) | 89 (4.4) | <0.01 * |
| Alcohol consumption | 523 (51.5) | 110 (11.0) | 633 (31.3) | <0.01 * |
| Current smoking | 110 (10.8) | 10 (1.0) | 120 (5.9) | <0.01 * |
| Diagnostic assessments of SO | ||||
| HGS (kg) | 32.47 ± 5.71 | 21.27 ± 3.85 | 26.90 ± 7.43 | <0.01 * |
| Five Times Sit-to-Stand Test (s) | 10.47 ± 2.99 | 11.81 ± 4.05 | 11.14 ± 3.62 | <0.01 * |
| ALM/W | 29.56 ± 3.21 | 23.91 ± 2.89 | 26.75 ± 4.16 | <0.01 * |
| Fat mass (%) | 0.27 ± 0.06 | 0.37 ± 0.06 | 0.32 ± 0.08 | <0.01 * |
| SO (%) | 88 (8.7) | 101 (10.1) | 189 (9.4) | 0.28 |
| Screening assessments of SO | ||||
| BMI (kg/cm2) | 23.96 ± 2.73 | 24.48 ± 2.82 | 24.22 ± 2.85 | <0.01 * |
| WC (cm) | 88.63 ± 8.45 | 86.13 ± 8.14 | 87.39 ± 8.39 | <0.01 * |
| CC (cm) | 34.34 ± 2.82 | 33.38 ± 2.88 | 33.86 ± 2.89 | <0.01 * |
| SARC-F score | 0.60 ± 1.05 | 1.40 ± 1.58 | 1.00 ± 1.40 | <0.01 * |
| SARC-CalF score | 4.60 ± 5.04 | 5.36 ± 5.33 | 4.97 ± 5.20 | <0.01 * |
| SARC-F (≥4) + high BMI or WC 1 | 14 (1.4) | 69 (6.9) | 83 (4.1) | <0.01 * |
| CC (<Ref. 2) + high BMI or WC | 149 (14.7) | 194 (19.3) | 343 (17.0) | <0.01 * |
| SARC-CalF (≥11) + high BMI or WC | 58 (5.7) | 138 (13.7) | 196 (9.7) | <0.01 * |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| SARC-F (≥4) + high BMI or WC 1 | |||||
| Men | 5.68 | 99.03 | 35.83 | 91.68 | 90.91 |
| Women | 17.82 | 94.35 | 26.17 | 91.09 | 86.62 |
| CC 2 + high BMI or WC | |||||
| Men | 34.09 | 59.48 | 7.42 | 90.45 | 57.27 |
| Women | 34.65 | 59.91 | 8.85 | 89.08 | 57.36 |
| SARC-CalF (≥11) + high BMI or WC | |||||
| Men | 13.64 | 95.04 | 20.56 | 92.12 | 88.04 |
| Women | 19.80 | 86.93 | 14.55 | 90.61 | 80.15 |
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|
| Sarc-F (≥6) + high BMI or WC 1 | |||||
| Men | 2.27 | 99.89 | 66.77 | 91.47 | 91.40 |
| Women | 8.08 | 98.45 | 36.93 | 90.51 | 89.32 |
| Sarc-F (≥5) + high BMI or WC | |||||
| Men | 3.37 | 99.78 | 59.82 | 91.55 | 91.40 |
| Women | 15.49 | 97.79 | 44.01 | 91.15 | 89.47 |
| Sarc-F (≥4) + high BMI or WC | |||||
| Men | 5.68 | 99.03 | 35.83 | 91.68 | 90.91 |
| Women | 17.82 | 94.35 | 26.17 | 91.09 | 86.62 |
| Sarc-F (≥3) + high BMI or WC | |||||
| Men | 6.82 | 98.83 | 28.67 | 91.72 | 90.42 |
| Women | 24.75 | 90.03 | 21.81 | 91.42 | 83.44 |
| Sarc-F (≥2) + high BMI or WC | |||||
| Men | 15.91 | 95.26 | 24.23 | 92.24 | 88.36 |
| Women | 42.57 | 79.96 | 19.27 | 92.53 | 76.18 |
| Sarc-F (≥1) + high BMI or WC | |||||
| Men | 44.32 | 83.19 | 20.08 | 94.00 | 79.81 |
| Women | 59.41 | 61.02 | 14.62 | 93.05 | 60.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Kim, M.; Soh, Y.; Won, C.W. Validation of Sarcopenic Obesity Screening Tools: A Cross-Sectional Analysis Based on ESPEN and EASO Criteria. Medicina 2025, 61, 1909. https://doi.org/10.3390/medicina61111909
Choi S, Kim M, Soh Y, Won CW. Validation of Sarcopenic Obesity Screening Tools: A Cross-Sectional Analysis Based on ESPEN and EASO Criteria. Medicina. 2025; 61(11):1909. https://doi.org/10.3390/medicina61111909
Chicago/Turabian StyleChoi, Seongmin, Miji Kim, Yunsoo Soh, and Chang Won Won. 2025. "Validation of Sarcopenic Obesity Screening Tools: A Cross-Sectional Analysis Based on ESPEN and EASO Criteria" Medicina 61, no. 11: 1909. https://doi.org/10.3390/medicina61111909
APA StyleChoi, S., Kim, M., Soh, Y., & Won, C. W. (2025). Validation of Sarcopenic Obesity Screening Tools: A Cross-Sectional Analysis Based on ESPEN and EASO Criteria. Medicina, 61(11), 1909. https://doi.org/10.3390/medicina61111909





